Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10874
Peer-review started: February 5, 2015
First decision: March 26, 2015
Revised: May 13, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: October 14, 2015
Processing time: 253 Days and 22.5 Hours
AIM: To evaluate the long-term efficacy adefovir (ADV)-based combination therapies in entecavir (ETV)-resistant chronic hepatitis B (CHB) patients.
METHODS: Fifty CHB patients with genotypic resistance to ETV at 13 medical centers in South Korea were included for the analysis. All the patients received rescue therapy with the combination of ADV plus ETV (ADV/ETV, n = 23) or ADV plus lamivudine (LMV) (ADV/LMV, n = 27) for more than 12 mo. Patients were monitored at least every 3-4 mo during ADV-based combination therapy by clinical examination as well as biochemical and virological assessments. Hepatitis B virus (HBV) DNA levels were measured by real-time PCR and logarithmically transformed for analysis. Cumulative rates of virologic response (VR; HBV DNA < 20 IU/mL) were calculated using the Kaplan-Meier method, and the difference was determined by a log-rank test. Multivariate logistic regression and Cox proportional hazards models were used to identify independent risk factors significantly associated with short-term and long-term VR, respectively.
RESULTS: Baseline median HBV DNA levels were 5.53 (2.81-7.63) log10 IU/mL. The most commonly observed ETV genotypic mutation sites were rt184 and rt202. Patients were treated for a median of 27 (12-45) mo. Overall, cumulative VR rates at 6, 12, 24, and 36 mo were 26%, 36%, 45%, and 68%, respectively. Patients treated with the ADV/ETV combination showed higher cumulative VR rates (35%, 43%, 65%, and 76%, respectively) than those with the ADV/LAM combination (18%, 30%, 30%, and 62%, respectively; P = 0.048). In the multivariate analysis, low baseline HBV DNA levels (< 5.2 log10 IU/mL) and initial virologic response at 3 mo (IVR-3; HBV DNA < 3.3 log10 IU/mL after 3 mo) were independent predictive factors for VR. Patients with favorable predictors achieved cumulative VR rates up to 90% at 36 mo. During the same period, the cumulative incidence of virologic breakthrough was as low as 6% in patients with the both favorable predictors.
CONCLUSION: If tenofovir is not available, ADV/ETV combination could be considered in ETV-resistant patients with low HBV DNA titers, and may be continued if IVR-3 is achieved.
Core tip: Studies regarding optimal treatment strategies for entecavir-resistant chronic hepatitis B are sparse. Tenofovir may be the best option, but it is still not available in many countries. Where tenofovir is not available, adefovir plus entecavir can be considered an alternative treatment option in patients with favorable predictive factors. These factors included lower baseline hepatitis B virus (HBV) DNA levels (< 5.2 log10 IU/mL) and reduction of HBV DNA < 3.3 log10 IU/mL after 3 mo of treatment in our study. The present study will guide the treatment of entecavir-resistant chronic hepatitis B.
- Citation: Kim HS, Yim HJ, Jang MK, Park JW, Suh SJ, Seo YS, Kim JH, Kim BH, Park SJ, Lee SH, Kim SG, Kim YS, Lee JI, Lee JW, Kim IH, Kim TY, Kim JW, Jeong SH, Jung YK, Park H, Group SGHOBOARS. Management of entecavir-resistant chronic hepatitis B with adefovir-based combination therapies. World J Gastroenterol 2015; 21(38): 10874-10882
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10874
Chronic hepatitis B virus (HBV) infection remains an important global health problem, and 15%-40% of infected patients may develop cirrhosis-related complications and/or hepatocellular carcinoma (HCC)[1]. Over the past decades, there have been great advances in the management of chronic hepatitis B (CHB) owing to the development of oral nucleos(t)ide analogues (NAs)[2]. The sustained suppression of serum HBV DNA by these agents has been associated with the prevention of liver disease progression and inhibition of HCC development[3,4]. However, a major shortcoming of these NAs is the high rate of virological relapse when treatment is discontinued[5,6]. Therefore, long-term or indefinite treatment with NAs is needed. Unfortunately, the risk of drug resistance increases in proportion to the duration of NAs therapy[7]. For example, cumulative lamivudine (LMV) resistance rates were reported to be 23% and 71% after 1 and 4 years of LMV therapy, respectively[8,9]. Moreover, NAs discontinuation sometimes results in hepatitis flares that may lead to fulminant hepatic failure and death[10]. Thus, the benefits of therapy are attenuated and subsequent therapeutic options may be limited.
Of the NAs, entecavir (ETV) is one of the most potent and safest antiviral agents for HBV infection, with a superior potency to LMV and adefovir (ADV)[11-13]. A previous study showed that the cumulative probability of ETV resistance in treatment naïve patients remained at only 1.2% after up to 5 years of treatment[14]. However, the rate is higher in LMV-resistant patients[15,16], and it may increase to 51% after 5 years of ETV therapy[14]. Resistance to ETV appears to occur through a two-hit mechanism with an initial selection of the M204V/I mutation followed by amino acid substitutions at rtT184, rtS202, or rtM250[17]. Consequently, for CHB patients with LMV resistance, current international guidelines recommend switching to tenofovir disoproxil fumarate (TDF), adding on TDF, or adding on ADV, but not switching to ETV monotherapy[18,19]. However, earlier international guidelines had recommended switching to 1 mg of ETV per day as a treatment option for CHB patients infected with HBV resistant to LMV due to insufficient clinical data[2,20]. As a result of sequential ETV monotherapy in LMV-resistant patients, resistance to ETV developed in a substantial number of patients currently.
For patients with an ETV-resistant CHB, switching to or adding on TDF or TDF-emtricitabine combination therapy are considered as therapeutic options, and combination therapy with ADV plus NAs may still be used in countries where TDF is not available[19,21,22]. It has been shown that both ADV and TDF are active in vitro against ETV-resistant HBV infection, but clinical data on the efficacy of ADV or TDF in patients infected with ETV-resistant HBV strains are limited[21,23-26].
Although there have been few reports on the short-term effects of ADV combination therapy for ETV-resistant HBV infection, especially for that developed after LMV-ETV sequential monotherapy[23,24,27], there is little available clinical information regarding the long-term effects of ADV combination therapy in such patients. Therefore, this study aimed to evaluate the long-term efficacy of combined ADV regimens over 48 wk in CHB patients with ETV resistance.
A total of 50 CHB patients with genotypic ETV resistance, who subsequently received rescue ADV-based combination therapy for more than 12 mo at 13 medical centers in South Korea between January 2008 and October 2012, were enrolled in this retrospective cohort study. ETV resistance was documented in all patients by genotypic analyses at the time of switching to ADV-based combination therapy. We excluded patients infected with other viruses such as hepatitis C virus, human immunodeficiency virus, or hepatitis D virus and those with other concomitant liver diseases such as alcoholic liver disease, autoimmune liver disease, or HCC. All patients were monitored at least every 3-4 mo during ADV-based combination therapy by clinical examination as well as biochemical and virological assessments.
The study was approved by the Institutional Review Boards of each institution, and informed written consent was obtained from all study participants, or their legal guardian. The protocol conforms to the ethical guidelines of the Declaration of Helsinki.
Routine biochemical tests were performed using standard laboratory procedures. Hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), hepatitis B e antigen (HBeAg), and antibody to HBeAg (anti-HBe) levels were measured using a microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, IL, United States). Serum HBV DNA levels were measured by the COBAS TaqMan PCR assay (Roche, Branchburg, NJ, United States; lower limit of detection: 20 IU/mL). Genotypic resistance to LMV, ADV, and ETV was determined by direct sequencing (TRUGENE HBV, Siemens Health Care Diagnostic Solutions, Tarrytown, NY, United States) or restriction fragment mass polymorphism analysis, as previously described[28].
Primary non-response was defined as a failure to reduce serum HBV DNA levels by > 1 log10 IU/mL after 3 mo of treatment[29]. Initial virologic response at 3 mo (IVR-3) and virologic response (VR) were defined as an HBV DNA level < 3.3 log10 IU/mL after 3 mo of treatment[28,30] and an undetectable HBV DNA level (< 20 IU/mL) during treatment, respectively. A biochemical response was defined as normalization of serum alanine aminotransferase (ALT) levels. Virological breakthrough (VBT) was defined as an increase in serum HBV DNA level > 1 log10 IU/mL from the nadir during therapy.
HBV DNA levels were logarithmically transformed for analysis. Continuous variables were analyzed using the Mann-Whitney U-test, whereas categorical variables were analyzed using the χ2 test. A repeated measure analysis was used to compare HBV DNA level reductions according to ADV combination regimens. Cumulative rates of VR and VBT were calculated using the Kaplan-Meier method, and the difference was determined by a log-rank test. Multivariate logistic regression and Cox proportional hazards models were used to identify independent risk factors significantly associated with short-term and long-term VR, respectively. Candidate variables with a P-value < 0.1 on univariate analysis were entered into the regression analysis. A P-value < 0.05 was considered significant. Statistical analyses were performed using SPSS, version 16 (SPSS Inc., Chicago, IL, United States) and the statistical review of the study was performed by a biomedical statistician.
A total of 50 patients who met the inclusion criteria were analyzed. The patients’ baseline characteristics are summarized in Table 1. Thirty-seven (74%) patients were men and the median age was 46.5 (22-74) years. Twelve patients (24%) had liver cirrhosis and 47 patients (94%) were positive for HBeAg. The median HBV DNA level was 5.53 (2.81-7.63) log10 IU/mL and 18 patients had elevated serum ALT levels above the upper limit of normal (40 IU/L). The most commonly observed ETV genotypic mutation sites were rt184 and rt202. The median duration of ETV therapy was 24 (13-58) mo. Out of the total 50 patients, 27 received ADV/LMV combination therapy and 23 received ADV/ETV combination therapy. The median duration of ADV combination therapy was 27 (12-45) mo.
| Variables | Total (n = 50) |
| Age (yr)1 | 46.5 (22-74) |
| Male | 37 (74) |
| HBeAg-positive | 47 (94) |
| Cirrhosis | 12 (24) |
| Antiviral history before ETV (naïve/clevudine/LMV) | 2/2/46 (4/4/92) |
| Duration of ETV (mo)1 | 24 (13-58) |
| Serum ALT (IU/L)1 | 31 (5-1704) |
| Serum total bilirubin level (mg/dL)1 | 0.84 (0.28-4.30) |
| Serum albumin level (g/dL)1 | 4.2 (3.6-5.1) |
| INR1 | 1.01 (0.87-1.30) |
| Serum HBV DNA level (log10 IU/mL)1 | 5.53 (2.81-7.63) |
| Duration of ADV combination therapy (mo)1 | 27 (12-45) |
| Site of ETV-resistant mutations added on rtM204V/I | |
| rt184 | 19 (38) |
| rt202 | 22 (44) |
| rt173 | 1 (2) |
| rt169 + rt184 | 1 (2) |
| rt184 + rt202 | 6 (12) |
| rt184 + rt250 | 1 (2) |
| Patients with elevated ALT level above ULN | 18 (36) |
| Rescue therapy regimens [(ADV + LMV)/(ADV + ETV)] | 27/23 (54/46) |
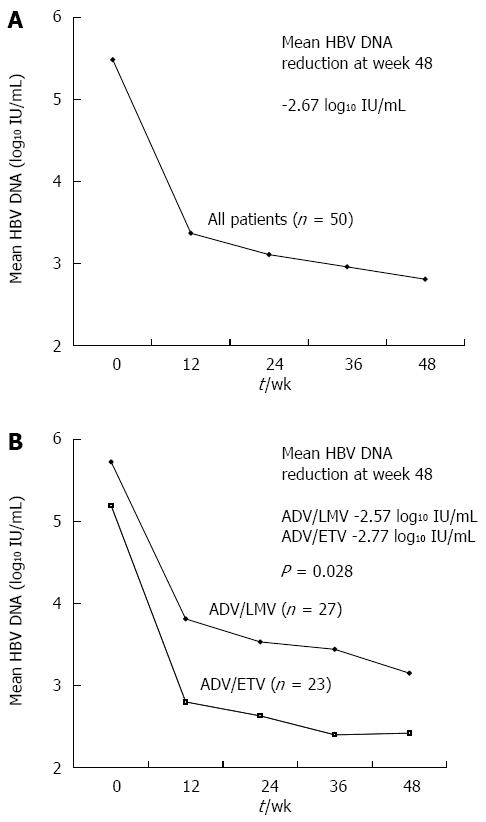
Figure 1 shows the changes in mean HBV DNA levels during the first 12 mo of treatment. After the start of ADV combination therapy, serum HBV DNA levels declined continuously with overall mean changes of -2.14 log10 IU/mL, -2.37 log10 IU/mL, and -2.67 log10 IU/mL at months 3, 6, and 12, respectively. The mean reduction in serum HBV DNA levels from baseline to month 12 was significantly greater in the ADV/ETV combination group than in the ADV/LMV combination group (-2.77 vs -2.57 log10 IU/mL, P = 0.028) by repeated measure analysis (Figure 1). During the first year of treatment, VR (HBV DNA levels < 20 IU/mL) and primary non-response were observed in 18 (36%) and 9 (18%) patients, respectively. Eight of the 18 patients who showed elevated serum ALT levels at baseline experienced normalization of serum ALT levels (44.4%). During the first year of ADV combination therapy, HBeAg loss occurred in 6 (12.8%) of the 47 HBeAg positive patients. Of these, one patient experienced HBeAg seroconversion.
During the long-term treatment period that lasted a median of 27 mo, VR, HBeAg loss, and biochemical response were achieved in an additional 9, 3, and 6 patients, respectively.
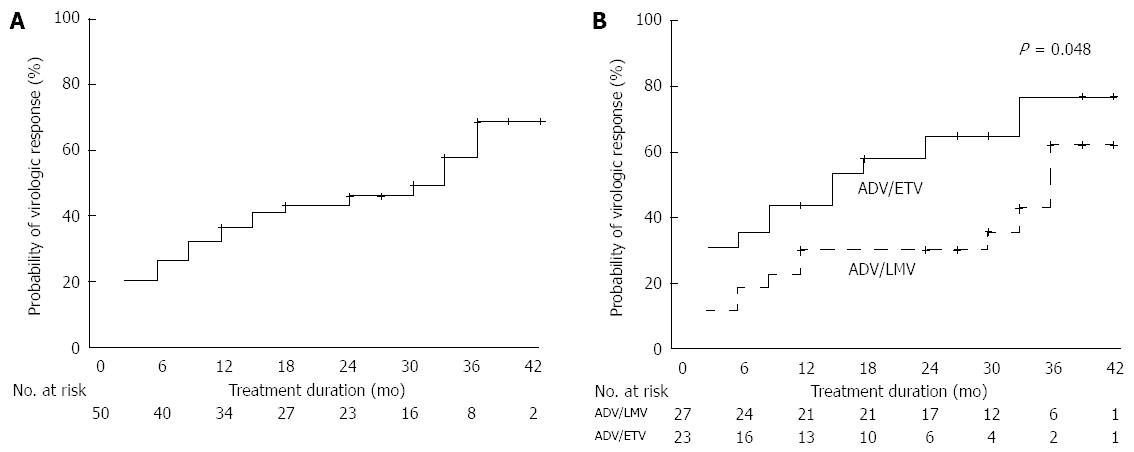
Cumulative VR rates at 6, 12, 24, and 36 mo were 26%, 36%, 45%, and 68%, respectively (Figure 2A). Cumulative VR rates at 6, 12, 24, and 36 mo were, respectively, 35%, 43%, 65%, and 76% in the ADV/ETV combination group and 18%, 30%, 30%, and 62% in the ADV/LMV combination group. There was a significant difference between the two groups (P = 0.048; Figure 2B).
Of the clinical features, a longer duration of ETV treatment prior to ADV combination therapy, low serum HBV DNA levels, and the achievement of IVR-3 were considered favorable factors for VR after 1-year of treatment. Other factors such as age, sex, cirrhosis, HBeAg status, serum ALT levels, international normalized ratio (INR), serum bilirubin levels, serum albumin levels, type of ETV resistance mutation, and type of ADV combination regimen were not significantly associated with VR (Table 2).
| Patients without VR (n = 32) | Patients with VR (n = 18) | P value | |
| Age (yr)1 | 47 (22-70) | 42.5 (33-74) | 0.413 |
| Male | 22 (68.8) | 15 (83.3) | 0.328 |
| HBeAg-positive | 31 (96.9) | 16 (88.9) | 0.291 |
| Cirrhosis | 8 (25) | 4 (22.2) | 1.000 |
| Duration of ETV therapy (mo)1 | 24 (13-48) | 36 (17-58) | 0.003 |
| Serum ALT level (IU/L)1 | 34.5 (12-918) | 29 (5-1704) | 0.210 |
| Serum total bilirubin level (mg/dL)1 | 0.84 (0.31-1.99) | 0.79 (0.28-4.30) | 0.869 |
| Serum albumin level (g/dL)1 | 4.2 (3.6-5.1) | 4.3 (3.6-4.9) | 0.691 |
| INR1 | 1.01 (0.93-1.23) | 1.02 (0.87-1.30) | 0.848 |
| Serum HBV DNA level (log10 IU/mL)1 | 6.16 (3.85-7.63) | 4.24 (2.81-7.08) | < 0.001 |
| Site of ETV-resistant mutations | 0.441 | ||
| rt184 | 12 (37.5) | 7 (38.9) | |
| rt202 | 14 (43.8) | 8 (44.4) | |
| rt173 | 0 (0) | 1 (5.6) | |
| rt169 + rt184 | 0 (0) | 1 (5.6) | |
| rt184 + rt202 | 5 (15.6) | 1 (5.6) | |
| rt184 + rt250, | 1 (3.1) | 0 (0) | |
| Presence of IVR-3 | 7 (21.9) | 17 (94.4) | < 0.001 |
| Rescue therapy regimens (ADV/LMV vs ADV/ETV) | 19 vs 13 (59.4 vs 40.6) | 8 vs 10 (44.4 vs 55.6) | 0.382 |
A multivariate logistic regression model was used to identify independent risk factors significantly associated with VR during the first year. In the univariate analysis, duration of ETV treatment prior to ADV combination therapy, serum HBV DNA levels, and IVR-3 were candidate variables for multivariate analysis (P < 0.1). In the multivariate analysis, IVR-3 and serum HBV DNA levels remained independent predictors of VR (Table 3).
| RR | 95%CI | P value | |
| Duration of ETV therapy (mo) | 1.039 | 0.936-1.153 | 0.473 |
| Serum HBV DNA level (< 5.2 log10 IU/mL) | 7.614 | 1.160-49.986 | 0.034 |
| Presence of IVR-3 | 24.862 | 2.398-257.781 | 0.007 |
A Cox proportional hazards model was used to identify independent risk factors significantly associated with long-term VR. The results were similar to the 1-year results detailed above (Table 4).
| Univariate analysis | Multivariate analysis | |||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Age (yr) | 1.011 | 0.973-1.050 | 0.586 | |||
| Sex (male) | 1.156 | 0.488-2.740 | 0.741 | |||
| HBeAg positivity (-) | 1.905 | 0.568-6.383 | 0.296 | |||
| Disease status (LC) | 0.775 | 0.293-2.054 | 0.609 | |||
| Duration of ETV (mo) | 1.077 | 1.036-1.119 | < 0.001 | 1.022 | 0.970-1.076 | 0.419 |
| Serum ALT (IU/L) | 1.000 | 0.998-1.002 | 0.976 | |||
| Serum total bilirubin level (mg/dL) | 1.405 | 0.774-2.550 | 0.264 | |||
| Serum albumin level (g/dL) | 1.214 | 0.384-3.836 | 0.741 | |||
| INR | 0.137 | 0.001-22.543 | 0.445 | |||
| Serum HBV DNA level (< 5.2 log10 IU/mL) | 5.084 | 2.231-11.581 | < 0.001 | 2.870 | 1.049-7.854 | 0.040 |
| Type of ETV-resistant mutation (rtT184 ) | 0.780 | 0.359-1.693 | 0.529 | |||
| Presence of IVR-3 | 8.822 | 3.228-24.114 | < 0.001 | 4.417 | 1.402-13.918 | 0.011 |
| Rescue therapy regimens (ADV/ETV) | 2.007 | 0.928-4.338 | 0.077 | 1.678 | 0.683-4.119 | 0.259 |
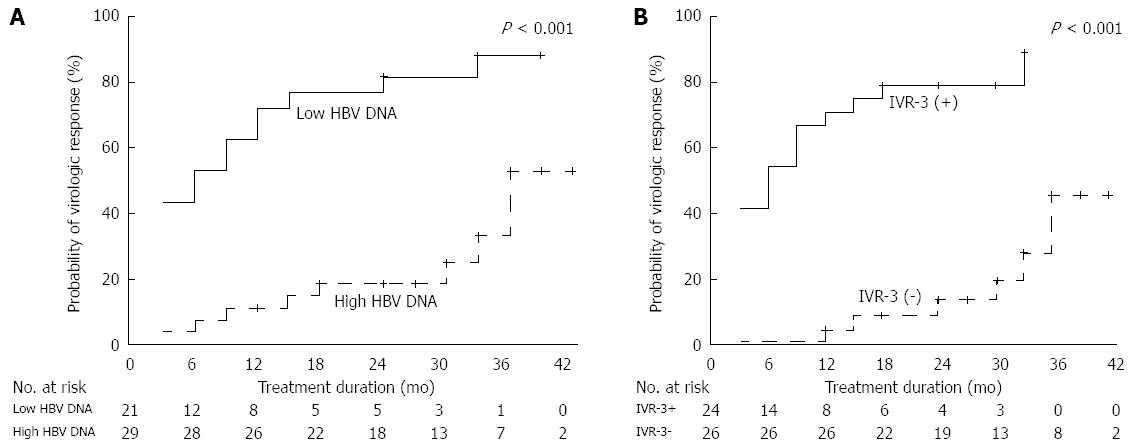
Twenty-one patients (42%) had low baseline serum HBV DNA levels (< 5.2 log10 IU/mL) and IVR-3 was achieved in 24 of 50 (48%) patients. Patients with a low serum HBV DNA level or IVR-3 had a significantly higher probability of achieving VR. Cumulative VR rates at 6, 12, 24, and 36 mo were 52%, 71%, 81%, and 87% in patients with low baseline serum HBV DNA levels and 7%, 10%, 18%, and 52% in patients with high baseline serum HBV DNA levels, respectively(P < 0.001; Figure 3A). Cumulative VR rates at 6, 12, 24, and 36 mo were 0%, 4%, 13%, and 46% in patients without IVR-3 and 54%, 71%, 80%, and 90% in patients with IVR-3, respectively (P < 0.001; Figure 3B). VR was achieved in only 18% (4/22) of patients without favorable predictors (no IVR-3 and a high HBV DNA level) and in 73% (8/11) of patients with one predictor. However, patients with two favorable predictors achieved VR in 88% of cases (15/17). During the treatment period, the respective cumulative incidence of VR at 36 mo according to the increasing number of favorable predictors was 38%, 85%, and 88%. There was a significant difference among the groups (P < 0.001; Figure 4).
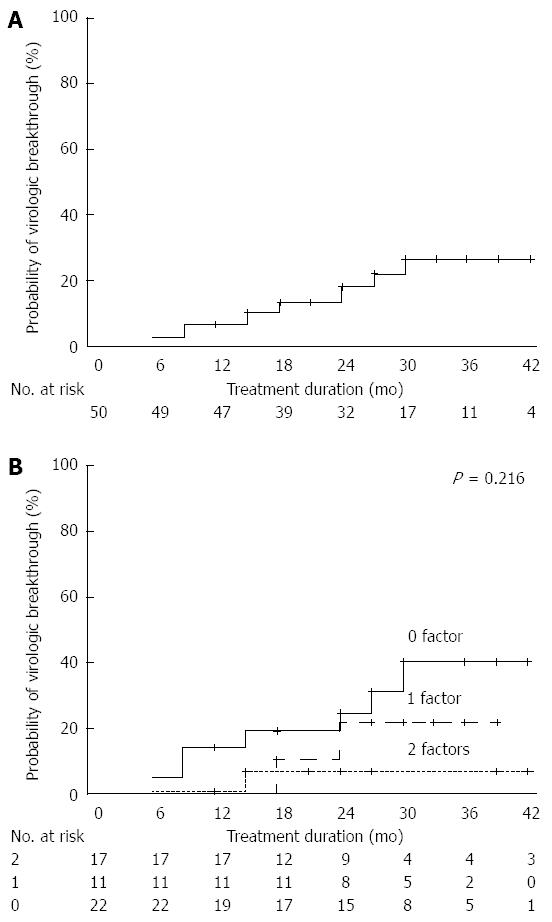
VBT was observed in 10 patients during the follow-up period. Cumulative VBT rates at 6, 12, 24, and 36 mo were 2%, 6%, 18%, and 26%, respectively (Figure 5A). Only one patient with VR (3.7%, 1/27) and one patient with two favorable predictors (4.5%, 1/22) experienced VBT. During the treatment period, the respective cumulative incidence of VBT at 36 mo according to the increasing number of favorable predictors was 40%, 21%, and 6% (Figure 5B).
Although highly potent NAs with optimal genetic resistance profiles (ETV and TDF) have been introduced, prior NAs with lower genetic barriers continue to cause drug resistance, which is an important clinical problem. In particular, sequential monotherapy leads to the emergence of multi-drug resistant mutants, a matter of great concern in the management of CHB patients. So far, few studies have evaluated the efficacy of ADV combination therapy for ETV-resistant HBV infection. However, previous studies included small numbers of patients and/or patients with concurrent ADV resistance[21,23,27]. To our knowledge, this is one of the largest studies and the first long-term follow-up study (up to 4 years) of the efficacy of ADV-based combination therapy in ETV-resistant CHB patients.
Previous studies showed VR rates of about 50% to ADV/ETV combination therapy in patients with LMV- and ETV-resistant HBV infection[21,23,24]. In the present study, however, 27 of 50 (54%) patients showed a VR with respective cumulative VR rates of 36% and 68% at 12 and 36 mo. The reason for the relatively high VR in our study may be due to the difference in the study population and follow-up duration compared to previous studies. Our study excluded patients with prior ADV exposure in order to accurately evaluate the antiviral efficacy of ADV-based regimens in those with resistance to ETV, and the patients were followed up for a median of 27 mo (up to 4 years).
This study demonstrated that the antiviral efficacy of ADV/ETV combination therapy is superior to that of ADV/LMV combination therapy in patients with ETV resistance. During the first year of therapy, the mean reduction in serum HBV DNA levels was significantly greater in the ADV/ETV combination group than in the ADV/LMV combination group (-2.77 vs -2.57 log10 IU/mL, P = 0.028) by repeated measure analysis. In addition, during the long-term follow-up period, the respective cumulative VR rates at 12 and 36 mo were 43% and 76% in the ADV/ETV combination group and 30% and 62% in the ADV/LMV combination group. There was a significant difference between the two groups (P = 0.048). This is the first such finding in ETV-resistant CHB patients; previous studies did not demonstrate the superiority of ADV/ETV combination therapy over ADV/LMV combination therapy in LMV- and ETV-resistant patients[24,27]. However, in a previous study, ADV/ETV combination therapy was used as rescue therapy in only 18 patients[24], which is a relatively small number for a comparison of the efficacy of the ADV/ETV and ADV/LMV regimens.
Another interesting finding of this study is the prognostic role of lower baseline HBV DNA levels and IVR-3, which are predictive factors for short-term and long-term VR. ADV-based combination therapy has proven to be highly effective in patients with lower baseline HBV DNA levels or IVR-3. In fact, cumulative VR rates in patients with lower baseline HBV DNA levels or IVR-3 were very high, reaching 90% at 36 mo. In clinical practice, the ADV/ETV combination can be considered for ETV-resistant CHB patients with lower HBV DNA levels, and IVR-3 may help determine whether ADV/ETV combination therapy could be maintained or should be switched to TDF-based regimens.
A VBT was observed in 10 out of 50 patients during the follow-up period, with a cumulative VBT rate of 26% at 36 mo. Interestingly, only one patient with favorable predictors experienced VBT during the follow-up period, with a 6% cumulative VBT rate at 36 mo. No ADV mutations were found in this patient, and serum HBV DNA levels declined again despite maintaining therapy. This indicates a clinically useful long-term efficacy of ADV-based combination therapy in ETV-resistant patients in the presence of favorable predictors of VR such as a lower HBV DNA level and IVR-3.
TDF is a potent HBV inhibitor with a high genetic barrier to resistance and doesn’t exhibit cross resistance with LMV or ETV[22,31]. In recent studies, TDF/ETV combination therapy showed excellent efficacy in patients with multi-drug resistance (MDR) and resulted in a relatively high rate of complete VR at an early time point, even in patients with triple resistance to LAM, ETV, and ADV[25,26]. When considering the potencies of TDF and ADV, a TDF/ETV combination should be superior to an ADV/ETV combination in CHB patients with MDR although comparative data of this is lacking. As there are countries where TDF is still not available, ADV/ETV combination could be considered an alternative option.
Our study has some limitations. First, the sample size was relatively small. However, considering the difficulty of including ETV-resistant CHB patients, the present study would be accepted as a valuable multicenter study and the largest one evaluating ADV-based combination therapy in ETV-resistant CHB patients. Second, the study was performed retrospectively. In future, a prospective study based on TDF mono- or combination therapy should be considered in ETV resistant CHB patients depending on TDF availability.
In conclusion, an ADV/ETV combination was superior to an ADV/LMV combination, and ADV-based combination therapy was effective in patients with favorable predictors.
In countries where tenofovir is not available, the ADV/ETV combination could be considered an alternative treatment option in ETV-resistant patients with a low HBV DNA titer, and may be continued if IVR-3 is achieved.
Antiviral resistance to hepatitis B virus (HBV) leads to attenuation of the therapeutic benefits and limits subsequent treatment options. Entecavir (ETV) is one of the most potent and the safest antiviral agents with high genetic barrier. Studies regarding optimal treatment strategies ETV-resistant chronic hepatitis B (CHB) are sparse.
Both adefovir (ADV) and tenofovir (TDF) are active against ETV-resistant HBV infection in vitro, but clinical data on the efficacy of ADV or TDF in those patients are lacking. Therefore, additional study is needed to determine optimal treatment strategies in ETV-resistant CHB patients.
Previous few studies regarding the efficacy of ADV combination therapy for ETV-resistant CHB were conducted in small numbers of patients and evaluated short- term efficacy. This study is one of the largest studies and the first long-term follow-up study (up to 4 years). Furthermore, it shows predictive factors for virologic response (VR), which will be useful for guidance of the treatment strategy.
This study results suggest the ADV/ETV combination therapy could be considered an alternative treatment option in ETV-resistant CHB patients, especially in those with favorable predictive factors.
Initial virologic response at 3 mo (IVR-3) is defined as an HBV DNA level < 3.3 log10 IU/mL after 3 mo of treatment and demonstrated as a predictive factor for VR.
Here the authors report original data on long term efficacy of ADV-based combination therapies, i.e., ADV/ETV and ADV/LMV, on 50 CHB patients with genotypic resistance to ETV. They find higher rates of virological response in patients treated with ADV/ETV vs ADV/LMV and they identify low baseline HBV DNA levels and IVR-3 as independent predictive factor for VR. Although its interest is limited to countries where TDF is not available or not reimbursed, this study will be the largest one on this topic, hence worthy of attention and consideration.
| 1. | Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191-211, v. [PubMed] |
| 2. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 3. | Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006;11:669-679. [PubMed] |
| 4. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Lee SJ, Joo MK, Kim CH, Choi JH, Jung YK, Yim HJ, Yeon JE, Park JJ, Kim JS. Durability of antiviral response in HBeAg-positive chronic hepatitis B patients who maintained virologic response for one year after lamivudine discontinuation. Dig Dis Sci. 2009;54:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Kim YJ, Kim K, Hwang SH, Kim SS, Lee D, Cheong JY, Cho SW. Durability after discontinuation of nucleos(t)ide therapy in chronic HBeAg negative hepatitis patients. Clin Mol Hepatol. 2013;19:300-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Bartholomeusz A, Locarnini SA. Antiviral drug resistance: clinical consequences and molecular aspects. Semin Liver Dis. 2006;26:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. [PubMed] |
| 9. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [PubMed] |
| 10. | Kim JK, Hwang SG, Park H, Choi HY, Cho HJ, Ko KH, Hong SP, Park PW, Kim NK, Rim KS. [Clinical outcomes after discontinuation of Lamivudine in chronic hepatitis B patients with Lamivudine resistant HBV mutant]. Korean J Hepatol. 2005;11:227-242. [PubMed] |
| 11. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [PubMed] |
| 12. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [PubMed] |
| 13. | Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 15. | Preda CM, Baicus C, Negreanu L, Tugui L, Olariu SV, Andrei A, Zambatu I, Diculescu MM. Effectiveness of entecavir treatment and predictive factors for virologic response. Rev Esp Enferm Dig. 2014;106:305-311. [PubMed] |
| 16. | Yim HJ, Seo YS, Yoon EL, Kim CW, Lee CD, Park SH, Lee MS, Park CK, Chae HB, Kim MY. Adding adefovir vs. switching to entecavir for lamivudine-resistant chronic hepatitis B (ACE study): a 2-year follow-up randomized controlled trial. Liver Int. 2013;33:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Warner N, Locarnini S. Mechanisms of hepatitis B virus resistance development. Intervirology. 2014;57:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (2)] |
| 20. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 21. | Yang HJ, Lee JH, Kim YJ, Yoon JH, Lee HS. Antiviral efficacy of combination therapy with entecavir and adefovir for entecavir/lamivudine-resistant hepatitis B virus with or without adefovir resistance. J Med Virol. 2012;84:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Yim HJ, Hwang SG. Options for the management of antiviral resistance during hepatitis B therapy: reflections on battles over a decade. Clin Mol Hepatol. 2013;19:195-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Jeon JW, Shin HP, Lee JI, Joo KR, Cha JM, Park JJ, Lim JU, Lim K, Kim S. Efficacy of entecavir and adefovir combination therapy for patients with lamivudine- and entecavir-resistant chronic hepatitis B. Dig Dis Sci. 2012;57:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lee YB, Lee JH, Choi WM, Cho YY, Yoo JJ, Lee M, Lee DH, Cho Y, Yu SJ, Kim YJ. Efficacy of adefovir-based combination therapy for patients with Lamivudine- and entecavir-resistant chronic hepatitis B virus infection. Antimicrob Agents Chemother. 2013;57:6325-6332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Lee YB, Lee JH, Lee DH, Cho H, Ahn H, Choi WM, Cho YY, Lee M, Yoo JJ, Cho Y. Efficacy of entecavir-tenofovir combination therapy for chronic hepatitis B patients with multidrug-resistant strains. Antimicrob Agents Chemother. 2014;58:6710-6716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, Schollmeyer J, Zoulim F, Wedemeyer H, Sterneck M. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012;56:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Yim HJ, Lee HJ, Suh SJ, Seo YS, Kim CW, Lee CD, Park SH, Lee MS, Park CK, Chae HB. Adefovir and lamivudine combination therapy in patients with entecavir-resistant chronic hepatitis B: antiviral responses and evolution of mutations. Intervirology. 2014;57:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Park JW, Kim HS, Seo DD, Jang JS, Shin WG, Kim KH, Jang MK, Lee JH, Kim HY, Kim DJ. Long-term efficacy of entecavir in adefovir-refractory chronic hepatitis B patients with prior lamivudine resistance. J Viral Hepat. 2011;18:e475-e481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593-1608.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 550] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 30. | Lee JM, Kim HJ, Park JY, Lee CK, Kim do Y, Kim JK, Lee HW, Paik YH, Lee KS, Han KH. Rescue monotherapy in lamivudine-resistant hepatitis B e antigen-positive chronic hepatitis B: adefovir versus entecavir. Antivir Ther. 2009;14:705-712. [PubMed] |
| 31. | Jung SK, Kim KA, Ha SY, Lee HK, Kim YD, Lee BH, Paik WH, Kim JW, Bae WK, Kim NH. Tenofovir disoproxil fumarate monotherapy for nucleos(t)ide analogue-naïve and nucleos(t)ide analogue-experienced chronic hepatitis B patients. Clin Mol Hepatol. 2015;21:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: De Vincentis A, Preda CM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN