Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10853
Peer-review started: June 8, 2015
First decision: July 10, 2015
Revised: July 25, 2015
Accepted: August 31, 2015
Article in press: August 31, 2015
Published online: October 14, 2015
Processing time: 129 Days and 11.1 Hours
AIM: To investigate whether dimethyl sulfoxide (DMSO) inhibits gut inflammation and barrier dysfunction following zymosan-induced systemic inflammatory response syndrome and multiple organ dysfunction syndrome.
METHODS: Sprague-Dawley rats were randomly divided into four groups: sham with administration of normal saline (SS group); sham with administration of DMSO (SD group); zymosan with administration of normal saline (ZS group); and zymosan with administration of DMSO (ZD group). Each group contained three subgroups according to 4 h, 8 h, and 24 h after surgery. At 4 h, 8 h, and 24 h after intraperitoneal injection of zymosan (750 mg/kg), the levels of intestinal inflammatory cytokines [tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-10] and oxides (myeloperoxidase, malonaldehyde, and superoxide dismutase) were examined. The levels of diamine oxidase (DAO) in plasma and intestinal mucosal blood flow (IMBF) were determined. Intestinal injury was also evaluated using an intestinal histological score and apoptosis of intestinal epithelial cells was determined by deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The intestinal epithelial tight junction protein, ZO-1, was observed by immunofluorescence.
RESULTS: DMSO decreased TNF-α and increased IL-10 levels in the intestine compared with the ZS group at the corresponding time points. The activity of intestinal myeloperoxidase in the ZS group was higher than that in the ZD group 24 h after zymosan administration (P < 0.05). DMSO decreased the content of malondialdehyde (MDA) and increased the activity of superoxide dehydrogenase (SOD) 24 h after zymosan administration. The IMBF was lowest at 24 h and was 49.34% and 58.26% in the ZS group and ZD group, respectively (P < 0.05). DMSO alleviated injury in intestinal villi, and the gut injury score was significantly lower than the ZS group (3.6 ± 0.2 vs 4.2 ± 0.3, P < 0.05). DMSO decreased the level of DAO in plasma compared with the ZS group (65.1 ± 4.7 U/L vs 81.1 ± 5.0 U/L, P < 0.05). DMSO significantly preserved ZO-1 protein expression and localization 24 h after zymosan administration. The TUNEL analysis indicated that the number of apoptotic intestinal cells in the ZS group was much higher than the ZD group (P < 0.05).
CONCLUSION: DMSO inhibited intestinal cytokines and protected against zymosan-induced gut barrier dysfunction.
Core tip: We examined whether the administration of dimethyl sulfoxide (DMSO) inhibited zymosan-induced intestinal inflammation and barrier dysfunction to provide an experimental basis for the use of DMSO in protecting intestinal barrier function. We found that DMSO can inhibit intestinal cytokines and protect against zymosan-induced gut barrier dysfunction.
- Citation: Li YM, Wang HB, Zheng JG, Bai XD, Zhao ZK, Li JY, Hu S. Dimethyl sulfoxide inhibits zymosan-induced intestinal inflammation and barrier dysfunction. World J Gastroenterol 2015; 21(38): 10853-10865
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10853.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10853
A large number of bacteria and viruses is found in the human intestine, and the intestinal mucosal barrier is the most important defense mechanism in the body. Intestinal mucosal barrier integrity can separate the luminal content from the body and prevent intestinal bacteria and endotoxin translocation. The intestinal mucosal barrier is composed of a mechanical barrier, immunological barrier, biological barrier, and chemical barrier. A decline in intestinal mucosal barrier function allows luminal bacteria, toxins, and other macromolecules, such as antigens, into the body, which is a key initiation factor in intestinal inflammation and deterioration. Increased permeability exposes the mucosal immune system in the intestinal lumen to foods and bacterial antigens, which stimulate the immune system and lead to the occurrence of gut inflammation.
A growing body of evidence indicates that intestinal ischemia plays a critical role in the development of excessive inflammatory-induced organ dysfunction[1,2]. When intestinal permeability and tight junction proteins are damaged, the gut becomes a source of pro-inflammatory mediators, which may amplify systemic inflammatory response syndrome (SIRS) and induce a septic state and distant organ failure. Moreover, it can lead to multiple organ dysfunction syndrome (MODS) and even death[3-5]. In the pathogenesis of MODS induced by an uncontrolled systemic inflammatory response, the intestine is the first organ to be affected and is one of the most easily damaged organs in the pathological process.
Research shows that when the intestine is ischemic, infected, or inflamed, bacteria and their toxins can rapidly activate originally static functions of intestinal innate macrophages to produce large amounts of pro-inflammatory cytokines. These pro-inflammatory factors cause further aggregation of monocytes and polymorphonuclear leukocytes in the intestinal microcirculation and intestinal tissue and release more inflammatory cytokines, oxygen free radicals, and inhibit gastrointestinal motility medium[1,6]. This response causes excessive inflammation of the intestine, mucosal edema, intestinal barrier dysfunction, and intestinal paralysis, triggering intestinal bacteria and endotoxin translocation and gut-derived sepsis and MODS. Thus, effectively inhibiting the production of intestinal pro-inflammatory cytokines and reducing the production of inflammatory cytokines and oxygen free radicals to protect intestinal tissue from excessive inflammatory damage is significantly important. However, available drugs that protect gut barrier function due to excessive inflammatory response are limited.
Dimethyl sulfoxide (DMSO), a hydrophile-lipophile molecule, has anti-inflammatory, analgesic, diuretic, and vasodilatation activity, improves the microcirculation, and affects platelet aggregation hypertonicity[7]. Due to its anti-inflammatory properties, DMSO has also been evaluated in the treatment of inflammatory diseases such as cystitis and arthritis[8]. In addition, DMSO has been approved by the United States Food and Drug Administration for the treatment of interstitial cystitis by bladder instillation[9,10]. Therefore, this study aimed to determine whether the administration of DMSO inhibited zymosan-induced intestinal inflammation and barrier dysfunction and to provide an experimental basis for the use of DMSO in protecting intestinal barrier function.
Male Sprague-Dawley rats (8-10 wk, 251.5 ± 8.7 g) were purchased from the Experimental Animal Center of Military Medical Sciences of the Chinese People’s Liberation Army (PLA). The rats were housed in mesh cages in a room maintained at 25 °C, illuminated by a 12:12 h light-dark cycle, and provided with standard rodent chow and water ad libitum. The rats were fasted overnight and allowed free access to water up to 4 h before surgery. The Committee of Scientific Research of the First Hospital Affiliated to the Chinese PLA General Hospital, China approved all the research protocols. The experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals of the National Research Council, China.
After sterilization of the abdomen, an intraperitoneal injection of high-dose zymosan (750 mg/kg) was administered followed by a subcutaneous injection of DMSO (3 mL/kg, diluted in saline 1:2) or normal saline (3 mL/kg) 1 h after zymosan administration. The animals were allowed to breathe spontaneously under a nose cone scavenging system, using a veterinary anesthesia delivery system (Kent Scientific TOPO, Torrington, CT, United States). Rectal temperature was maintained at 37 °C with a heating pad and a heating lamp. Following the injection of zymosan, the animals developed acute peritonitis. The rats were very ill during the first day, as shown by ruffled fur, skin folds, lethargy, diarrhea, high body temperature, and decreased body weight[11].
Zymosan (Sigma Chemical, St. Louis, MO, United States) was accurately weighed, and the appropriate volume of sterile saline was added to produce a zymosan suspension of 60 mg/mL. A high frequency magnetic stirrer was used to stir the suspension until blended. Disinfection was carried out in a 100 °C water bath for 80 min, and the suspension was then cooled to room temperature. The suspension was heated to 40 °C, and high-frequency vibration blending was performed before use. The zymosan suspension was then injected intraperitoneally at the dose of 750 mg/kg.
All the animals underwent the same procedure and were then randomly divided into four groups, weighed, and scored. In the ZS group and ZD group, an intraperitoneal injection of high-dose zymosan (750 mg/kg) was administered followed by a subcutaneous injection of DMSO (3 mL/kg, diluted in saline 1:2) in the ZD group and normal saline (3 mL/kg) in the ZS group 1 h after zymosan administration. In the SS group and SD group, an intraperitoneal injection of normal saline was administered in each group and then 1 h later a subcutaneous injection of DMSO (3 mL/kg, diluted in saline 1:2) was administered in the ZD group and normal saline (3 mL/kg) in the ZS group. The SS group and SD group were treated as the surgery and drug controls, respectively. Each group was divided into three subgroups, according to 4 h, 8 h, and 24 h after injury. In total, there were 12 subgroups (four groups with four time points each), with eight samples in each subgroup. Because the model has a 50% mortality rate at 24 h, each subgroup consisted of 16-20 rats. From the surviving rats, eight animals from each subgroup were randomly selected for the final analysis.
The rats were anesthetized by inhalation of 3% isoflurane (Yeeran Technology Limited, Beijing, China), and the aorta was punctured and exsanguinated at 4 h, 8 h, and 24 h after surgery. For diamine oxidase (DAO), blood was collected, and plasma was obtained by centrifuging the blood at 10000 g for 10 min at 4 °C. The animals were sacrificed, and the distal small intestine was harvested. Segments of distal small intestine were fixed in 4% paraformaldehyde for histologic evaluation, deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis, and immunofluorescent staining. Segments of the distal small intestine stored at -40 °C for enzyme linked immunosorbent assay (ELISA).
Intestine tissue (100 mg) in 1 mL phosphate-buffered saline (PBS) was homogenized at 4 °C with a Polytron homogenizer. After centrifugation at 10000 g at 4 °C for 10 min, the supernatants were collected. Tumor necrosis factor alpha (TNF-α) and interleukin (IL)-10 in the intestine supernatants were quantified with a commercial ELISA kit (Nanjing Jiancheng Corp., China) according to the manufacturer’s instructions. Intestinal TNF-α and IL-10 levels were expressed as picograms per milligram of protein.
Intestinal tissue myeloperoxidase (MPO) activity was determined using a kit according to the manufacturer’s instructions. The tissue homogenate and reagent were placed in a water bath at 60 °C for 10 min after being thoroughly mixed. The absorbance value of each tube was then determined at 460 nm immediately after removal from the water bath. The activity of MPO in the intestine was calculated according to the following formula: MPO (U/weight grams) = [determination optical density (OD) value - control OD value]/11.3 × sample volume (g).
Intestinal malonaldehyde (MDA) was determined using a kit according to the manufacturer’s instructions. The tissue homogenate and reagent were placed in a water bath at 95 °C for 40 min after being thoroughly mixed. After cooling, the mixture was centrifuged at 4000 r/m for 10 min. The absorbance value of each supernatant was then determined at 532 nm immediately after removal from the water bath. The content of MDA in the intestine was calculated according to the following formula: MDA (nmol/mgProt) = {[(determination tube absorbance - blank tube absorbance)/(standard tube absorbance - blank tube absorbance)] × standard concentrations}/protein content.
Intestinal superoxide dismutase (SOD) activity was determined using a kit according to the manufacturer’s instructions. The tissue homogenate and reagent were placed in a water bath at 37 °C for 40 min after being thoroughly mixed. After 10 min at room temperature, the absorbance value of each supernatant was determined at 550 nm. The activity of SOD in the intestine was calculated according to the following formula: SOD (U/mL) = [(control tube absorbance - determination tube absorbance)/control tube absorbance]/(50% × reaction system dilution multiple × sample dilution multiple).
A laser Doppler flowmeter (Perimed AB; Stockholm, Sweden) was used to monitor intestinal mucosal blood flow (IMBF) at 4 h, 8 h, and 24 h after surgery. The probe of the blood flow meter was aimed at the proximal jejunum, and the laser was focused on the mesentery. The flow signal was measured for 30 s, and a 10-s stable signal was selected to calculate the mean value expressed in the blood perfusion unit (BPU).
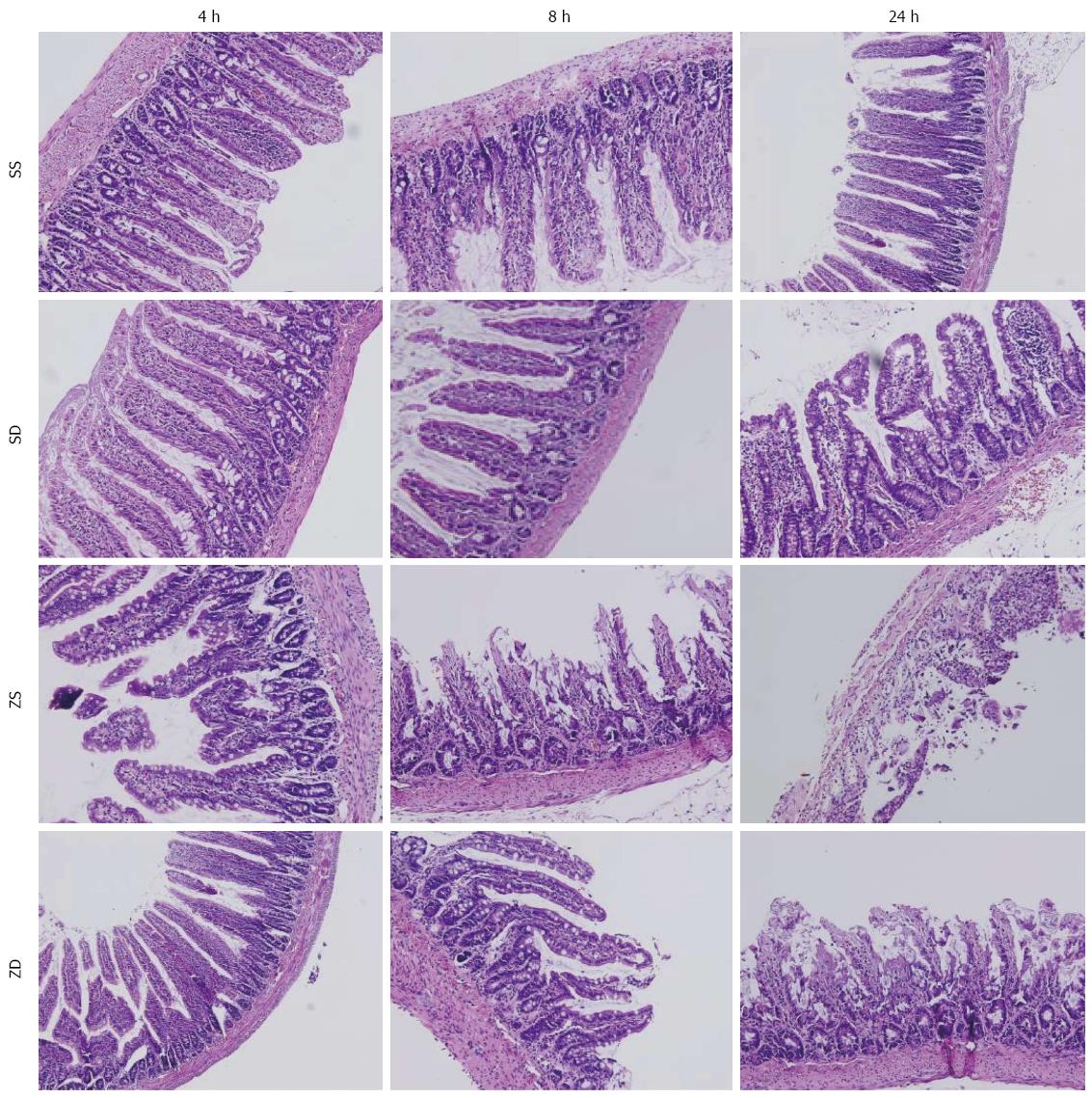
Segments of the distal ileum were fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and sectioned. Hematoxylin and eosin staining of the intestine was performed after deparaffinization and rehydration. Two pathologists, who were blinded to the experimental groups, viewed and evaluated the sections under a light microscope. Three randomly selected fields from each specimen were graded using a scoring system that characterized gut injury on a scale of 0 to 4, as developed by Chiu et al[12].
Determination of DAO activity was performed to assess gut barrier function. The activity of DAO was evaluated using an assay kit (Jiancheng Biotech Ltd., Nanjing, China) according to the manufacturer’s instructions.
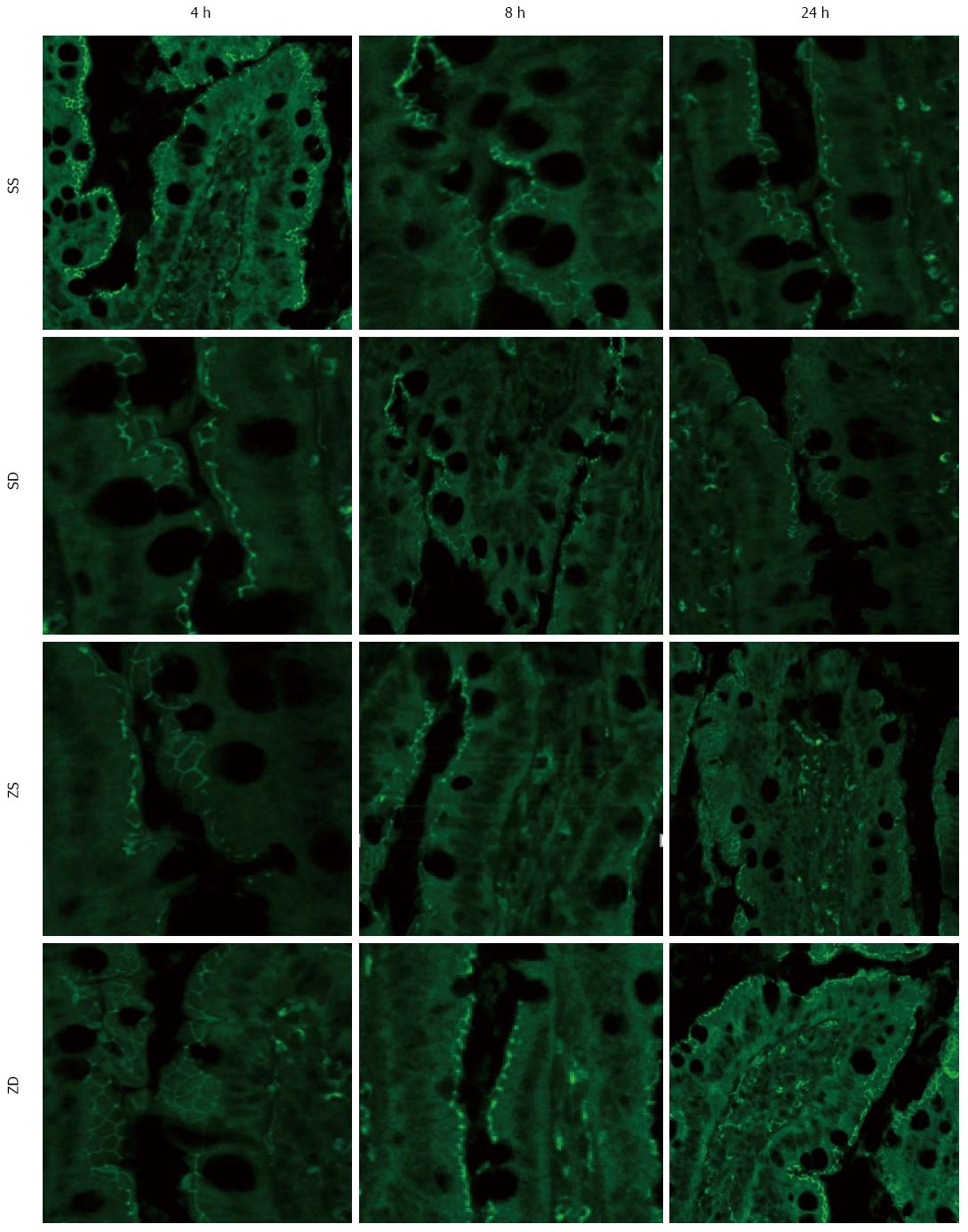
After deparaffinization, the intestine sections were rehydrated and incubated in citrate buffer (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for heat-induced antigen retrieval. After three washes with PBS, the sections were incubated with 3% bovine serum albumin (BSA) (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30 min to block nonspecific binding sites. The sections were then incubated with the ZO-1 antibody (1:100; Life Technologies, Gaithersburg, MD, United States) at 4 °C overnight. The following day, after washing with PBS three times, the sections were treated with Alexa Fluor 488 secondary goat anti-rabbit antibody in 1% BSA for 1 h at room temperature followed by three washes with PBS. Sections were mounted using Antifade Solution (Applygen Technologies Inc., Beijing, China). The negative control was incubated with PBS instead of the ZO-1 antibody, and the other steps were the same as above. Images were viewed using an Olympus fluorescence microscope (BX51-DP71, Center Valley, PA, United States) with exposure-matched settings.
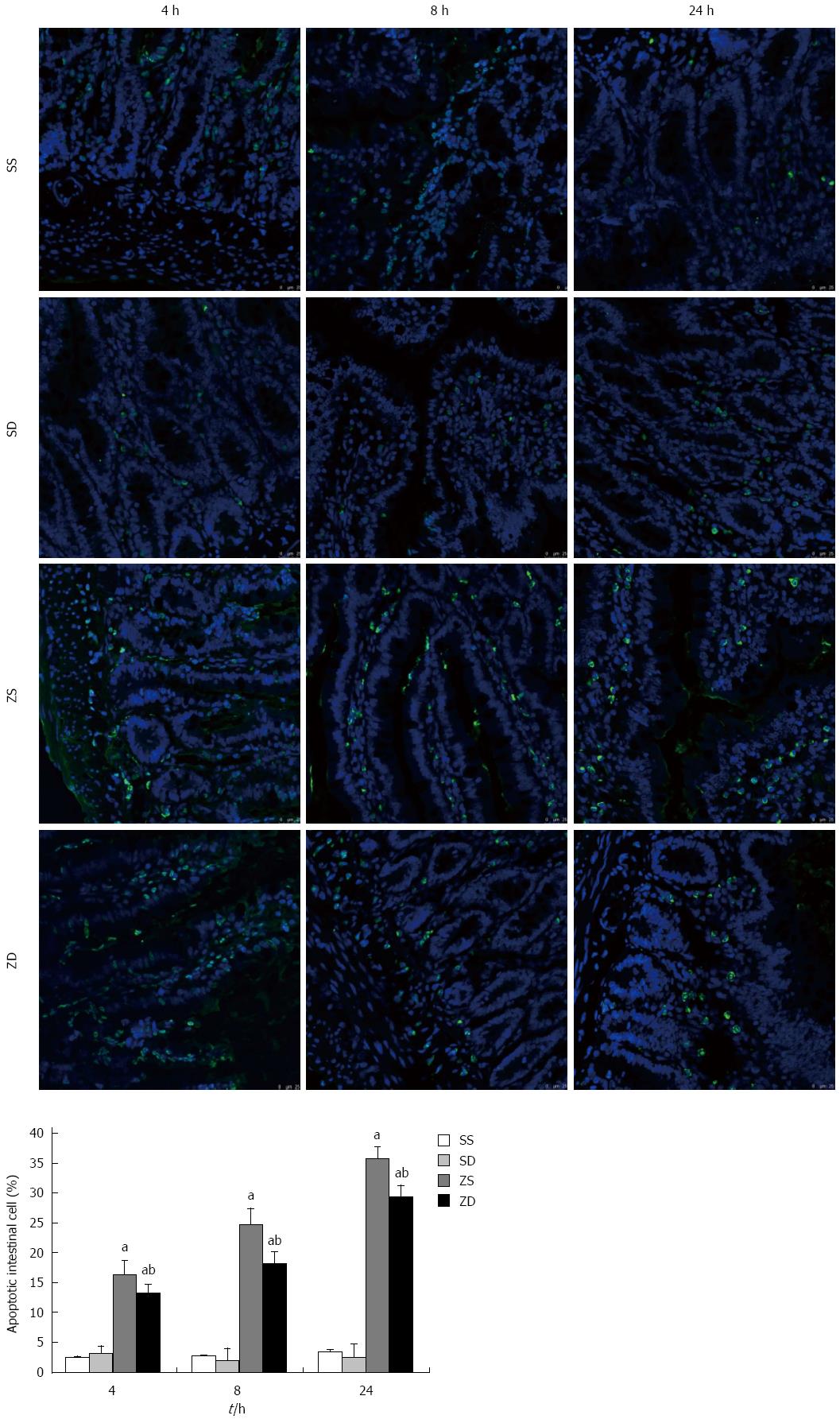
TUNEL analysis was performed using the In Situ Cell Death Detection kit (Roche Diagnostics GmbH., Penzberg, Germany) according to the manufacturer’s instructions. Segments of the distal ileum were fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and sectioned. The sections were incubated with pepsin digestion liquid in a wet box for 60 min after deparaffinization and rehydration. After two washes, 100 L DNase 1 (1500 U/mL) was added to the positive control group and incubated in a wet box for 20 min. Fifty μL TUNEL reaction mixture solution (50 L enzyme solution + 450 L label solution) was added to the positive control group and the experimental group, and 50 L label solution was added to the negative control group. The sections were incubated in the dark in the wet box for 60 min. Differential interference contrast microscopy images were then obtained at 400 × magnification following the random selection of intestinal mucosa in five non-overlapping regions. The number of apoptotic intestinal mucosa cells and total intestinal mucosa cells were counted, and then the cell apoptosis rate was determined by the following equation: cell apoptosis rate = the number of apoptotic cells/total cells × 100%.
Data were analyzed using a commercial statistical software package (SPSS Statistics 17.0, Chicago, IL, United States). Continuous variables were expressed as mean ± SE. Statistically significant differences were determined using one way analysis of variance (ANOVA). Dunnett’s test was used to compare within groups and SNK-q analysis was used to compare between groups. If variables were non-normally distributed, the Kruskal-Wallis H test was used. In all tests, a P value < 0.05 was considered statistically significant.
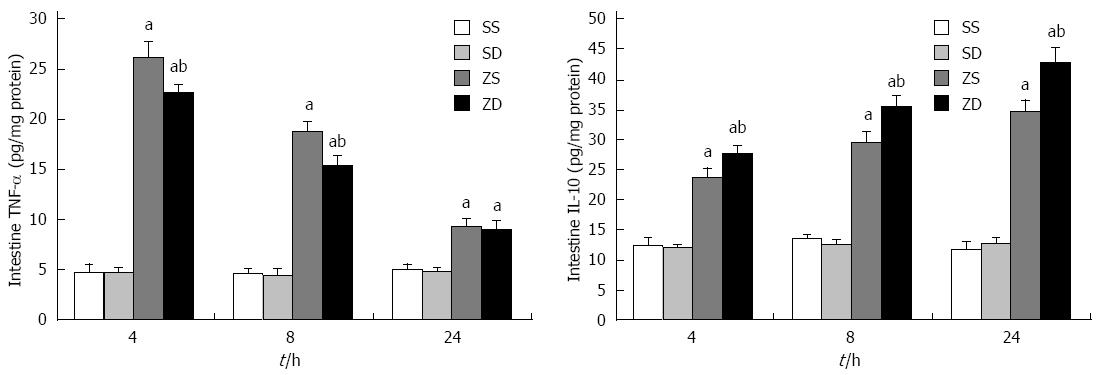
Figure 1 illustrates the effect of DMSO on TNF-α and IL-10 levels in rat intestine following intraperitoneal administration of zymosan. Zymosan induced increases in TNF-α and IL-10 in intestinal homogenates relative to saline treated controls, and DMSO reduced TNF-α levels and increased IL-10 levels in zymosan treated animals. The TNF-α and IL-10 levels in group ZS and group ZD were significantly higher than those in group SS and group SD (P < 0.05) following zymosan administration. The content of TNF-α in group ZD was significantly lower than that in group ZS at 4 h and 8 h (P < 0.05), while the IL-10 level in group ZD was higher than that in group ZS at all time points.
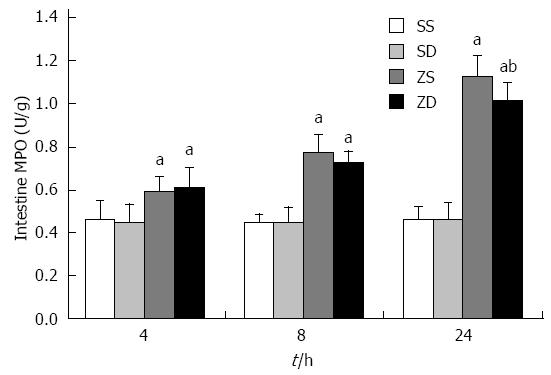
The activity of MPO in group ZS and group ZD was significantly different from that in group SS and group SD. A significant decrease in MPO in group ZD compared with group ZS was observed at 24 h (P < 0.05). This indicated that DMSO reduced the accumulation of neutrophils in the gut (Figure 2).
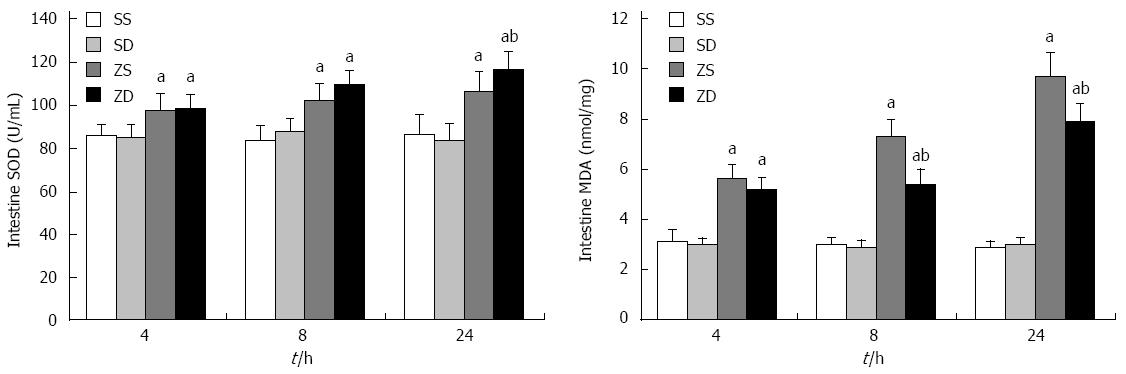
The MDA content and SOD activity were significantly increased after intraperitoneal injection of zymosan. Both MDA content and SOD activity increased with time and were highest at 24 h. Furthermore, MDA content in group ZD was lower than that in group ZS, and SOD activity was highest at 24 h in group ZD (P < 0.05). This indicated that DMSO decreased MDA content, increased SOD activity, and reduced the damage caused by lipid peroxidation (Figure 3).
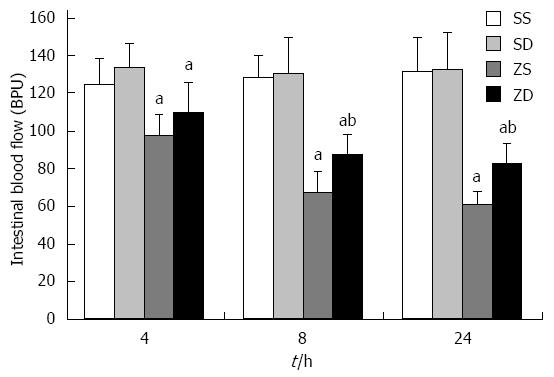
IMBF in group SS and group SD was not significantly different at 4 h, 8 h, and 24 h. IMBF in group ZS and group ZD was significantly lower than that in group SS and group SD (P < 0.05) after zymosan administration. The lowest level in group ZS and group ZD, was 46.29% of that in group SS and 62.21% of that in group SD, respectively, at 24 h. The levels of IMBF in group ZD were significantly higher than those in group ZS at 8 h and 24 h (P < 0.05). These results indicated that DMSO improved IMBF and intestinal perfusion (Figure 4).
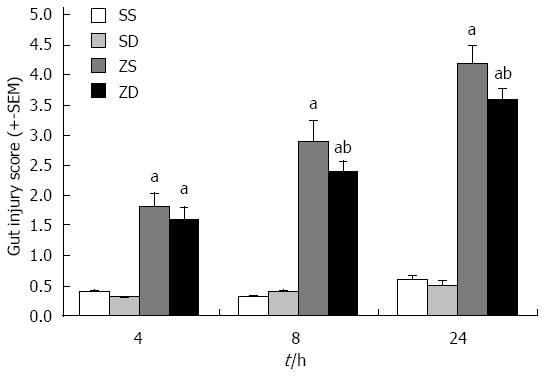
Histologic evaluation of intestinal mucosa was performed based on Chiu’s grading system[12]. Histopathologic analysis of the sham group (sham + SS and sham + SD) showed a normal mucosal pattern. The villi were packed, tall, and intact. Compared with the sham group, intraperitoneal injection of zymosan caused significant mucosal damage. The intestinal villi became erosive, hyperemic, edematous, and atrophic. The villous stroma was full of inflammatory cells, and epithelial cell villi showed necrosis and exfoliation. These effects increased with time. No significant difference was observed between group ZS and group ZD at 4 h (P > 0.05). However, DMSO treatment significantly attenuated mucosal damage at 24 h (P < 0.05) (Figures 5 and 6).
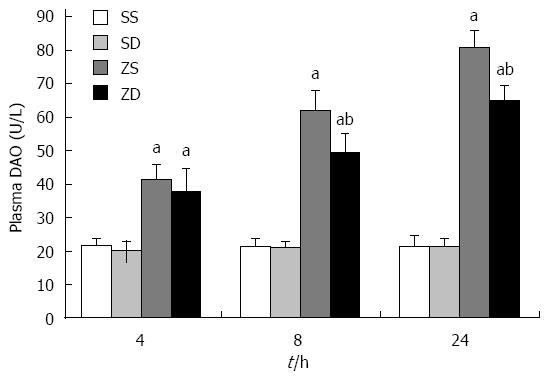
The activity of DAO in group SS and group SD was not significantly different. The activity of DAO in group ZS and group ZD was significantly higher than that in group SS and group SD (P < 0.05) after zymosan administration. The activity was highest at 24 h in group ZS (81.10 ± 5.01 U/L) and group ZD (65.09 ± 4.74 U/L) and increased to 73.58% and 67.08% of group SS (21.43 ± 3.12 U/L) and group SD (21.43 ± 3.12 U/L), respectively. The activity of DAO in group ZD was significantly lower than that in group ZS (P < 0.05) at 8 h and 24 h. These results indicate that DMSO reduced the release of DAO into the bloodstream and protected intestinal structure and function (Figure 7).
To assess the effects of DMSO on the expression of ZO-1, a tight junction protein, immunoflourescence was performed. Exposure-matched fluorescent intensity correlated with ZO-1 protein expression after immunostaining. In the sham group, ZO-1 was densely and continuously distributed along the apical membrane of epithelial cells (Figure 8). The expression pattern of ZO-1 was similar in group SS and group SD at all time points. Intraperitoneal administration of zymosan caused a loss of ZO-1 expression at 8 h (Figure 8), and zymosan-induced loss of ZO-1 was more pronounced at 24 h, resulting in a low expression of ZO-1 at the cell periphery (Figure 8). The pattern of ZO-1 expression in group ZS was lower than that in group SS at 8 h and 24 h. Following treatment with DMSO, the loss of ZO-1 was attenuated, and the level of ZO-1 continually improved 8 and 24 h after intraperitoneal administration of zymosan (Figure 8). Intraperitoneal zymosan (ZS group) resulted in a significant reduction in intestinal ZO-1 expression and DMSO treatment (ZD group) attenuated the degradation of ZO-1 at 8 and 24 h.
The rate of apoptosis in intestinal tissues in group SS and group SD were not significantly different, and the rate of apoptosis in intestinal cells in group ZS and group ZD was significantly higher than that in the sham control group (all P < 0.05). The rate of apoptosis increased with time after zymosan administration. The rate of apoptosis in intestinal tissues in group ZD was significantly lower than that in group ZS (P < 0.05) at all time points. These results indicate that DMSO may inhibit intestinal cell apoptosis (Figure 9).
MODS refers to the clinical syndrome of simultaneous or sequential dysfunction of two or more organs leading to an unstable internal environment after severe trauma, shock, and infection[13]. Under physiological conditions, the body maintains a balance between pro-inflammatory and anti-inflammatory reactions, as a protective response against foreign invasion. Pro-inflammatory factors initiate and promote inflammation and injury to the body through the release of “aggressive” inflammatory mediators. Anti-inflammatory cytokines are released by pro-inflammatory cytokines and are involved in defense and promote anti-inflammatory reactions and tissue repair. However, when the pro-inflammatory or inflammatory reaction is too strong or too weak, the body is in a state of immune hyperfunction or immune suppression, the inflammatory response cannot be controlled, and homeostasis is disrupted. Infection, trauma, and ischemia reperfusion injury results in the excessive activation of inflammatory cells, such as macrophages, neutrophils, and endothelial cells, with the excessive release of inflammatory mediators. This causes the “waterfall effect”, inducing a SIRS, and, if not treated, leads to multiple organ dysfunction[14-16]. Therefore, the balance between pro-inflammatory mediators and anti-inflammatory factors determines the prognosis of the disease, and the imbalance between the two types of cytokines is an important cause of further development of SIRS and MODS.
Zymosan is a substance derived from the cell wall of the yeast Saccharomyces cerevisiae. Intraperitoneal injection of zymosan in mice or rats leads to systemic inflammation and organ damage by inducing a wide range of inflammatory mediators of the complement system[17], prostaglandins and leukotrienes[18], platelet aggregation factor[19], oxygen radicals[20], and lysosomal enzymes[21]. In the mid-1980s, the zymosan-induced generalized inflammation (ZIGI) model was first introduced by Goris[22]. To date, the ZIGI model is recognized as the best model as it resembles human MODS and has been widely used to study systemic inflammation in relation to organ failure. Cuzzocera[23,24] administered intraperitoneal zymosan to animals, inducing acute peritonitis and multiple organ damage within 18 h. Inflammatory lesions play a role in the process of systemic inflammation and multiple organ damage induced by zymosan. Zymosan induces the excessive release of inflammatory mediators, damages vascular endothelial cells, and slows blood flow. Inflammatory cells and platelets adhere to the endothelium, and leukocytes migrate into the gap, releasing a variety of inflammatory transmitters, that damage the endothelial barrier and tissue.
After intraperitoneal injection of zymosan, pro-inflammatory factors and anti-inflammatory factors in the blood increase significantly, and the uncontrolled synthesis and release of these factors induce SIRS. In recent years, research has shown that DMSO can inhibit the activation of nuclear factor-kappa B (NF-κB) stimulated by lipopolysaccharide in mouse macrophages[25] and intestinal Caco-2 cells[26], lower mRNA expression of cytokines, and reduce the biological activity of TNF. DMSO inhibits the activation of rat NF-κB in sepsis, the expression of the intracellular adhesion molecule-1 (ICAM-1) gene, and the expression of inflammatory factors, such as TNF-α[27]. Following selective inhibition of the nod-like receptor family pryin domain containing-3 (NLRP3) inflammatory complex, inhibition of mature IL-1, casP1, casP1 activity, and ASC pyroptosomes, DMSO was further confirmed to have anti-inflammatory effects in animal sepsis and inflammatory bowel disease models[28].
TNF-α is the primary initiator of the inflammatory cytokine cascade during SIRS, and IL-10 is considered to be the most important anti-inflammatory cytokine in vivo. The increase in TNF-α, a positive feedback response following activation of NF-κB, upregulates the expression and release of cytokines, such as IL-2 and ICAM-1, and plays a role in causing inflammatory damage to tissues. DMSO reduces the formation of inflammatory mediators, localizes the inflammatory response, and controls the systemic inflammatory response in the appropriate range, preventing the development of SIRS and even MODS. IL-10 inhibits the activity of NF-κB in at least two ways: (1) DMSO prevents the dissociation of NF-κB and protein IkappaB (Iκβ) by inhibiting the activity of Iκβ kinase; and (2) DMSO inhibits the combination of NF-κB with the DNA transcriptional regulatory region, thereby inhibiting the transcription of corresponding inflammatory factors[29,30].
In the present study, zymosan induced peritonitis, ascites leakage, and intestinal edema and significantly reduced intestinal blood flow, decreased the expression of intestinal tight junction protein ZO-1, increased intestinal permeability, which were correlated with the release of pro-inflammatory factors such as TNF-α. After the administration of DMSO, intestinal inflammatory factor TNF-α was significantly decreased, and IL-10, blood flow, and expression of ZO-1 were increased, indicating that DMSO decreased the synthesis and release of inflammatory factor TNF-α and increased the release of IL-10 to alleviate inflammatory damage caused by these factors. Previous studies have shown that macrophage Toll-like receptor 2 combined with zymosan leads to activation of NF-κB and generation of the pro-inflammatory factor TNF-α[31,32]. In this study, DMSO inhibited the synthesis and release of TNF-α, indicating that DMSO is likely to inhibit the activation of NF-κB and reduce the synthesis and release of TNF-α.
The activity of MPO in tissues reflects the aggregation of neutrophils at inflammatory sites. After intraperitoneal injection of zymosan, intestinal MPO activity significantly increased with time, as demonstrated by the aggregation of neutrophils in the intestine. However, the effect of DMSO on neutrophil activity and white blood cell count was not significant, with only some effects at 24 h. DMSO may inhibit the oxidative stress reaction mediated by neutrophils to alleviate injury caused by zymosan.
After intraperitoneal injection of zymosan, viscera microcirculation blood flow decreased, and the tissue was ischemic and anoxic and produced a large number of free radicals. Therefore, the animals continued to be in a state of oxidative stress, endogenous antioxidant enzyme activity was reduced, and the body's reduction/oxidation system balance was disrupted. MDA is the main metabolite in lipid peroxidation, and its content can reflect the degree of lipid peroxidation and indirectly reflect the degree of oxidative damage[33]. Previous research showed that DMSO can reduce MDA and NO levels, inhibit or increase the level of glutathione, and alleviate liver injury and ischemia reperfusion-induced transaminase release[34,35]. DMSO can also reduce renal damage caused by HgCl2[36]. In addition, SOD is an important antioxidant enzyme in organisms and the primary enzyme involved in scavenging free radicals. Oxygen free radicals can activate NF-κB, thus a reduction in oxidative products and an improvement in antioxidant enzymes decrease the serum and tissue levels of pro-inflammatory cytokines and protect organ function[37]. In this study, intraperitoneal injection of zymosan increased MDA content and SOD activity in the intestine, while subcutaneous injection of DMSO suppressed the increase in MDA and SOD. These results demonstrate that DMSO can reduce the damage caused by visceral lipid peroxidation mediated by oxygen free radicals.
Cell apoptosis regulates body development and maintains a stable internal environment via a series of genes that control the process of active cell death. Intestinal mucosal epithelial cell proliferation, differentiation, and apoptosis are processes of dynamic change. However, the balance is disrupted in inflammatory bowel disease, where the occurrence and scope of epithelial cell apoptosis is higher than that in normal tissue[38]. Epithelial cell apoptosis is mainly due to activation of the Fas/Fas ligand (FasL) signal transduction pathway and both Bcl-2 and Bax[39,40]. In this study, intestinal epithelial cell apoptosis was increased following intraperitoneal administration of zymosan and was reduced by DMSO, however, the specific mechanism involved in the effect of DMSO requires further study.
In conclusion, DMSO reduced intestinal tissue injury after intraperitoneal injection of zymosan, restored intestinal blood flow, and protected intestinal function. The mechanism likely involves regulation of the balance between pro-inflammatory and anti-inflammatory factors, inhibition of peroxidation in organs, oxygen free radical scavenging, reduction in intestinal epithelial cell apoptosis, and alleviation of intestinal function damage.
The authors thank Dr. Jiang-Yang Lu, Yi-Duo Jin, Xiu-Li Ma, Hui-Zhen Ding, Yi Yang, and Dong-Mei Yang from the Department of Pathology, First Hospital Affiliated to the PLA General Hospital, for their technical assistance. The authors also thank Hui-Ping Zhang, Ming-Juan Gao, and Xiao-Na Zhang for their assistance with the experiments.
When severe injury occurs, the blood supply to the intestinal tract is sharply reduced, which results in gut barrier dysfunction. The incidence of serious complications is increased following dysfunction of the gut barrier. This promotes bacterial translocation and the local production of cytokines. Bacteria and their endotoxins move into the circulation and remote organs, contributing to subsequent local and systemic inflammation. This may lead to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS). Therefore, protecting the intestinal barrier function is important. Thus, interventions such as drugs to prevent excessive inflammation and the reduction/oxidation reaction are of great significance in controlling SIRS and MODS induced by zymosan.
The current treatment options for SIRS and MODS are limited, therefore, it is important to identify alternative therapies. Dimethyl sulfoxide (DMSO) has been found to have anti-inflammatory, analgesic, diuretic, and vasodilatation activities, to improve the microcirculation, and to affect platelet aggregation hypertonicity. In addition, DMSO has been studied in the treatment of inflammatory diseases, such as cystitis and arthritis. DMSO can reduce malonaldehyde (MDA) and nitric oxide (NO) level, and alleviate liver injury. DMSO can also reduce renal damage caused by HgCl2. However, whether DMSO can protect intestinal function in SIRS and MODS and its specific mechanism are unclear.
The most important novel findings in this study are that DMSO inhibits zymosan-induced intestinal inflammation and barrier dysfunction. Regulation of the balance between pro-inflammatory and anti-inflammatory reactions and inhibition of excessive oxidation are considered possible mechanisms underlying DMSO regulation of zymosan-induced intestinal barrier function.
These study results provide possible mechanisms of DMSO regulation of intestinal barrier function after zymosan-induced systemic inflammatory response syndrome and MODS.
Intestinal barrier function: refers to the function of the intestine in preventing harmful substances, such as bacteria and toxins, from entering the intestinal mucosa, other organs, and the blood circulation. The normal intestinal mucosal barrier is composed of a mechanical barrier, chemical barrier, immunologic barrier, and biological barrier. Inflammation of the intestine: Intestinal ischemia, infection, and inflammation can activate intestinal inflammatory cells to release many cytokines and oxygen free radicals and inhibit gastrointestinal motility medium, resulting in excessive inflammation, mucosal edema, and intestinal barrier damage. DMSO is a hydrophile-lipophile molecule and is widely used as a solvent for biological compounds. It has anti-inflammatory, analgesic, diuretic, and vasodilatation activity, improves the microcirculation, and affects platelet aggregation hypertonicity. Zymosan: zymosan is a substance derived from the cell wall of the yeast Saccharomyces cerevisiae. It is composed of polysaccharide chains of various molecular weights and contains approximately 73% polysaccharides, 15% proteins, and 7% lipids and inorganic components. When injected into animals, it results in inflammation by inducing a wide range of inflammatory mediators.
This is a well written and set up study. The authors provide a sufficient overview of the study background and clearly present the hypothesis of the study. The aim of the study was fulfilled. The results are presented sufficiently well and discussed well. The nine figures clearly and correctly present the results.
| 1. | Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449-453. [PubMed] |
| 2. | Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141-145. [PubMed] |
| 3. | Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res. 1998;79:39-46. [PubMed] |
| 4. | Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177-196. [PubMed] |
| 6. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [PubMed] |
| 7. | Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035-1041. [PubMed] |
| 8. | Kloesch B, Liszt M, Broell J, Steiner G. Dimethyl sulphoxide and dimethyl sulphone are potent inhibitors of IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Life Sci. 2011;89:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sant GR. Intravesical 50% dimethyl sulfoxide (Rimso-50) in treatment of interstitial cystitis. Urology. 1987;29:17-21. [PubMed] |
| 10. | Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis--a practical approach. Urology. 1997;49:105-107. [PubMed] |
| 11. | Jansen MJ, Hendriks T, Verhofstad AA, Lange W, Geeraedts LM, Goris RJ. Gradual development of organ damage in the murine zymosan-induced multiple organ dysfunction syndrome. Shock. 1997;8:261-267. [PubMed] |
| 12. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1453] [Article Influence: 25.9] [Reference Citation Analysis (6)] |
| 13. | Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151:1799-1806. [PubMed] |
| 14. | Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150. [PubMed] |
| 15. | Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 16. | Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1101] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 17. | Pillemer L, Ecker EE. Anticomplementary factor in fresh yeast. J Biol Chem. 1941;137:139-142. |
| 18. | Humes JL, Sadowski S, Galavage M, Goldenberg M, Subers E, Bonney RJ, Kuehl FA. Evidence for two sources of arachidonic acid for oxidative metabolism by mouse peritoneal macrophages. J Biol Chem. 1982;257:1591-1594. [PubMed] |
| 19. | Roubin R, Mencia-Huerta JM, Landes A, Benveniste J. Biosynthesis of platelet-activating factor (PAF-acether). IV. Impairment of acetyl-transferase activity in thioglycollate-elicited mouse macrophages. J Immunol. 1982;129:809-813. [PubMed] |
| 20. | Nauseef WM, Root RK, Newman SL, Malech HL. Inhibition of zymosan activation of human neutrophil oxidative metabolism by a mouse monoclonal antibody. Blood. 1983;62:635-644. [PubMed] |
| 21. | Bonney RJ, Wightman PD, Davies P, Sadowski SJ, Kuehl FA, Humes JL. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978;176:433-442. [PubMed] |
| 22. | Goris RJ, Boekholtz WK, van Bebber IP, Nuytinck JK, Schillings PH. Multiple-organ failure and sepsis without bacteria. An experimental model. Arch Surg. 1986;121:897-901. [PubMed] |
| 23. | Imperatore F, Cuzzocrea S, De Lucia D, Sessa M, Rinaldi B, Capuano A, Liguori G, Filippelli A, Rossi F. Hyperbaric oxygen therapy prevents coagulation disorders in an experimental model of multiple organ failure syndrome. Intensive Care Med. 2006;32:1881-1888. [PubMed] |
| 24. | Impellizzeri D, Mazzon E, Di Paola R, Paterniti I, Bramanti P, Cuzzocrea S. Effect of NADPH-oxidase inhibitors in the experimental model of zymosan-induced shock in mice. Free Radic Res. 2011;45:820-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kelly KA, Hill MR, Youkhana K, Wanker F, Gimble JM. Dimethyl sulfoxide modulates NF-kappa B and cytokine activation in lipopolysaccharide-treated murine macrophages. Infect Immun. 1994;62:3122-3128. [PubMed] |
| 26. | Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, During A. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011;206:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Chang CK, Llanes S, Schumer W. Inhibitory effect of dimethyl sulfoxide on nuclear factor-kappa B activation and intercellular adhesion molecule 1 gene expression in septic rats. J Surg Res. 1999;82:294-299. [PubMed] |
| 28. | Ahn H, Kim J, Jeung EB, Lee GS. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology. 2014;219:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868-31874. [PubMed] |
| 30. | Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64-73. [PubMed] |
| 31. | Frasnelli ME, Tarussio D, Chobaz-Péclat V, Busso N, So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther. 2005;7:R370-R379. [PubMed] |
| 32. | Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res. 2003;58:95-130. [PubMed] |
| 33. | Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515-540. [PubMed] |
| 34. | Stein HJ, Oosthuizen MM, Hinder RA, Lamprechts H. Oxygen free radicals and glutathione in hepatic ischemia/reperfusion injury. J Surg Res. 1991;50:398-402. [PubMed] |
| 35. | Akyürek N, Kafali EM, Muhtaroglu S. The effects of dimethylsulfoxide on experimental hepatic ischemia. Swiss Surg. 2000;6:23-27. [PubMed] |
| 36. | Jo SK, Hu X, Yuen PS, Aslamkhan AG, Pritchard JB, Dear JW, Star RA. Delayed DMSO administration protects the kidney from mercuric chloride-induced injury. J Am Soc Nephrol. 2004;15:2648-2654. [PubMed] |
| 37. | Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock. 2010;34:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152-159. [PubMed] |
| 39. | Martin CA, Panja A. Cytokine regulation of human intestinal primary epithelial cell susceptibility to Fas-mediated apoptosis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G92-G104. [PubMed] |
| 40. | Lu J, Caplan MS, Saraf AP, Li D, Adler L, Liu X, Jilling T. Platelet-activating factor-induced apoptosis is blocked by Bcl-2 in rat intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G340-G350. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Vorobjova T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S