Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10367
Peer-review started: January 16, 2015
First decision: April 24, 2015
Revised: May 23, 2015
Accepted: August 28, 2015
Article in press: August 31, 2015
Published online: September 28, 2015
Processing time: 256 Days and 13.3 Hours
AIM: To evaluate the efficacy of ursodeoxycholic acid (UDCA) as a chemotherapeutic agent for the treatment of hepatocellular carcinoma (HCC).
METHODS: BALB/c nude mice were randomized into four groups 24 h before subcutaneous injection of hepatocarcinoma BEL7402 cells suspended in phosphate buffered saline (PBS) into the right flank. The control group (n = 10) was fed a standard diet while treatment groups (n = 10 each) were fed a standard daily diet supplemented with different concentrations of UDCA (30, 50 and 70 mg/kg per day) for 21 d. Tumor growth was measured once each week, and tumor volume (V) was calculated with the following equation: V = (L × W2) × 0.52, where L is the length and W is the width of the xenograft. After 21 d, mice were killed under ether anesthesia, and tumors were excised and weighed. Apoptosis was evaluated through detection of DNA fragmentation with gel electrophoresis and the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. Western blot analysis was performed to determine the expression of apoptosis-related proteins BAX, BCL2, APAF1, cleaved caspase-9, and cleaved caspase-3.
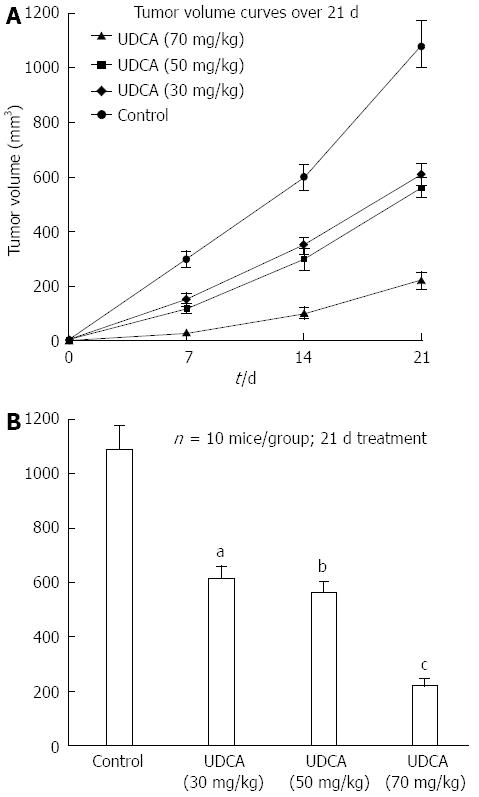
RESULTS: UDCA suppressed tumor growth relative to controls. The mean tumor volumes were the following: control, 1090 ± 89 mm3; 30 mg/kg per day, 612 ± 46 mm3; 50 mg/kg per day, 563 ± 38 mm3; and 70 mg/kg per day, 221 ± 26 mm3. Decreased tumor volumes reached statistical significance relative to control xenografts (30 mg/kg per day, P < 0.05; 50 mg/kg per day, P < 0.05; 70 mg/kg per day, P < 0.01). Increasing concentrations of UDCA led to increased DNA fragmentation observed on gel electrophoresis and in the TUNEL assay (control, 1.6% ± 0.3%; 30 mg/kg per day, 2.9% ± 0.5%; 50 mg/kg per day, 3.15% ± 0.7%, and 70 mg/kg per day, 4.86% ± 0.9%). Western blot analysis revealed increased expression of BAX, APAF1, cleaved-caspase-9 and cleaved-caspase-3 proteins, which induce apoptosis, but decreased expression of BCL2 protein, which is an inhibitor of apoptosis, following administration of UDCA.
CONCLUSION: UDCA suppresses growth of BEL7402 hepatocellular carcinoma cells in vivo, in part through apoptosis induction, and is thus a candidate for therapeutic treatment of HCC.
Core tip: Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer worldwide. Prognosis of HCC patients remains poor, however, due to the lack of effective therapies. In this study, ursodeoxycholic acid (UDCA) was investigated as a potential chemotherapeutic agent in a mouse model of HCC. Tumor growth was inhibited by increasing concentrations of UDCA over a 21-d period, and the effect was elicited through apoptosis. UDCA is thus a candidate chemopreventive and chemotherapeutic agent for HCC.
- Citation: Liu H, Xu HW, Zhang YZ, Huang Y, Han GQ, Liang TJ, Wei LL, Qin CY, Qin CK. Ursodeoxycholic acid induces apoptosis in hepatocellular carcinoma xenografts in mice. World J Gastroenterol 2015; 21(36): 10367-10374
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10367
Worldwide, hepatocellular carcinoma (HCC) ranks as the sixth most common cancer, with over half a million new cases diagnosed each year[1]. HCC is particularly prevalent in Asia, where it is the third leading cause for cancer-related death[2,3]. The incidence is even higher in China[4], where HCC ranks as the second cause of cancer-related death[5-7]. Despite advances in surgical and chemo-radiotherapies, the prognosis of HCC patients remains poor. Thus it is crucial to develop new therapeutic options for this disease.
A potential chemotherapeutic agent is ursodeoxycholic acid (UDCA), a metabolite produced by intestinal bacteria, which is currently used in the treatment of liver disease. It has been reported to have a variety of therapeutic effects based upon its ability to reduce oxidative stress. UDCA has also been shown to lower biliary and serum concentrations of hydropholic bile acids, as well as tumor necrosis factor-α in chronic cholestasis[8,9]. Thus, UDCA appears to improve symptoms of nonalcoholic steatohepatosis (NASH)[10,11]. UDCA has been most successfully used for the treatment of primary biliary cirrhosis, which is thought to be mediated in part through immunosuppression[12]. In fact, the prognosis of primary biliary cirrhosis has improved not only due to earlier detection of the disease, but also to the increased use of UDCA for treatment[13]. Interestingly, while it has been reported to have anti-apoptotic properties, recent studies have demonstrated that UDCA reduced the frequency of colonic carcinogenesis by inhibiting interleukin-1 beta and deoxycholic acid-induced activation of NF-kappaB and AP-1[14]. Furthermore, UDCA has been shown to induce apoptosis of HCC cells in vitro[15]. In our own studies, we have previously demonstrated that UDCA selectively inhibits proliferation and induces apoptosis in the HCC cell lines, HepG2 and BEL7402, in vitro by blocking the cell cycle and regulating the expression of genes involved in programmed cell death, such as BAX/BCL2[16].
Although a number of studies have demonstrated that UDCA inhibits cell proliferation and induces apoptosis in various types of cancer cells in vitro, little is known about the effect of UDCA on cancer cells in vivo, in particular with regard to HCC. Here, the efficacy of UDCA as a possible therapy was investigated in a mouse model of HCC. The results demonstrated that in BALB/c nude mice, UDCA suppressed the growth of subcutaneously injected BEL7402 cells, derived from a hepatocellular carcinoma, through the induction of apoptosis.
All animal experiments were carried out under an Institutional Animal Care and Use Committee-approved protocol.
BEL7402 cells (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China) were derived from a specimen obtained from a 53 year-old male patient with HCC in 1974. Cells were maintained in DMEM (Life Technologies, Grand Island, NY, United States) containing 10% fetal bovine serum supplemented with penicillin (100 units/mL) and streptomycin (100 μg/mL) and cultured at 37 °C in a humidified chamber with 5% CO2.
Six-week-old male immunodeficient BALB/c mice (n = 40) were obtained from the experimental animal center of Shandong University (Shandong, China). The animals were housed in sterile filter-capped microisolator cages and provided with a sterilized diet and water. HCC BEL7402 cells (1 × 106/0.2 mL/mouse) were suspended in phosphate buffered saline (PBS) and injected subcutaneously into the right flank of mice. Mice were randomized into four groups one day before the injection of tumor cells. Group 1 (control, n = 10) was fed a standard diet; Group 2 (n = 10), a standard diet supplemented with UDCA (Sigma, St. Louis, MO, United States) at 30 mg/kg per day; Group 3 (n = 10), a standard diet supplemented with UDCA at 50 mg/kg per day; and Group 4 (n = 10), a standard diet supplemented with UDCA at 70 mg/kg per day. Body weights of animals in each group were measured before initiation of the experiment and after 21 d. Tumor growth was measured once each week over the 21 d, and tumor volume (V) was calculated as V = (L×W2) × 0.52, where L is the length and W is the width of a xenograft. After 21 d, mice were killed under ether anesthesia. The tumors were excised and weighed. A portion of the tumor was snap-frozen for protein analysis, and the remaining tissue was fixed in phosphate buffered formalin to obtain sections for histological analysis and immunohistochemistry.
DNA was isolated from homogenized tissues or cells harvested and rinsed twice with ice-cold PBS. Samples were treated with proteinase K (0.1 g/L; Sigma) in 0.3 mL of buffer containing Tris-HCl (10 mmol/L, pH 7.4), EDTA (25 mmol/L), and SDS (0.5%) at 37 °C for 12 h. DNA was extracted with an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) and precipitated in NaOAc (3 mol/L) and 2 volumes of ice-cold absolute ethanol. The precipitated DNA was rinsed once with 70% ethanol, resolubilized in TE buffer (Tris-HCl 10 mmol/L and EDTA 1 mmol/L, pH 8.0), and incubated with RNase I (10 g/L) for 1 h at 37 °C. Genomic DNA (10 mg/well) and markers were run on 1.5% agarose gels containing ethidium bromide (0.1 g/L) for 2 h at 60 V and were visualized with ultraviolet light.
Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) using the ApoTag Plus Peroxidase in situ Apoptosis Detection Kit (Chemicon, Temecula, CA, United States) according to the manufacturer’s instructions. In brief, tissue sections were deparaffinized, rehydrated through a graded alcohol series, and rinsed in distilled water. The tissue sections were incubated with proteinase K for 20 min at room temperature and subsequently incubated with terminal deoxynucleotidyl transferase (TdT) buffer containing 0.3 U/L TdT (Life Technologies) and 0.04 nmol/L biotinylated dUTP (Boehringer Mannheim GmbH, Mannheim, Germany) in a humidified chamber for 1 h at 37 °C. Slides were rinsed with PBS, and signal was amplified with horseradish peroxidase-conjugated streptavidin. Sections were counterstained with hematoxylin for 30 s. Cells undergoing apoptosis contained dark brown staining nuclei, and the number of TUNEL-positive cells was determined by analyzing 1000 cells in randomly selected fields of three sections for each group. A section from rat mammary gland provided by the manufacturer was used as a positive control.
Xenograft tissue was lysed for 40 min on ice in buffer containing 50 mmol/L NaCl, 0.5% Triton X-100, 50 mmol/L Tris-HCl (pH 7.4), 25 mmol/L NaF, 20 mmol/L EDTA, 1 mmol/L DTT, 1 mmol/L Na3VO4, and protease inhibitors at a concentration of 10 mg/mL (Roche, Mannheim, Germany). Protein lysates were centrifuged at 14800 g for 15 min to remove cellular debris. Supernatants were collected, and protein concentrations were measured with the bicinchoninic acid assay (BCA; Life Technologies). Protein (20 mg) was loaded onto a 4% to 12% NuPAGE gel (Life Technologies) and transferred onto a polyvinylidene difluoride membrane following electrophoresis. Membranes were blocked for 1 h with 5% nonfat dry milk in PBS with 0.1% Tween 20 (PBST), incubated with primary antibodies (cleaved caspase-3 and cleaved caspase-9: Cell Signaling, Beverly, MA, United States; anti-mouse BAX, anti-human BCL2, anti-human APAF1, and anti-actin: Santa Cruz Biotechnology, Dallas, TX, United States) in 5% bovine serum albumin/0.1% PBST overnight at 4 °C, rinsed three times for 5 min each with PBST, and then incubated with a horseradish peroxidase-conjugated secondary antibody in 5% nonfat dry milk/PBST for 1 h at room temperature. Blots were rinsed with PBST three times, and transferred proteins were visualized with Super Signal chemiluminescent substrate (Life Technologies).
Preparation of mitochondrial and cytosolic fractions was performed as previously described[11]. For isolation of mitochondria, the tumor tissue was minced on ice, resuspended in 10 mL of ice-cold Buffer A (200 mmol/L mannitol, 50 mmol/L sucrose, 10 mmol/L KCl, 1 mmol/L EDTA, 10 mmol/L HEPES-KOH (pH 7.4), 0.1% bovine serum albumin, 10 μg/mL aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride), and homogenized with a glass Dounce homogenizer and a tight Teflon pestle. Homogenates were centrifuged at 600 g for 15 min at 4 °C to pellet debris, and the supernatants collected and centrifuged at 3500 g for 15 min at 4 °C to pellet mitochondria. Floating lipid layers were aspirated, and the mitochondrial pellets were resuspended in Buffer A. Suspensions were centrifuged at 1500 g for 5 min at 4 °C, and the supernatants were recentrifuged at 5500 g for 10 min. The last two steps were repeated twice, and used as mitochondrial protein lysates for Western blot analysis. For preparation of cytosolic extracts, the tumor was homogenized in ice-cold buffer (20 mmol/L HEPES-KOH (pH 7.0), 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L sodium EDTA, 1 mmol/L sodium EGTA, 1 mmol/L dithiothreitol, 250 mmol/L sucrose, 10 mg/mL aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride) with a Dounce homogenizer. Supernatants were centrifuged at 14000 g for 15 min in a microcentrifuge. The resulting supernatants were used for Western blot analysis. For detection of cytochrome c, cytosolic and mitochondrial proteins (20 mg) were separated on SDS-PAGE, transferred to polyvinylidene difluoride membrane, and incubated with anti-cytochrome c antibody (Santa Cruz Biotechnology). β-actin (AC-15; Sigma) was used as a cytoplasm-specific marker and oxidative complex 1 protein (20C11; Life Technologies) as a mitochondrion-specific marker.
SPSS version 11.0 (SPSS, Inc.; Chicago, IL, United States) was used for data processing. All data are expressed as the mean ± SD. The student’s t-test was used for the comparison between two groups. A P-value < 0.05 was considered statistically significant.
BEL7402 cells were subcutaneously injected into mice and growth of xenografts treated with UDCA was monitored over 21 d. The effect of UDCA treatment at different doses was first investigated as a function of the weight of the animals and tumor volume. Animals were weighed on day 0 and a mean weight was calculated for each experimental group and the controls. The mean body weight for all groups was 17.8 ± 1.8 g. On day 21, body weights generally had decreased as xenografts developed with the most dramatic decrease in untreated controls. The mean body weight was 14.5 ± 1.5 g, 15.7 ± 1.6 g, 16.7 ± 1.7 g and 17.6 ± 1.8 g for controls and the UDCA groups at 30, 50 and 70 mg/kg per day, respectively. Statistical analysis demonstrated that body weight was significantly different between the treatment groups and controls (30 mg/kg per day, P < 0.05; 50 mg/kg per day, P < 0.05; 70 mg/kg per day, P < 0.01).
As expected, tumor volume (1090 ± 89 mm3) increased significantly in control animals over the 21 d. In the experimental groups, growth of tumors was inhibited with increasing doses of UDCA (Figure 1A). Differences between the mean volumes of treated tumors and controls were statistically significant at each dose: 30 mg/kg per day, 612 ± 46 mm3, P < 0.05; 50 mg/kg per day, 563 ± 38 mm3, P < 0.05; and 70 mg/kg per day, 221 ± 26 mm3, P < 0.01 (Figure 1B).
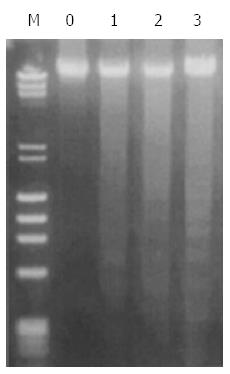
Apoptosis is one of the mechanisms underlying UDCA growth inhibition in vitro. To determine whether UDCA elicits growth inhibition in vivo through apoptosis, DNA from treated xenografts was isolated and examined by agarose gel electrophoresis for the presence of the characteristic DNA ladder. DNA ladders were observed in genomic DNA isolated from tumors treated with UDCA after 21 d. Furthermore, the DNA fragmentation increased with increasing UDCA dose (Figure 2).
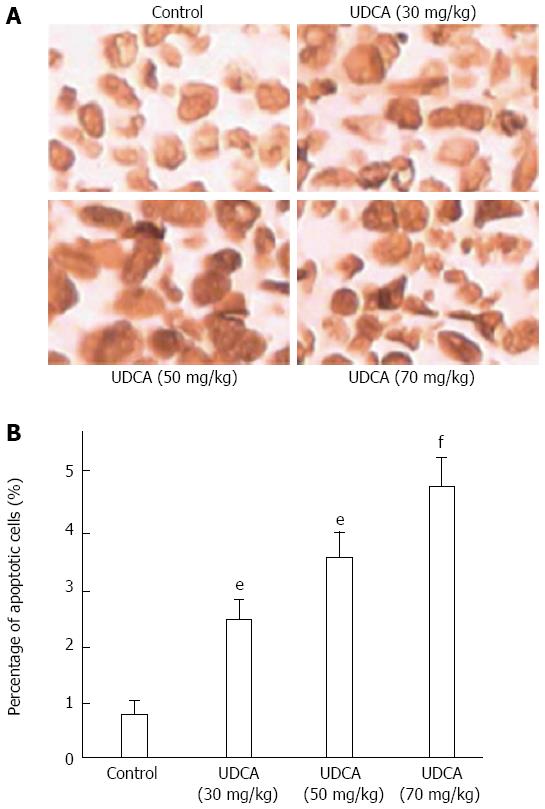
Apoptosis induced by UDCA was further evaluated with the TUNEL assay which enables the detection of fragmented DNA in situ. The TUNEL assay revealed that the mean percentage of apoptotic cells increased with increasing UDCA dose: control, 1.6% ± 0.3%; 30 mg/kg per day, 2.9% ± 0.5%; 50 mg/kg per day, 3.15% ± 0.7%, and 70 mg/kg per day, 4.86% ± 0.9%. The increased percentage of apoptotic cells in treated vs control xenografts was significant (P = 0.041, 0.029, and 0.016 for 30 mg/kg per day UDCA, 50 mg/kg per day UDCA, and 70 mg/kg per day UDCA, respectively) (Figure 3).
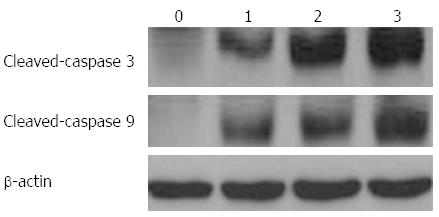
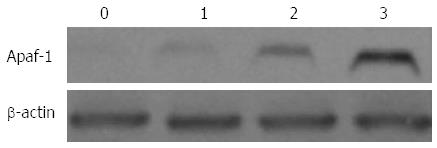
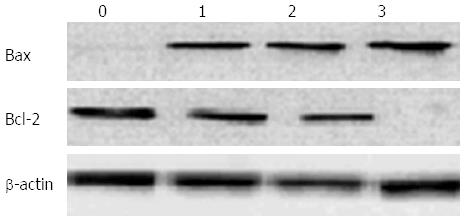
Several proteins mediating and regulating apoptosis were examined by Western blot in order to further elucidate the mechanism of UDCA-induced cell death in BEL7402 xenografts. With the administration of UDCA, the protein levels of BAX, apoptotic protease-activating factor-1 (APAF1), cleaved caspase-9, and cleaved caspase-3, proteins executing apoptosis, were up-regulated, whereas BCL2, a protein known to inhibit cell death, was down-regulated. The expression of these proteins furthermore increased with increasing doses of UDCA (Figures 4-6).
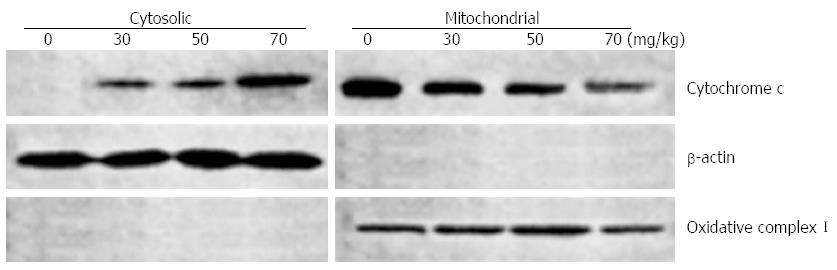
Release of the mitochondrial protein cytochrome c into the cytosol signals the involvement of the mitochondria in programmed cell death. In order to localize cytochrome c during UDCA induced apoptosis, cytosolic and mitochondrial protein extracts were prepared from treated and control xenografts and analyzed by Western blot. UDCA treatment resulted in release of cytochrome c into the cytoplasm of BEL7402 relative to control xenografts (Figure 7).
The prognosis of HCC remains poor worldwide due to a high recurrence rate despite state of the art treatments. Based on previous work in vitro, UDCA was investigated here as a potential chemotherapeutic agent for the treatment of HCC in vivo. First, the results indicated that UDCA suppressed growth of HCC in vivo; continued UDCA administration, initiated one day ahead of implantation of the BEL7402 cells in nude mice, significantly inhibited the growth of xenografts compared to controls. Second, UDCA inhibition of tumor development was mediated in part through apoptosis. Finally, UDCA treatment at the doses used did not lead to the development of life threatening lesions or toxic side effects in the mice. Taken together, results from both in vitro and in vivo experiments support further investigation of UDCA as a chemotherapeutic agent for HCC.
UDCA, a hydrophilic bile acid, has been previously found to have therapeutic effects. First, UDCA was recognized as an effective agent in the treatment of primary biliary cirrhosis, from various biochemical and physiological aspects[12]. Second, UDCA is known as a cytoprotective agent. UDCA prevents apoptosis induced by a variety of stress stimuli including cytotoxic bile acids such as deoxycholic acid (DCA), and it has been shown to antagonize DCA-induced apoptosis in human colon cancer cells[17,18] and it is a chemopreventive agent in the azoxymethane model of experimental colonic carcinogenesis[19]. Finally, it has been reported that UDCA and its derivatives induce apoptosis in several cancer cell lines, such as human HCC cells[20], human prostate cancer cells[21], human cervical carcinoma cells[22] and human breast carcinoma cells[23]. Thus, UDCA and its derivatives may inhibit carcinogenesis through different mechanisms in a variety of tissue types.
The appearance of a DNA ladder and the results of the TUNEL assay indicate that the inhibitory properties of UDCA in vivo are mediated in part through the induction of apoptosis. Additional experiments determined that molecular components of the apoptotic machinery associated with mitochondria were in fact altered under UDCA treatment. For example, UDCA induced the expression of BAX but down-regulated the expression of BCL2. BAX protein has a proapoptotic effect causing release of cytochrome c[24-26] and increasing outer membrane permeability[27]. In contrast, the BCL2 protein is anti-apoptotic and prevents both the loss of mitochondrial membrane potential and the efflux of cytochrome c[24-26]. Furthermore, BCL2 proteins are directly associated with the mitochondrial membrane and effectively regulate its integrity[28,29]. In addition, cytochrome c was localized in the cytosolic fraction, indicating its release from the mitochondria. Cytochrome c is a crucial mediator of the pathway, as it leads to the activation of a complex (apoptosome) of caspase-9 and caspase-3 through the adaptor protein APAF1[30,31]. Once released, cytochrome c promotes the activation of pro-caspase-9 directly within the apoptosome complex[31,32]. The formation of the APAF1/caspase-9 apoptosome is a crucial event in the apoptotic cascade[33].
Based on these results, UDCA induced apoptosis appears to be mediated through a mitochondrial pathway. The mitochondrial apoptotic pathway has also been reported to play an important role in the apoptosis of other types of human carcinoma cells[34,35]. Thus, the identification of new drugs that stabilize the formation of an active apoptosome complex is a possible strategy for effective treatment of HCC as well as other cancers[36].
In conclusion, oral administration of UDCA was effective in suppressing the growth of BEL7402 xenografts in mice. Our results support further investigation of UDCA as a candidate for the treatment of liver cancer.
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, and the third leading cause of cancer-related deaths. Novel treatment strategies are necessary. A candidate therapy is administration of ursodeoxycholic acid (UDCA), which has been shown to be of therapeutic value in liver disease and to induce apoptosis in cancer cell lines. UDCA is effective in the treatment of primary biliary cirrhosis, mediated in part through immunosuppression, and nonalcoholic steatohepatosis. In addition, UDCA has been shown to inhibit the development of colonic carcinogenesis and induce apoptosis specifically in the HCC cells HepG2 and BEL7402, as opposed to the normal human hepatic line L-02 in vitro.
HCC is a major cause of morbidity and mortality worldwide. Despite advances in surgical and chemo-radiotherapies, the prognosis remains poor for HCC patients. Therefore, it is crucial to develop novel therapies for the disease. While a number of studies have demonstrated efficacy of UDCA on cancer cells in vitro, little is known about the role of UDCA in vivo, especially with regard to HCC. This study is the first to evaluate the potential efficacy of UDCA in the treatment of HCC in vivo.
Previous work from our group has established that UDCA selectively inhibits proliferation and induces apoptosis of the human HCC cell lines HepG2 and BEL7402 in comparison to the normal human hepatic cell line L-02 in vitro. This study is the first to evaluate the potential efficacy of UDCA as a chemotherapeutic agent through oral administration in BALB/c nude mice bearing subcutaneous xenografts derived from BEL7402. Through several state of the art methods, such as DNA ladder detection, TUNEL, and Western blot analysis, UDCA was found to suppress tumor growth and induce apoptosis through a mitochondrial pathway. Further delineation of the molecular basis of the effect might be exploited in the future to develop additional therapies for HCC.
UDCA has been previously established to elicit therapeutic effects in the treatment of liver diseases, such as primary biliary cirrhosis and nonalcoholic steatohepatosis. Recent studies have also indicated that UDCA reduced the frequency of colonic carcinogenesis and induced apoptosis of HCC cells in vitro. The results further demonstrate that UDCA suppresses development of liver cancer in vivo. The results thus indicate that patients with HCC might benefit from treatment with UDCA as a chemotherapeutic agent.
Ursodeoxycholic acid, a hydrophilic bile acid produced by intestinal bacteria, has been found to have several therapeutic effects.
The aim of the study was to evaluate the potential efficacy of UDCA as a chemotherapeutic agent for the treatment of HCC. Oral administration of UDCA suppressed the growth of xenografts derived from the HCC cell line BEL7402 in BALB/c nude mice. The research findings are of interest to the scientific and medical community as the prognosis of the large number of people affected worldwide by this disease remains poor. The data presented are clear and support the conclusions.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 2. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3128] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 4. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 5. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1827] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 6. | Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF, Zhang J. Growth inhibition and apoptosis induction of tanshinone II-A on human hepatocellular carcinoma cells. World J Gastroenterol. 2004;10:2024-2028. [PubMed] |
| 7. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1067] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 8. | Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: where are we and where should we go? Eur J Gastroenterol Hepatol. 2005;17:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 361] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Padda S, Ramirez FC, Shernhoff NJ. The effect of ursodeoxycholic acid treatment on liver tests in patients with non alcohol induced steatohepatitis(NASH). Am J Gastroenterol. 1999;94:A334. |
| 12. | Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, Fukui H, Ishizaka S. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 155] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Crosignani A, Battezzati PM, Invernizzi P, Selmi C, Prina E, Podda M. Clinical features and management of primary biliary cirrhosis. World J Gastroenterol. 2008;14:3313-3327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Shah SA, Volkov Y, Arfin Q, Abdel-Latif MM, Kelleher D. Ursodeoxycholic acid inhibits interleukin 1 beta [corrected] and deoxycholic acid-induced activation of NF-kappaB and AP-1 in human colon cancer cells. Int J Cancer. 2006;118:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhu L, Shan LJ, Liu YJ, Chen D, Xiao XG, Li Y. Ursodeoxycholic acid induces apoptosis of hepatocellular carcinoma cells in vitro. J Dig Dis. 2014;15:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Liu H, Qin CY, Han GQ, Xu HW, Meng M, Yang Z. Mechanism of apoptotic effects induced selectively by ursodeoxycholic acid on human hepatoma cell lines. World J Gastroenterol. 2007;13:1652-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Im E, Martinez JD. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J Nutr. 2004;134:483-486. [PubMed] |
| 18. | Centuori SM, Martinez JD. Differential regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci. 2014;59:2367-2380. [PubMed] |
| 19. | Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, Roy H, Khare S, Brasitus TA. Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res. 1994;54:5071-5074. [PubMed] |
| 20. | Baek JK, Kim JA, Kang CM, Lee , YS , Kim KW. Induction of apoptosis by bile acids in HepG2 human hepatocellular carcinoma cells. Korean J Pharmcol. 1997;1:107-115. |
| 21. | Choi YH, Im EO, Suh H, Jin Y, Yoo YH, Kim ND. Apoptosis and modulation of cell cycle control by synthetic derivatives of ursodeoxycholic acid and chenodeoxycholic acid in human prostate cancer cells. Cancer Lett. 2003;199:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Im E, Choi SH, Suh H, Choi YH, Yoo YH, Kim ND. Synthetic bile acid derivatives induce apoptosis through a c-Jun N-terminal kinase and NF-kappaB-dependent process in human cervical carcinoma cells. Cancer Lett. 2005;229:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Im EO, Choi YH, Paik KJ, Suh H, Jin Y, Kim KW, Yoo YH, Kim ND. Novel bile acid derivatives induce apoptosis via a p53-independent pathway in human breast carcinoma cells. Cancer Lett. 2001;163:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | He J, Xiao Y, Casiano CA, Zhang L. Role of mitochondrial cytochrome c in cocaine-induced apoptosis in coronary artery endothelial cells. J Pharmacol Exp Ther. 2000;295:896-903. [PubMed] |
| 25. | Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997-5002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1240] [Article Influence: 44.3] [Reference Citation Analysis (12)] |
| 26. | Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 673] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 27. | Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 418] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3963] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 29. | Bruce JIe. Plasma membrane calcium pump regulation by metabolic stress. World J Biol Chem. 2010;1:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, Cohen GM. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM, Chen LG, Ren YJ, Yao HB, Yang Q, He XJ. Oridonin induces apoptosis in gastric cancer through Apaf-1, cytochrome c and caspase-3 signaling pathway. World J Gastroenterol. 2012;18:7166-7174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5532] [Article Influence: 190.8] [Reference Citation Analysis (0)] |
| 33. | Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549-11556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1507] [Cited by in RCA: 1517] [Article Influence: 56.2] [Reference Citation Analysis (12)] |
| 34. | Liu J, Qin CK, Lv W, Zhao Q, Qin CY. OSU-03012, a non-Cox inhibiting celecoxib derivative, induces apoptosis of human esophageal carcinoma cells through a p53/Bax/cytochrome c/caspase-9-dependent pathway. Anticancer Drugs. 2013;24:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Liu H, Qin CK, Han GQ, Xu HW, Ren WH, Qin CY. Synthetic chenodeoxycholic acid derivative, HS-1200, induces apoptosis of human hepatoma cells via a mitochondrial pathway. Cancer Lett. 2008;270:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | D’Amelio M, Tino E, Cecconi F. The apoptosome: emerging insights and new potential targets for drug design. Pharm Res. 2008;25:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Qin L, Stournaras C S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN