Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.9887
Peer-review started: March 21, 2015
First decision: April 13, 2015
Revised: May 16, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: September 14, 2015
Processing time: 178 Days and 6 Hours
AIM: To elucidate the effects of dexamethasone on hypoxia-induced epithelial-to-mesenchymal transition (EMT) in colon cancer.
METHODS: Human colon cancer HCT116 and HT29 cells were exposed to normoxic (21%) and hypoxic (1%) conditions. First, the effect of dexamethasone on cell viability was examined by MTT cell proliferation assay. In order to measure the expression levels of EMT markers (Snail, Slug, Twist, E-cadherin, and integrin αVβ6) and hypoxia-related genes [Hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF)] by dexamethasone, quantitative real-time polymerase chain reaction and western blot analysis were performed. Furthermore, the morphological changes of colon cancer cells and the expression pattern of E-cadherin by dexamethasone were detected through immunocytochemistry. Finally, the effects of dexamethasone on the invasiveness and migration of colon cancer cells were elucidated using matrigel invasion, migration, and wound healing migration assays.
RESULTS: Under hypoxia, dexamethasone treatment inhibited HIF-1α protein level and its downstream gene, VEGF mRNA level in the colon cancer cell lines, HCT116 and HT29. In addition, the presence of dexamethasone down-regulated the mRNA levels of hypoxia-induced Snail, Slug, and Twist, all transcriptional factors of EMT, as well as hypoxia-induced integrin αVβ6 protein level, a well-known EMT marker for colon cancer cells. Furthermore, reduced E-cadherin in hypoxic condition was found to be recoverable by treating with dexamethasone in both colon cancer cell lines. Similarly, under hypoxic conditions, dexamethasone restored the growth pattern and morphological phenotype reminiscent of colon cancer cells grown under normoxic conditions; dexamethasone blocked the migration and invasion of both colorectal cancer cell lines in hypoxia.
CONCLUSION: Our study suggested that dexamethasone has inhibitory effects on cell migration and invasion by suppressing EMT of colon cancer cell lines in hypoxic condition.
Core tip: In solid tumors, the heightened metabolism of cancer cells often leads to areas of hypoxia that can drive epithelial-mesenchymal transition (EMT). Dexamethasone is often given to patients with colon cancer to limit the negative side effects of chemotherapy. Our study investigated the effects of dexamethasone on hypoxia-induced EMT and found that it was sufficient to block the propensity for cells to undergo EMT by repressing the hypoxia-induced expression of Hypoxia-inducible factor-1α, vascular endothelial growth factor and other EMT markers. This evidence suggests that dexamethasone co-treatment may limit the migratory properties of colorectal cancer cells that subsist in the hypoxic regions of colorectal cancers.
- Citation: Kim JH, Hwang YJ, Han SH, Lee YE, Kim S, Kim YJ, Cho JH, Kwon KA, Kim JH, Kim SH. Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer. World J Gastroenterol 2015; 21(34): 9887-9899
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/9887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.9887
Among newly diagnosed cases of colorectal cancer in the United States, approximately 20% are fully metastatic and an additional 37% are lymph node-positive at presentation[1]. Thus, development of effective chemotherapeutic adjuvants is important to decrease cancer recurrence and increase cure rates in these patients.
Many human cancers contain hypoxic regions resulting from the increased consumption of oxygen by rapidly proliferating tumor cells, as well as the structural and functional irregularities of intratumoral blood vessels[2]. Intratumoral hypoxia promotes the accumulation of hypoxia-inducible factor-1α (HIF-1α), which is immediately degraded by proteasomal ubiquitination under normoxic conditions[2,3]. HIF-1α is a critical regulator of tumor invasion and metastasis[4-7]. For metastasis to occur, cancer cells must detach from the primary tumor and then invade and migrate via the connective tissues, blood, and lymphatic vessels. The process by which epithelial cells lose their cell polarity and cell-cell adhesions and thereby acquire migratory and invasive properties of mesenchymal cells is termed as epithelial-to-mesenchymal transition (EMT)[8,9]. The EMT is an important molecular step in cancer progression that provides cancer cells with a more aggressive phenotype. Notably, this process is potentiated by hypoxia in the tumor microenvironment[7].
The synthetic glucocorticoid dexamethasone (DEX) is widely used in the treatment of many diseases, particularly in hematologic malignancies where it has shown to have cytotoxic effects[10,11]. While DEX lacks this activity in solid tumors, patients are still treated with corticosteroids to prevent complications often associated with cancer therapy, including cancer-related pain, lack of appetite, edema, and electrolyte imbalance[12]. Although DEX is commonly prescribed as a co-medication in cancer treatment, its effects on the metastatic capacity of colorectal cancer are unknown. As such, this study aims to investigate the influence of DEX treatment on hypoxia-dependent EMT in colorectal cancer cell lines.
Human colon cancer cell lines, HCT116 and HT29, were purchased from the Korean Cell Line Bank (Seoul, South Korea) and grown in McCoy’s (Gibco Cell Culture, Carlsbad, CA, United States) supplemented with 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibco). For hypoxic conditions, both cell lines were maintained in a hypoxic incubator (New Brunswick Scientific, Edison, NJ, United States) with a humidified environment consisting of 1% O2, 5% CO2, and 94% N2.
DEX and deferoxamine (DFO) were purchased from Sigma-Aldrich (St. Louis, MO, United States) and dissolved in ethanol and water, respectively.
Cells were seeded in 96-well microassay plates and exposed to various concentrations of DEX for 24-72 h at 37 °C prior to the addition of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma-Aldrich) diluted 1:10 from a stock solution of 5 mg/mL in McCoy’s. After a 90 min incubation period, the MTT-containing medium was removed and replaced with 100 μL DMSO (Sigma-Aldrich) to dissolve the formazan crystals. Absorbance was then measured at 570 nm in a microplate reader and the IC50 values for DEX were calculated using non-linear regression analysis in GraphPad Prism software (version 3.05, San Diego, CA, United States).
Cells were lysed with either RIPA buffer [50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40] or whole cell lysate buffer (10 mmol/L HEPES pH 7.9, 400 mmol/L NaCl, 0.1 mmol/L EDTA, 5% Glycerol, 1 mmol/L DTT) to detect EMT markers and HIF-1α, respectively. Antibodies to HIF-1α (1:1000; Novus Biologicals, Littleton, CO, United States), β-actin (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, United States), E-cadherin (1:5000; Cell Signaling, Beverly, MA, United States), αVβ6 (1:1000; Abcam, Cambridge, United Kingdom) were then applied.
Total RNA was extracted with RNAiso Plus reagent (Takara Bio, Otsu, Japan) and cDNA was synthesized from 1 μg total RNA using the PrimeScript First Strand cDNA Synthesis Kit (Takara Bio). Real-time PCR was performed on a CFX 96 real-time system (Bio-Rad, Hercules, CA, United States) using SYBR Green I Universal PCR Master Mix (Takara Bio) in reactions with the following conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Samples were loaded in triplicate, and the experiment was repeated three times. Fold change of target gene expression was then calculated per the manufacturer’s instructions using GAPDH as an internal control. Primer sequences for PCR reactions are as follows: Snail 5’-CCCCAACTCGGAAGCCTAACT-3’ (forward) and 5’-GCTGGAAGGTAAACTCTGGATTAGA-3’ (reverse), Slug 5’-ACGCCCAGCTACCCAATG-3’ (forward) and 5’-CGCCCCAAAGATGAGGAGTA-3’ (reverse), Twist 5’-GCGCTGCGGAAGATCATC-3’ (forward) and 5’-GGTCTGAATCTTGCTCAGCTTGT-3’ (reverse), VEGF 5’-ATCTTCAAGCCATCCTGTGTGC-3’ (forward) and 5’-CAAGGCCCACAGGGATTTTC-3’ (reverse), GAPDH 5’-AGGTCGGAGTCAACGGATTTGG-3’ (forward) and 5’-ACAGTCTTCTGGGTGGCAGTGATG-3’ (reverse).
Cells were grown on coverslips coated with 0.1% gelatin prior to treatment with DEX for 5-7 d. Treated cells were then fixed in 3.7% paraformaldehyde at room temperature for 15 min, washed with phosphate-buffered saline (PBS), and permeabilized in 0.5% Triton X-100/PBS for 5 min. After blocking for 30 min in 1% BSA in PBS-T (0.1% Triton X-100/PBS), cells were incubated with E-cadherin antibody (1:500) diluted in 1% BSA/PBS-T for 1 h at room temperature, and washed with PBS prior to incubation with Alexa Fluor 555 Donkey Anti-Rabbit IgG antibody (Life Technologies, Carlsbad, CA, United States) for another 1 h at room temperature. Cells were then washed with PBS and mounted in Vectorshield with DAPI (Vector Laboratories Inc., Burlingame, CA, United States). Images were taken with a LSM710 confocal microscope (Carl Zeiss, Jena, Germany) at × 200 magnification.
Cells were seeded in 60 mm plates, incubated for 24 h, and then scratched using a pipette tip. After washing with PBS, cells were incubated in media containing 100 μmol/L DFO and/or 100-200 nmol/L DEX for 24 h. Images were taken at 0 and 24 h with an Olympus CFX41 microscope (Hamburg, Germany) at × 40 magnification and the percentage of migrating cells was quantified using Image J software. The experiment was repeated three times with independent samples. The percentage of wound closure was calculated by the equation: (% of wound closure) = [(Total area (0 h) - Total area (24 h)]/Total area (0 h) × 100.
After coating the lower surface of the transwells (Corning Incorporated, NY, United States) with 0.2% gelatin, cells (2 × 104) in DEX-containing, serum-free medium were seeded into the inner chamber and incubated under normoxic or hypoxic conditions for 24 h. Media containing 20% FBS with or without DEX was used as chemoattractant in the bottom chamber. After methanol fixation and hematoxylin/eosin (H/E) staining, cells on the upper surface were scraped away with a wet cotton swab, and those on the inner surface were mounted in mounting solution (Vectorshield, Vector Laboratories). Images were then obtained with a light microscope (Olympus DP72, Hamburg, Germany) at × 200 magnification. Experiments were independently performed in triplicate.
Cell invasion assays were performed using transwell inserts (Corning Incorporated). Briefly, the upper surfaces were coated with matrigel (BD Biosciences, San Jose, CA, United States) at 37 °C for 2 h, and the lower chambers were coated with 0.2% gelatin at room temperature for 1 h. Cells (2 × 104) in DEX-containing, serum-free medium were then added to the inner chamber and incubated under normoxic or hypoxic conditions for 48 h. Media supplemented with 20% FBS was used as a chemoattractant in the bottom chamber. After methanol fixation and H/E staining, cells on the upper surface were scraped away with a wet cotton swab, and those on the inner surface were counted under a light microscope (Olympus DP72). Experiments were performed in triplicate with independent samples.
Statistical analysis was carried out using SPSS 12.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, United States) for MS Windows®. The differences between groups were analyzed using the independent t-test. Two-tailed P-values of < 0.05 were considered to be statistically significant.
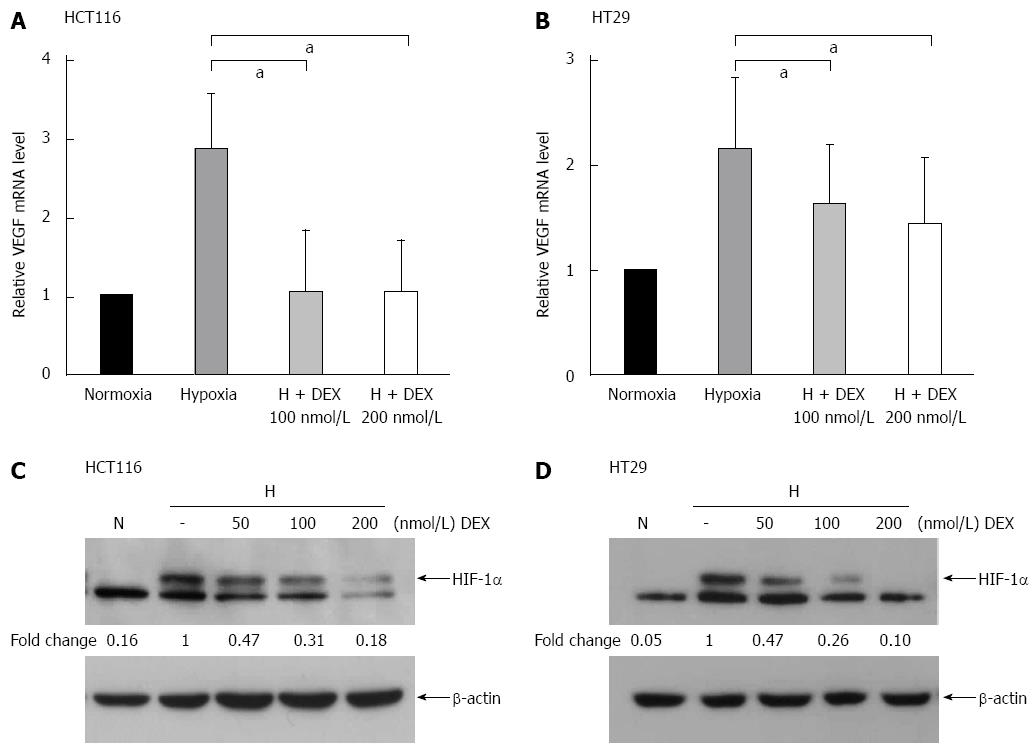
To determine the IC50 values of DEX in the colon cancer cell lines, HCT116 and HT29 cells were cultured with increasing concentrations of DEX for 24-72 h (Table 1). Notably, little change was observed on the IC50 over the time periods assessed. Next, to examine the effect of DEX on hypoxia-related gene expression, HCT116 and HT29 cells were treated with DEX and incubated under normoxic or hypoxic conditions for 24 or 72 h. As shown in Figure 1A, HCT116 cells treated with DEX under hypoxia exhibited a 67%-75% decrease in vascular endothelial growth factor (VEGF) mRNA compared to the hypoxia-only control (both P < 0.05 vs hypoxia controls). In addition, DEX-treated HT29 cells in hypoxic conditions displayed a 25%-35% reduction in VEGF mRNA when compared to the hypoxia-only control (both P < 0.05 vs hypoxia controls) (Figure 1B). The HIF-1α protein expression is kept low under normoxic conditions via ubiquitin-mediated proteasomal degradation; thus, we sought to investigate whether DEX treatment had any effect on HIF-1α protein level in either cell line. For this, cells were cultured under hypoxic conditions for 24 h in the presence of varying concentrations of DEX. Finally, Western blot analysis also demonstrated DEX’s inhibitory effect on HIF-1α protein expression by 53%-82% in HCT116 and 53%-90% in HT29 cells compared to the hypoxia-only controls (Figure 1C and D).
| Incubation time with DEX | |||
| 24 h | 48 h | 72 h | |
| HCT116 (mmol/L) | 0.916 | 0.340 | 0.191 |
| HT29 (mmol/L) | 1.130 | 0.259 | 0.129 |
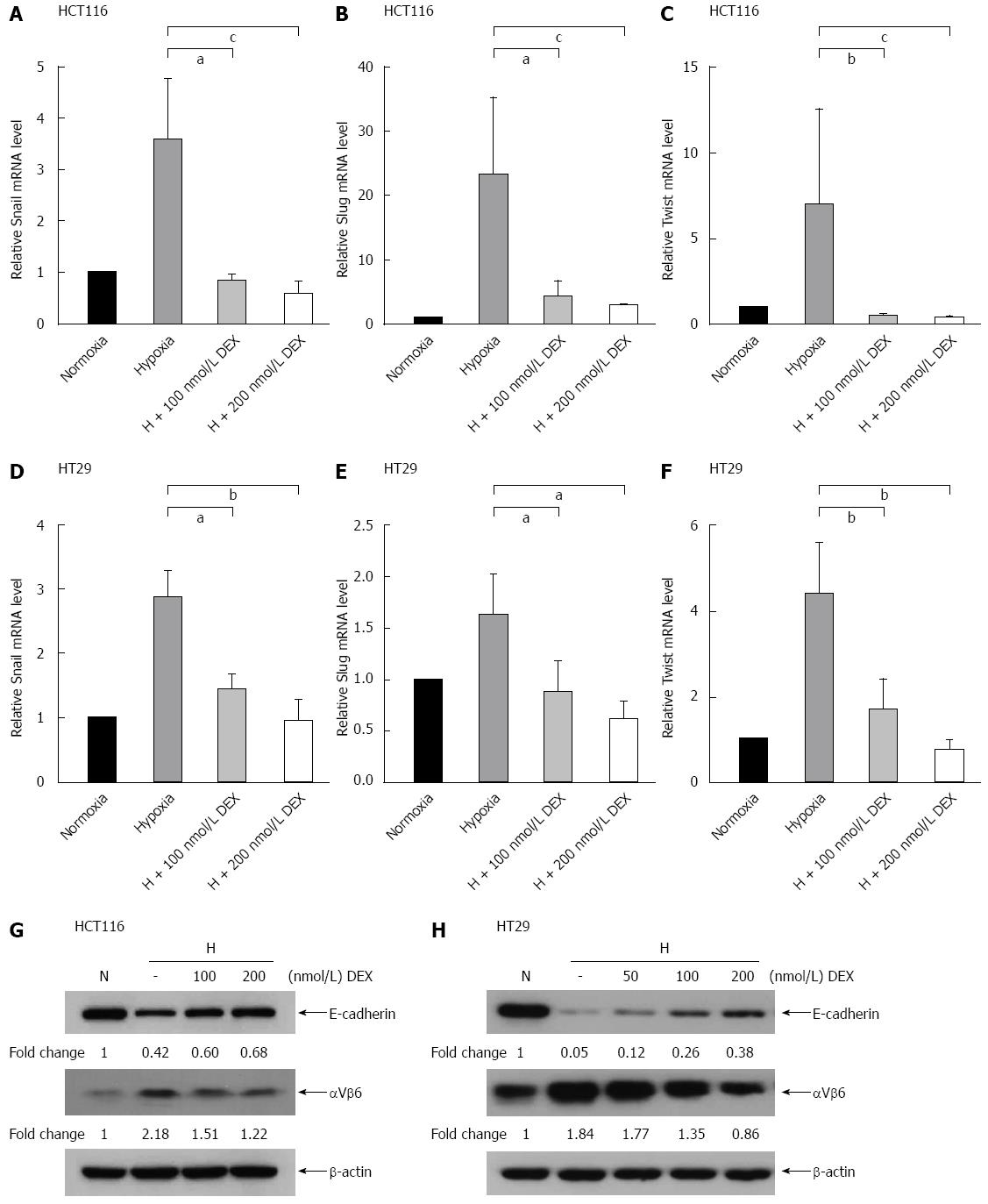
To monitor whether DEX regulates hypoxia-induced EMT in human colon cancer cells, we incubated HCT116 and HT29 cells with or without DEX under normoxic or hypoxic conditions for 24 or 72 h. The mRNA expression of Snail, Slug, and Twist was then examined by real-time quantitative polymerase chain reaction. While hypoxia alone augmented the mRNA levels of Snail, Slug, and Twist in HCT116 and HT29 cells, adding DEX resulted in the decrease of the mRNA levels as follows. HCT116 cells: Snail (73%-81%, P < 0.05 and P < 0.001 vs hypoxia controls), Slug (75%-85%, P < 0.05 and P < 0.001 vs hypoxia controls), and Twist (89%-93%, P < 0.01 and P < 0.001 vs hypoxia controls). HT29 cells: Snail (49%-65%, P < 0.05 and P < 0.01 vs hypoxia controls), Slug (46%-62%, both P < 0.05 vs hypoxia controls), and Twist (62%-81%, both P < 0.01 vs hypoxia controls) (Figure 2A-F).
Next, we explored the effect of DEX and hypoxia on expression of the EMT markers, E-cadherin and integrin αVβ6 at the protein level. As shown in Figure 2G and H, E-cadherin expression was reduced by hypoxia alone and recovered in a dose-dependent manner by the addition of DEX. Reversely, hypoxia-induced integrin αVβ6 was further depressed by DEX treatment under hypoxic conditions. Moreover, DEX-treated HCT116 and HT29 cells cultured under hypoxic conditions showed that E-cadherin was recovered by 26% and 33%, whereas integrin αVβ6 was reduced by 44% and 53%, respectively (Figure 2G and H).
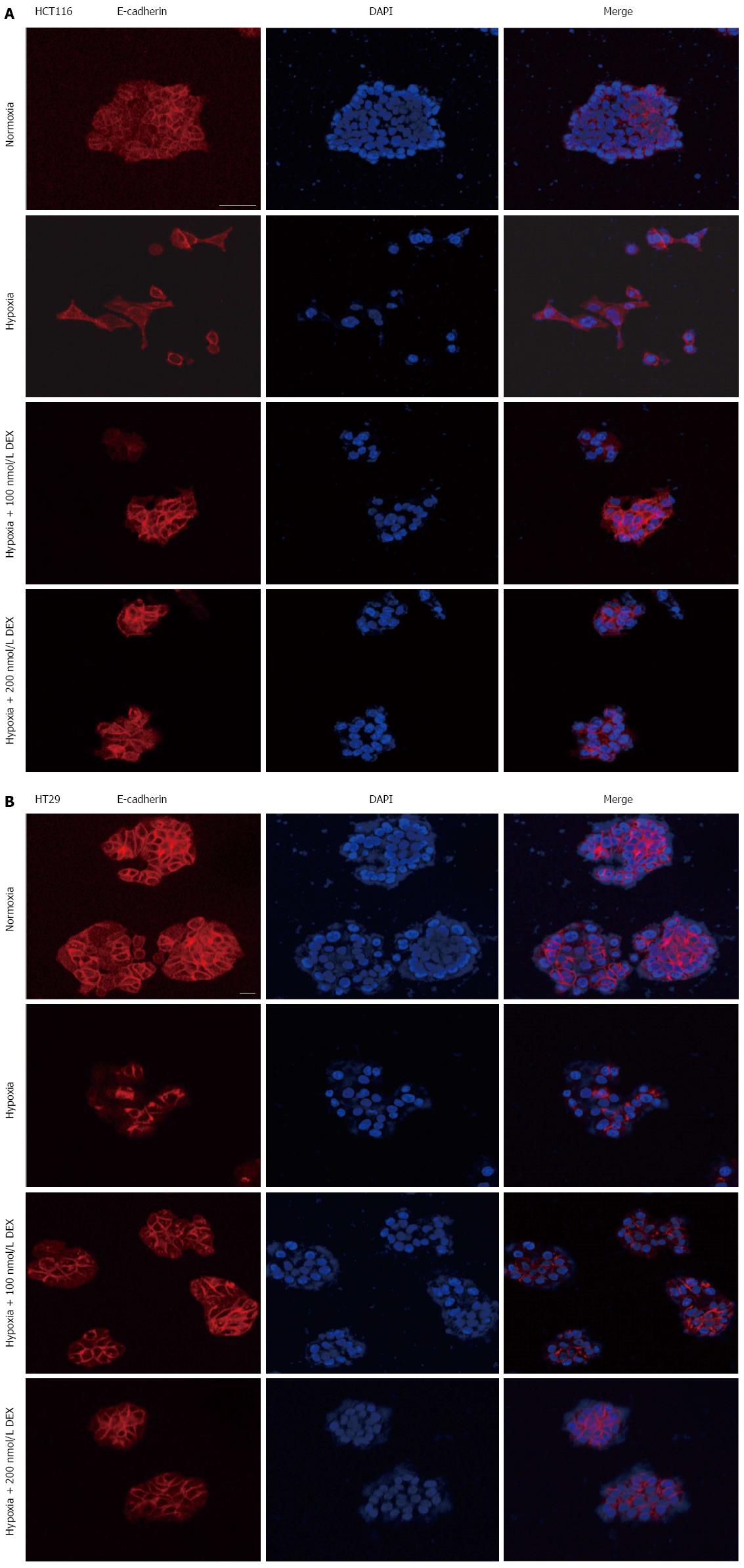
Since hypoxia has been shown to regulate the expression of EMT markers and change cell morphology, we assessed whether DEX could rescue the expression of E-cadherin and alter the morphology of cells cultured in the presence of hypoxia. To perform this experiment, HCT116 and HT29 cells were treated with DEX (100 and 200 nmol/L) and incubated under normoxic or hypoxic conditions for 5 or 7 d. Both HCT116 and HT29 cells were largely clustered together when cultured under normoxic conditions, whereas those exposed to hypoxia were scattered and displayed morphological changes, such as an elongated fibroblastic morphology (Figure 3A and B). Importantly, the presence of DEX partially restored the growth pattern and morphological phenotype reminiscent of cells grown under normoxic conditions. Furthermore, E-cadherin expression was increased by DEX compared to hypoxia control counterparts. These effects were present in both cell lines, HCT116 and HT29.
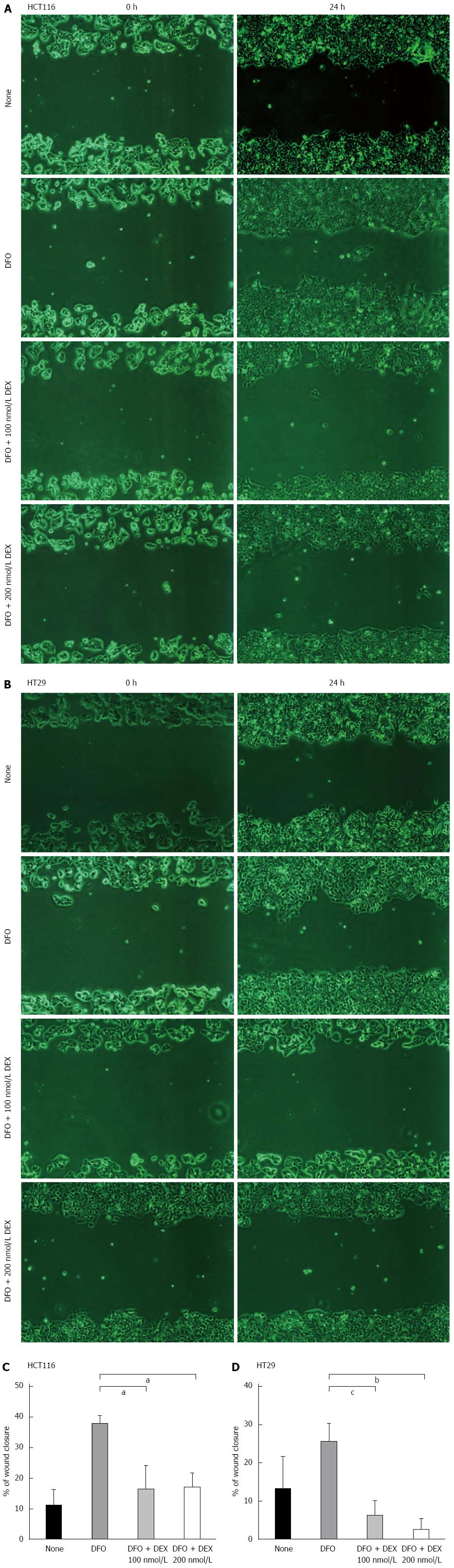
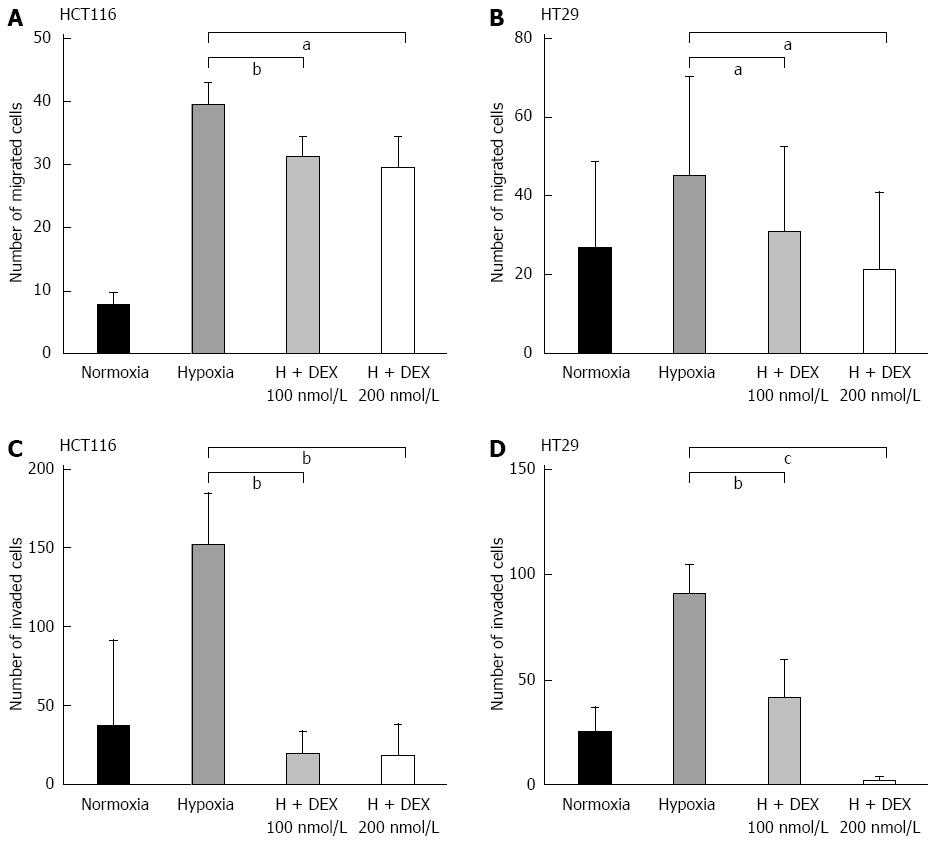
To examine the effect of DEX on epithelial cell migration, we performed wound healing assays in both cell lines. For these analyses, we used DFO to mimic hypoxic conditions. As shown in Figure 4, DFO-treated cells were highly migratory as compared to the untreated groups, whereas those co-treated with DEX and DFO exhibited a lower propensity for migration compared to DFO-only control cells. Additionally, in hypoxic conditions, DEX showed 55%-57% (both P < 0.05 vs DFO-only controls) and 77%-92% (P < 0.01 and P < 0.001 vs DFO-only controls) decrease on recovery of wounds in HCT116 and HT29 cells, respectively. To confirm this inhibitory effect of DEX on the migration of hypoxic colon cancer cells, we performed the migration assay using transwells. Consistently, the hypoxia-induced migration of HCT116 and HT29 cells decreased by 21%-25% (P < 0.05 and P < 0.01 vs hypoxia controls) and 37%-63% (both P < 0.05 vs hypoxia controls) in DEX-treated cells, respectively (Figure 5A and B).
Finally, we employed matrigel invasion assays to investigate the effect of DEX on colorectal cancer cell invasion. As shown in Figure 5C and D, hypoxic DEX-treated cells showed reductions of 87%-90% (both P < 0.01 vs hypoxia controls) and 56%-98% (P < 0.05 and P < 0.001 vs hypoxia controls) on the invasive capacity of HCT116 and HT29 cells compared to the hypoxia-only controls. This inhibitory effect of DEX on invasion was observed in both cell lines consistently.
Hypoxia is a hallmark of various human malignancies and is currently observed as an unfavorable prognostic factor often associated with other high-risk characteristics, such as therapy-resistant metastasis[13]. Thus, tumor hypoxia has been suggested to favor selection of the most aggressive/invasive neoplastic cells, and subsequently facilitate cancer progression[14,15].
In the current study, we used two colorectal cancer cell lines, HCT116 and HT29 to study the effect of DEX on hypoxia-induced EMT. Colon cancer can be classified into two major groups: MSI (microsatellite instability) and MSS (microsatellite stable, chromosome instability)[16]. HCT116 and HT29 were used for this study since they belong to MSI and MSS, respectively. In addition, we hypothesized that DEX blocks the migration and invasion of colorectal cancer cells by repressing EMT under hypoxic conditions. According to our results, DEX inhibited the hypoxia-related expression of HIF-1α and VEGF, as well as the EMT markers (Snail, Slug, Twist, and integrin αVβ6). Furthermore, DEX treatment rescued E-cadherin expression, the morphologic changes, and the migratory properties of colorectal cancer cells in hypoxia.
A number of molecules have been found to be involved in the process of EMT. Among them, Snail and Slug play key roles as transcriptional repressors that promote EMT[17]. More specifically, Snail is expressed in different human carcinoma and melanoma cells, has been detected at the invasive front of epidermoid carcinomas, and is related to breast carcinoma metastasis[18,19]. In addition, the ectopic expression of Snail represses E-cadherin expression to induce a mesenchymal phenotype[5,6,20]. These data suggest that Snail induces tumor invasion and metastasis. Additionally, Twist can function independently of Snail to suppress E-cadherin and is transcriptionally active during the EMT process in metastatic cancer cells[21-23]. Therefore, from our data which DEX inhibited the expression of Snail and Twist under hypoxic conditions, DEX may be involved in the loss of E-cadherin through Snail- and Twist-dependent pathways during hypoxia-induced EMT.
The loss of E-cadherin is a key event during EMT and the loss of cell-cell adhesion thereof. E-cadherin is a prototypical epithelial cell marker and is repressed during EMT events in early embryonic development, tissue fibrosis, and cancer metastasis[24]. Recent studies on colon cancer reported that only invasive cancer cells, which have undergone EMT to obtain a metastatic phenotype, have high expression of integrin αVβ6[25]. For these reasons, we selected E-cadherin and integrin αVβ6 as EMT markers for colon cancer cells and observed that while DEX increased E-cadherin protein expression in hypoxic cells, the reverse was true for integrin αVβ6.
There was no evidence to show a negative impact of DEX in patients with colorectal cancer. The beneficial effect of DEX during chemotherapy is under consideration. Some studies have identified that DEX promotes tumor proliferation and metastasis by blocking apoptosis and anti-tumor immunosurveillance and, thereby causing resistance to treatment agents[12,26-28], whereas others have reported on the benefits for DEX therapy[20,29,30]. These conflicting results of glucocorticoids may be explained by the differential expression of glucocorticoid receptor co-activators and corepressors in diverse cell types[27]. Corticosteroids activate or repress the transcription of many target genes by binding specific intracellular receptors that can vary between cell lineages. Thus, the observed difference in the efficacy of glucocorticoids on cell viability likely results from cell-type specific transcriptional regulation; however, the mechanistic understanding for this effect is unclear.
A recent report suggests that DEX as a preoperative prophylactic induces no difference in overall and disease-free survival among patients with colorectal cancer, and yet the rate of recurrence is higher among patients receiving DEX[31]. This result conflicts with our hypothesis; however, these results are based on observations after a single dose, and we are unable to make decision on the efficacy of DEX due to the small sample size and lack of a comparable study.
A limitation of this study is the lack of a detailed molecular mechanism underlying the regulation of EMT by DEX in colorectal cancer cells. Wagner et al[32] previously reported that DEX impairs HIF-1α function by causing an unusual protein distribution from the nucleus to the cytosol in hepatocellular carcinoma cells; however, our western blot analysis using the whole cell lysate from DEX-treated colorectal cancer cells showed that DEX blocked EMT by inhibiting HIF-1α stability, which can be explained by the cell-type specific activity of DEX. Therefore, further studies are necessary to determine how DEX controls HIF-1α stability and the expression of EMT markers under hypoxia.
Limiting the side effects of chemotherapy is clinically significant, as improved treatment outcome and prognosis can be achieved in patients undergoing chemotherapy in terms of recommended dose and duration of treatment. Given that DEX prevented hypoxia-dependent EMT of colon cancer cells, the use of DEX in colorectal cancer patients might be beneficial in mitigating tumor progression or metastasis. However, this finding should be interpreted with caution given the in vitro nature of this study.
The synthetic glucocorticoid dexamethasone (DEX) is widely used in the treatment of many diseases, particularly in hematologic malignancies where it has shown to have cytotoxic effects. While DEX lacks this activity in solid tumors, patients are still treated with corticosteroids to prevent complications often associated with cancer therapy, including cancer-related pain, lack of appetite, edema, and electrolyte imbalance. Although DEX is commonly prescribed as a co-medication in cancer treatment, the effect of DEX on the metastatic capacity of colorectal cancer is unknown.
The beneficial effect of DEX during chemotherapy is under consideration. The authors hypothesized that DEX blocks the migration and invasion of colorectal cancer cells by repressing epithelial-mesenchymal transition (EMT) under hypoxic conditions. According to this results, DEX inhibited the hypoxia-related expression of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF), as well as the EMT markers, Snail, Slug, Twist, and integrin αVβ6. Furthermore, DEX treatment rescued E-cadherin expression, mesenchymal morphology, and the migratory properties of colorectal cancer cells in hypoxia.
This study investigated the effects of DEX on hypoxia-induced EMT and found that it was sufficient to block the propensity for cells to undergo EMT by repressing the hypoxia-induced expression of HIF-1α and VEGF, as well as the EMT markers. This evidence suggests that DEX co-treatment may limit the migratory properties of colorectal tumors subsisting in the hypoxic regions of colorectal cancers. A limitation of this study is the lack of a detailed molecular mechanism underlying the regulation of EMT by DEX in colorectal cancer cells.
Limiting the side effects of chemotherapy is clinically significant, as improved treatment outcome and prognosis can be achieved in patients undergoing chemotherapy in terms of recommended dose and duration of treatment. Given that DEX prevented hypoxia-dependent EMT of colon cancer cells, its use in colorectal cancer patients might be beneficial in mitigating tumor progression or metastasis. However, this finding should be interpreted with caution given the in vitro nature of this study.
The authors address a clinically very interesting issue, considering the fact that most patients undergoing chemotherapeutic treatment for metastatic colorectal cancer also receive anti-emetic treatment with synthetic corticosteroids. These authors state that dexamethasone was sufficient to block the propensity for cells to undergo EMT by repressing the hypoxia-induced expression of HIF-1α. This study is easily read, addresses an interesting question, and adds to the existing evidence in the field.
P- Reviewer: Engelmann BE, Wang ZX S- Editor: Wang JL L- Editor: A E- Editor: Ma S
| 1. | Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute 2014; Available from: http://seer.cancer.gov/csr/1975_2011/. |
| 2. | Tang CM, Yu J. Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol. 2013;28:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 920] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 4. | Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826:272-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14:3792-3797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 8077] [Article Influence: 475.1] [Reference Citation Analysis (0)] |
| 10. | Bindreither D, Ecker S, Gschirr B, Kofler A, Kofler R, Rainer J. The synthetic glucocorticoids prednisolone and dexamethasone regulate the same genes in acute lymphoblastic leukemia cells. BMC Genomics. 2014;15:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Rutz HP. Effects of corticosteroid use on treatment of solid tumours. Lancet. 2002;360:1969-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1714] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 14. | Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 783] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 16. | Giacomini CP, Leung SY, Chen X, Yuen ST, Kim YH, Bair E, Pollack JR. A gene expression signature of genetic instability in colon cancer. Cancer Res. 2005;65:9200-9205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1338] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 18. | Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2519] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 19. | Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241-3246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Zhang L, Lei W, Wang X, Tang Y, Song J. Glucocorticoid induces mesenchymal-to-epithelial transition and inhibits TGF-β1-induced epithelial-to-mesenchymal transition and cell migration. FEBS Lett. 2010;584:4646-4654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2783] [Cited by in RCA: 3023] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 22. | Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, Neilson EG. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007;117:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Yu W, Kamara H, Svoboda KK. The role of twist during palate development. Dev Dyn. 2008;237:2716-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7888] [Article Influence: 464.0] [Reference Citation Analysis (1)] |
| 25. | Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Gündisch S, Boeckeler E, Behrends U, Amtmann E, Ehrhardt H, Jeremias I. Glucocorticoids augment survival and proliferation of tumor cells. Anticancer Res. 2012;32:4251-4261. [PubMed] |
| 27. | Zhang C, Kolb A, Mattern J, Gassler N, Wenger T, Herzer K, Debatin KM, Büchler M, Friess H, Rittgen W. Dexamethasone desensitizes hepatocellular and colorectal tumours toward cytotoxic therapy. Cancer Lett. 2006;242:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Zheng Y, Izumi K, Li Y, Ishiguro H, Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Mol Cancer Ther. 2012;11:2621-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Keith BD. Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy. BMC Cancer. 2008;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Münstedt K, Borces D, Bohlmann MK, Zygmunt M, von Georgi R. Glucocorticoid administration in antiemetic therapy: is it safe? Cancer. 2004;101:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Singh PP, Lemanu DP, Taylor MH, Hill AG. Association between preoperative glucocorticoids and long-term survival and cancer recurrence after colectomy: follow-up analysis of a previous randomized controlled trial. Br J Anaesth. 2014;113 Suppl 1:i68-i73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Wagner AE, Huck G, Stiehl DP, Jelkmann W, Hellwig-Bürgel T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem Biophys Res Commun. 2008;372:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |