Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9808
Peer-review started: February 22, 2015
First decision: March 26, 2015
Revised: April 14, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: September 7, 2015
Processing time: 204 Days and 21.7 Hours
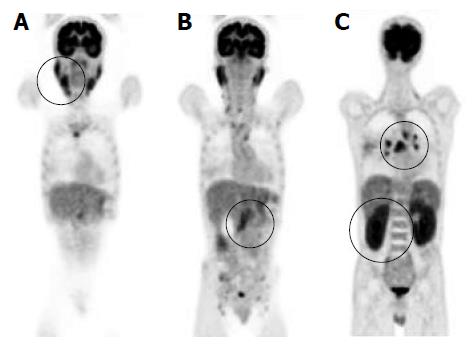
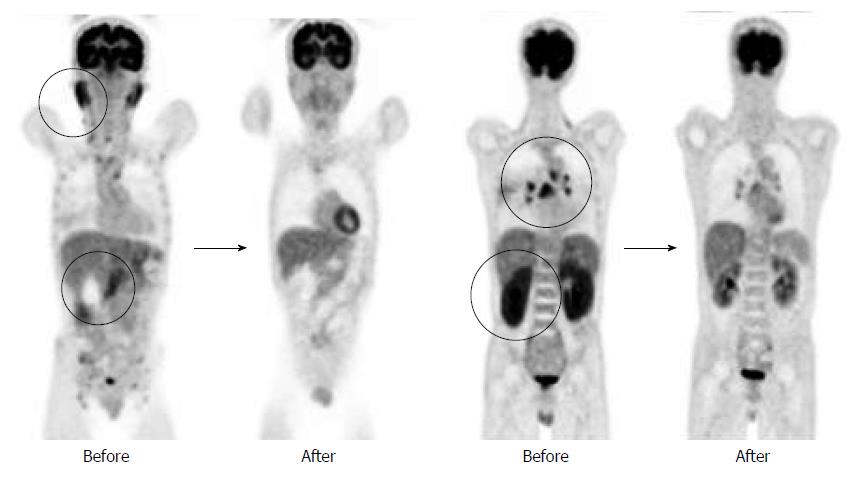
A 50-year-old male was referred to our hospital for the evaluation of hyperproteinemia. Fluorodeoxyglucose positron emission tomography revealed high fluorodeoxyglucose uptake in the pancreas, bilateral lacrimal glands, submandibular glands, parotid glands, bilateral pulmonary hilar lymph nodes, and kidneys. Laboratory data showed an elevation of hepatobiliary enzymes, renal dysfunction, and remarkably high immunoglobulin (Ig) G levels, without elevated serum IgG4. Abdominal computed tomography revealed swelling of the pancreatic head and bilateral kidneys. Endoscopic retrograde cholangiopancreatography showed an irregular narrowing of the main pancreatic duct in the pancreatic head and stricture of the lower common bile duct. Histological examination by endoscopic ultrasonography-guided fine-needle aspiration revealed findings of lymphoplasmacytic sclerosing pancreatitis without IgG4-positive plasma cells. Abnormal laboratory values and the swelling of several organs were improved by the treatment with steroids. The patient was diagnosed as having type 1 autoimmune pancreatitis (AIP) based on the International Consensus Diagnostic Criteria. Therefore, we encountered a case of compatible type 1 AIP without elevated levels of serum IgG4 or IgG4-positive plasma cells. This case suggests that AIP phenotypes are not always associated with IgG4.
Core tip: Type 1 autoimmune pancreatitis (AIP) is regarded as a pancreatic lesion of IgG4-related disease (IgG4-RD). However, the role of IgG4 in AIP or IgG4-RD phenotypes has not been established. This patient was diagnosed with compatible type 1 AIP according to the International Consensus Diagnostic Criteria without an elevation of serum IgG4 levels or IgG4-positive plasma cells. This case suggests that type 1 AIP phenotypes do not require IgG4 elevation.
- Citation: Nakano E, Kanno A, Masamune A, Yoshida N, Hongo S, Miura S, Takikawa T, Hamada S, Kume K, Kikuta K, Hirota M, Nakayama K, Fujishima F, Shimosegawa T. IgG4-unrelated type 1 autoimmune pancreatitis. World J Gastroenterol 2015; 21(33): 9808-9816
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9808.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9808
In 1995, Yoshida et al[1] proposed autoimmune pancreatitis (AIP) as a diagnostic entity and summarized the clinical features as follows: increased serum γ-globulin or immunoglobulin (Ig) G levels and the presence of autoantibodies; diffuse irregular narrowing of the main pancreatic duct (MPD) and enlargement of the pancreas; the occasional concurrent stenosis of the lower bile duct and other autoimmune diseases; mild symptoms, usually without attacks of acute pancreatitis; the effectiveness of steroid therapy; and histological findings of lymphoplasmacytic sclerosing pancreatitis (LPSP)[2]. With the accumulation of similar cases in Japan, the Japan Pancreas Society (JPS) proposed the world’s first clinical diagnostic criteria for AIP in 2002[3]. Thereafter, diagnostic criteria for AIP have been proposed in several countries after the report of an extensive number of cases worldwide. Therefore, the International Consensus Diagnostic Criteria (ICDC) were established to form an international standard.
In 2001, Hamano et al[4] reported that elevated serum IgG4 levels were highly specific and sensitive for the diagnosis of AIP. IgG4 has a pivotal role in the diagnosis and pathogenesis of AIP. Recently, the concept of IgG4-related disease (IgG4-RD) was proposed[5], and the diagnostic criteria for IgG4-RD were established in 2011[6]. IgG4-RD is a specific disease that presents with high serum levels of IgG4 and the enlargement of various organs including lacrimal glands, salivary glands, thyroid glands, lungs, pancreas, kidneys, and retroperitoneum. The histopathology of IgG4-RD includes remarkable IgG4-positive cell infiltration into plasma, fibrosis, and obliterative phlebitis. Type 1 AIP is considered to be a pancreatic lesion of IgG4-RD. However, the role of IgG4 in the phenotypic expression of AIP or IgG4-RD has not been clarified.
Here, we report a case of AIP compatible with type 1 AIP but without elevated levels of serum IgG4 and IgG4-positive plasma cells. This case suggests that the phenotype of type 1 AIP does not require an elevation of IgG4.
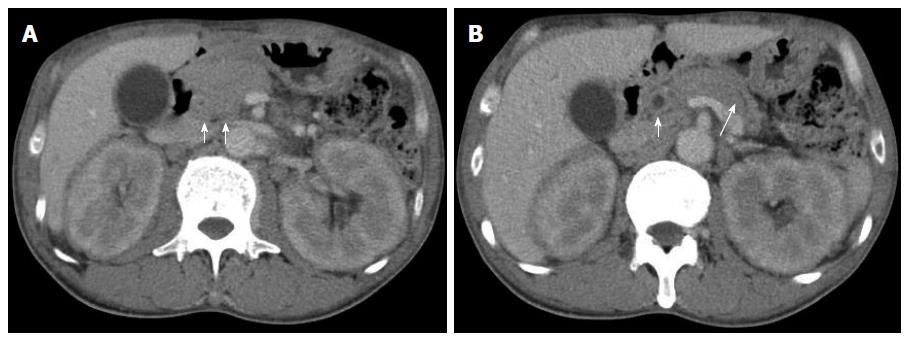
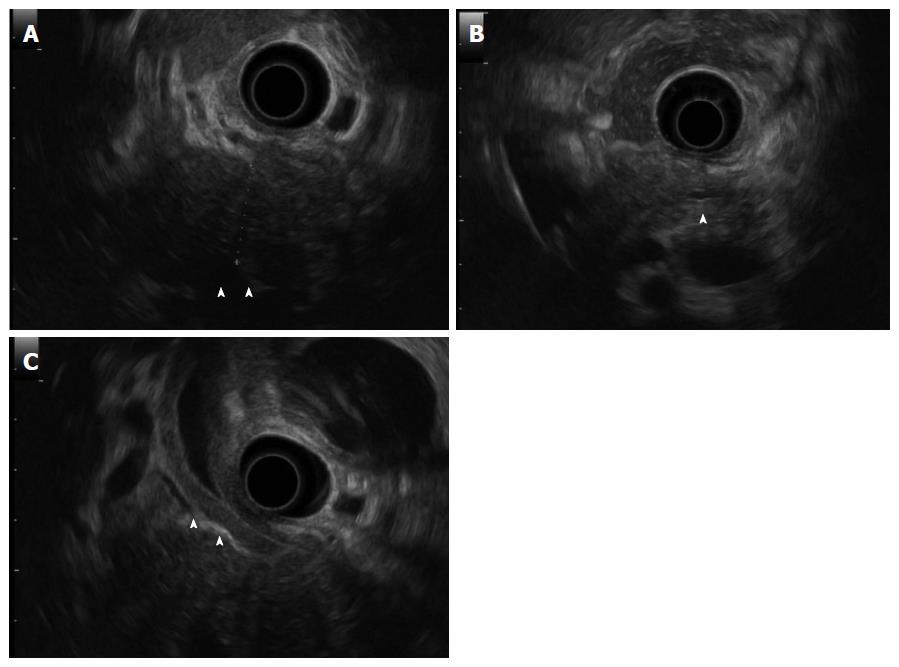
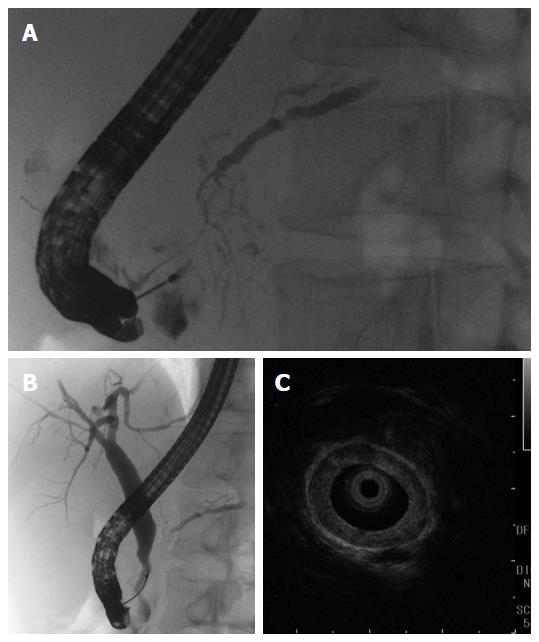
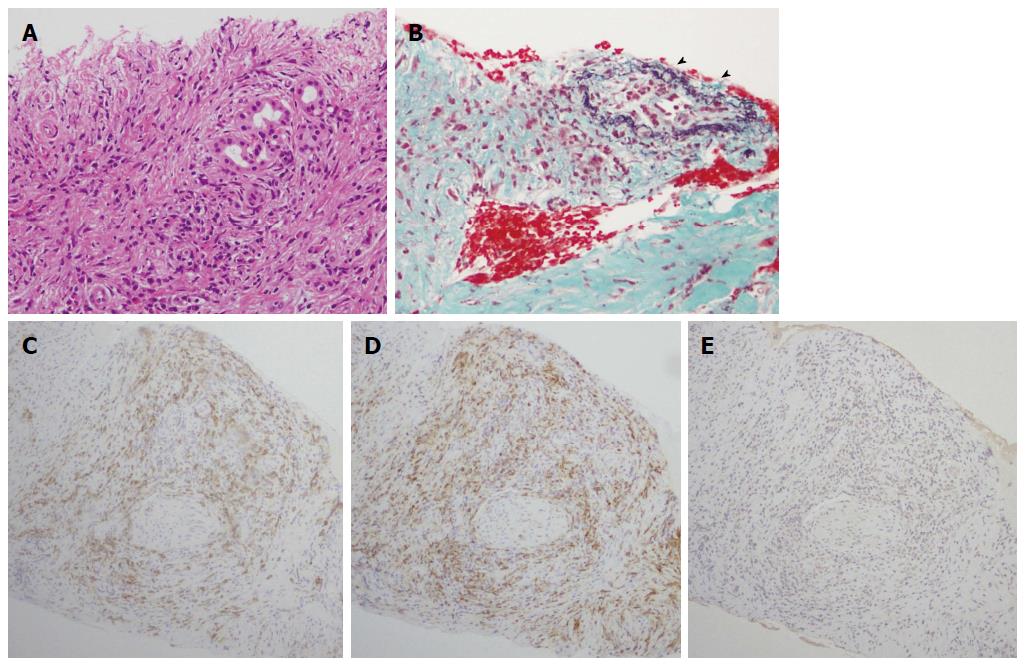
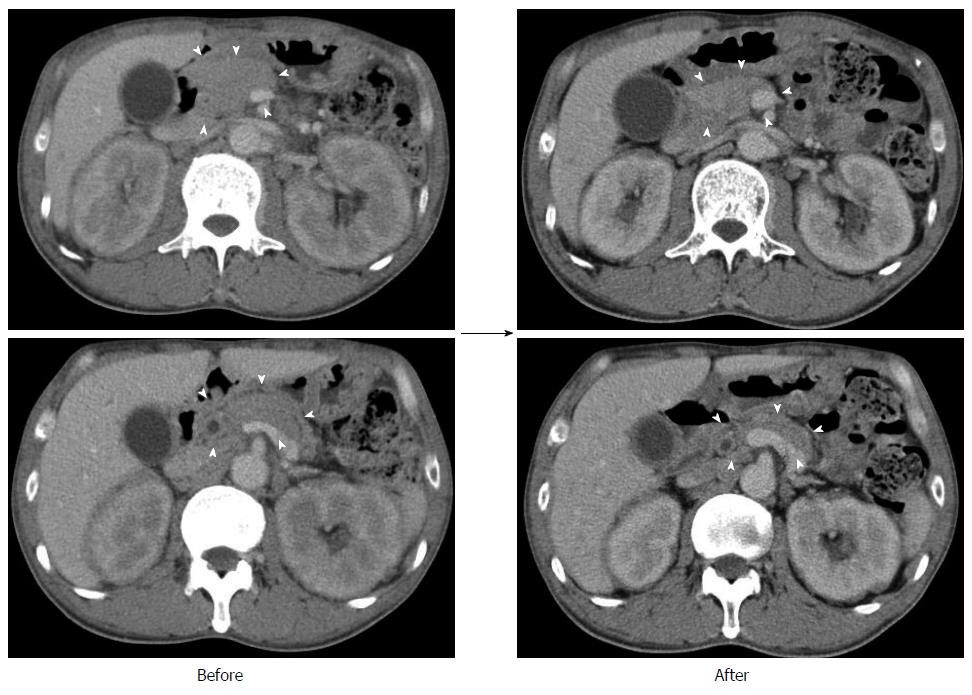
A 50-year-old male with a history of hypertension was referred to our hospital for the evaluation of hyperproteinemia [total protein (TP), 10.0 g/da; normal range, 6.7-8.1]. He was diagnosed with hypergammaglobulinemia, and a bone marrow biopsy and fluorodeoxyglucose (FDG)-positron emission tomography (PET) were performed to rule out multiple myeloma. Multiple myeloma was excluded based on the bone marrow results; however, FDG-PET revealed high FDG uptake in several enlarged organs (Figure 1A-C). The patient was admitted to our department for further examination of the pancreas, which was swollen and exhibited high FDG uptake. A physical examination showed elastic, painless, and persistent swelling of the salivary and lacrimal glands. Laboratory data revealed elevated levels of alkaline phosphatase (ALP 386 IU/L; normal range, 115-359) and γ-glutamyl transpeptidase (γ-GTP 101 IU/L; normal range, 10-47), renal dysfunction [creatinine (Cr), 1.36 mg/dL; normal range, 0.44-1.15], and elevated levels of pancreatic enzymes (Amylase 245 IU/L; normal range, 37-125; Lipase 339 IU/L; normal range, 6-48). The serum IgG levels were extremely high (6662 mg/dL; normal range, 870-1700); however, the serum IgG4 levels were normal (78.2 mg/dL; normal range, 4.8-105) (Table 1). Abdominal computed tomography (CT) revealed localized swelling of the head of the pancreas (Figure 2A), mild dilation of the upstream main pancreatic duct (MPD), a thickened common bile duct (CBD) wall, and swelling of the bilateral kidneys (Figure 2B). Endoscopic ultrasonography (EUS) showed hypoechoic swelling of the head of the pancreas and diffuse thickening of the CBD wall (Figure 3). Endoscopic retrograde cholangiopancreatography findings demonstrated irregular narrowing of the MPD in the head of the pancreas, dilation of the upstream MPD (Figure 4A), and a lower bile duct stricture (Figure 4B). Intraductal ultrasonography detected diffuse thickening of the bile duct wall (Figure 4C). We performed endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) using a 19-gauge needle (ExpectTM; Boston-Scientific). Histological examination of the aspirate revealed marked lymphoplasmacytic infiltration (Figure 5A), storiform fibrosis, and obliterative phlebitis (Figure 5B). Immunohistochemistry showed an infiltration of CD38-positive plasma cells (Figure 5C and D); however, the infiltrating plasma cells were negative for IgG4 (Figure 5E). Furthermore, percutaneous biopsies of the swollen salivary glands and kidneys were performed to evaluate Mikulicz disease and intestinal nephritis. The results revealed remarkable lymphoplasmacytic infiltration and fibrosis. The histological diagnoses were sialadenitis and interstitial nephritis; however, the absence of IgG4-positive plasma cells was similar to the histological findings in the pancreas.
| Test | Result | Reference range | Test | Result | Reference range | Test | Result | Reference range |
| WBC (103/μL) | 6.0 | 4.0-9.0 | TP (g/dL) | 10.0 | 6.7-8.1 | TG (mg/dL) | 42 | 30-150 |
| RBC (106/μL) | 3.24 | 4.27-5.70 | Albumin (%) | 26.2 | 54.6-66.1 | TC (mg/dL) | 85 | 130-220 |
| Hb (g/dL) | 9.6 | 14.0-18.0 | Alpha 1 (%) | 3.0 | 2.7-4.3 | GLU (mg/dL) | 110 | 68-109 |
| Ht (%) | 28.4 | 40.0-52.0 | Alpha 2 (%) | 6.5 | 6.2-10.5 | HbA1c (%) | 6.4 | 4.6-6.2 |
| Plt (103/μL) | 215 | 150.0-350.0 | Beta 1 (%) | 3.7 | 5.0-7.5 | CRP (mg/dL) | 0.5 | 0.0-0.3 |
| Beta 2 (%) | n.c. | 3.5-6.6 | ||||||
| T-Bil (IU/L) | 0.4 | 0.2-1.0 | Gamma (%) | 60.6 | 12.3-22.8 | IgG (mg/dL) | 6564 | 870-1700 |
| ALP (IU/L) | 386 | 115-359 | Alb (g/dL) | 2.4 | 3.8-5.3 | IgG1 (mg/dL) | 4863 | 320-748 |
| γ-GTP (IU/L) | 101 | 10-47 | BUN (mg/dL) | 18.0 | 8-20 | IgG2 (mg/dL) | 919 | 208-754 |
| AST (IU/L) | 28 | 8-38 | Cr (mg/dL) | 1.36 | 0.44-1.15 | IgG3 (mg/dL) | 703 | 6.6-88.3 |
| ALT (IU/L) | 18 | 4-43 | Na (mEq/L) | 136.0 | 136-141 | IgG4 (mg/dL) | 78.2 | 4.8-105 |
| LDH (IU/L) | 187 | 119-229 | K (mEq/L) | 3.8 | 3.5-5.1 | ANA (mg/dL) | (-) | |
| Lipase (IU/L) | 339 | 6-48 | Cl (mEq/L) | 105.0 | 98-107 | CEA (ng/dL) | 1.1 | 0.0-5.0 |
| Amylase (IU/L) | 245 | 37-125 | Ca (mg/dL) | 8.0 | 8.6-10.1 | CA19-9 (U/mL) | 11.3 | 0.0-37.0 |
Prednisolone was orally administered at a dose of 30 mg/d to treat AIP and intestinal nephritis. After 3 wk of 30 mg/d dosing, the patient was weaned on 25 mg/d for 2 wk followed by 20 mg/d for 7 wk. Renal dysfunction and elevated hepatobiliary enzymes, pancreatic enzymes, and IgG improved with steroid treatment (Table 2). In addition, imaging modalities revealed that the enlargement of several organs, such as the pancreas, kidneys, salivary glands, and submandibular glands, was improved (Figure 6). FDG uptake in various organs was also reduced on FDG-PET (Figure 7).
| Test | Result | Referencerange | Test | Result | Referencerange | Test | Result | Reference range |
| WBC (103/μL) | 9.3 | 4.0-9.0 | TP (g/dL) | 7.1 | 6.7-8.1 | TG (mg/dL) | 78 | 30-150 |
| RBC (106/μL) | 4.31 | 4.27-5.70 | Albumin (%) | 57.6 | 54.6-66.1 | TC (mg/dL) | 197 | 130-220 |
| Hb (g/dL) | 12.6 | 14.0-18.0 | Alpha 1 (%) | 4.7 | 2.7-4.3 | GLU (mg/dL) | 96 | 68-109 |
| Ht (%) | 38.4 | 40.0-52.0 | Alpha 2 (%) | 10.2 | 6.2-10.5 | HbA1c (%) | 5.8 | 4.6-6.2 |
| Plt (103/μL) | 203 | 150.0-350.0 | Beta 1 (%) | 5.7 | 5.0-7.5 | CRP (mg/dL) | 0.1 | 0.0-0.3 |
| Beta 2 (%) | 3.4 | 3.5-6.6 | ||||||
| T-Bil (IU/L) | 0.5 | 0.2-1.0 | Gamma (%) | 18.4 | 12.3-22.8 | IgG (mg/dL) | 1487 | 870-1700 |
| ALP (IU/L) | 292 | 115-359 | Alb (g/dL) | 3.9 | 3.8-5.3 | IgG4 (mg/dL) | 3.9 | 4.8-105 |
| γ-GTP (IU/L) | 30 | 10-47 | BUN (mg/dL) | 25.0 | 8-20 | CEA (ng/dL) | 1.3 | 0.0-5.0 |
| AST (IU/L) | 16 | 8-38 | Cr (mg/dL) | 0.98 | 0.44-1.15 | CA19-9 (U/mL) | 12.7 | 0.0-37.0 |
| ALT (IU/L) | 20 | 4-43 | Na (mEq/L) | 140 | 136-141 | |||
| LDH (IU/L) | 203 | 119-229 | K (mEq/L) | 4.4 | 3.5-5.1 | |||
| Lipase (IU/L) | 22 | 6-48 | Cl (mEq/L) | 104 | 98-107 | |||
| Amylase (IU/L) | 44 | 37-125 | Ca (mg/dL) | 9.3 | 8.6-10.1 |
The patient was diagnosed with AIP according to the ICDC, including pancreatic parenchymal imaging, irregular narrowing of MPD, other organ involvement (OOI), pancreas histology, and response to steroids; however, the serum IgG4 levels were not elevated. The patient was treated continuously with maintenance doses of prednisolone and was relapse-free after approximately 1 year.
The concept of AIP was proposed by Yoshida et al[1] in 1995. Recent studies suggested that there are two distinct subtypes of AIP: type 1 and type 2[7-9]. The histological descriptions of type 1 and 2 AIP are LPSP[10] and idiopathic duct-centric pancreatitis (IDCP) or granulocytic epithelial lesion (GEL)[11], respectively. The characteristic histological features of type 1 AIP are as follows: (1) marked lymphoplasmacytic infiltration with fibrosis and without granulocytic infiltration; (2) storiform fibrosis; (3) obliterative phlebitis; and (4) abundant [> 10 cells/high-power field (HPF)] IgG4-positive plasma cells. Type 1 AIP also has several specific clinical manifestations, such as increased serum levels of IgG4 and OOI and responsiveness to steroids[7]. Recently, IgG4-RD was recognized as a novel clinical entity with multiorgan involvement that is associated with the abundant infiltration of IgG4-positive plasma cells[5,6]. IgG4 may play a central role in the clinical features of IgG4-RD; however, the detailed pathophysiological mechanisms behind these effects are unclear.
This case was diagnosed with definitive type 1 AIP according to the ICDC and pancreas histology (LPSP; level 1). In this patient, parenchymal imaging, ductal imaging, and OOI were all level 2. Based on his response to steroid treatment, the only probable diagnosis was type 1 AIP. However, lymphoplasmacytic sclerosing pancreatitis without IgG4-positive plasma cells was detected in the pancreas fine needle aspirate specimens. ICDC recommends obtaining a core biopsy specimen or resection specimen for histological evaluation. However, we have previously reported that histological diagnosis of AIP is possible from an EUS-FNA specimen obtained using a 22G needle[12]. In the present case, we showed that EUS-FNA using a 19G needle provides an adequate tissue sample for histological diagnosis. Serum IgG4-negative AIP has been reported in several studies[13-16] and pediatric cases have also been reported[17]. These reports described that IgG4-negative AIP presents with weak symptoms such as abdominal pain and jaundice[13] as well as the appearance of OOI compared to IgG4-positive AIP[13,15,16]. The present case exhibited significant OOI in several organs, including the pancreas; however, there was no elevation of the serum IgG4 levels or IgG4-positive plasma cell infiltration in the pancreas, salivary gland, or kidneys. In addition, the histological findings in this case revealed type I AIP without abundant IgG4-positive plasma cells. Since this case was not type 2 AIP and there was no IgG4-related type 1 AIP activity, the pathophysiological mechanism of this case may be different from the IgG4-negative AIP cases reported previously.
The mechanism by which IgG4 is produced is unclear. Several studies examining the mechanism of IgG4 production have been reported, including a T cell-dependent pathway and a T cell-independent pathway. For the T cell-dependent pathway, recent studies have described the increased production of regulatory T cell (Treg) and IL-10 in IgG4-RD tissues[18-20]. In addition, IL-10 was secreted by inducible costimulatory molecule (ICOS)-positive Tregs. The increased number of ICOS-positive Tregs correlated with the production of IL-10, which may influence the switching of B cells into IgG4-producing plasmacytes and the production of serum IgG4[21]. In contrast, the T cell-independent pathway involves the activation of toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) in monocytes by microbial antigens, which induces the production of IgG4 via the activation of B cell-activation factor (BAFF)-mediated signaling pathways[22,23].
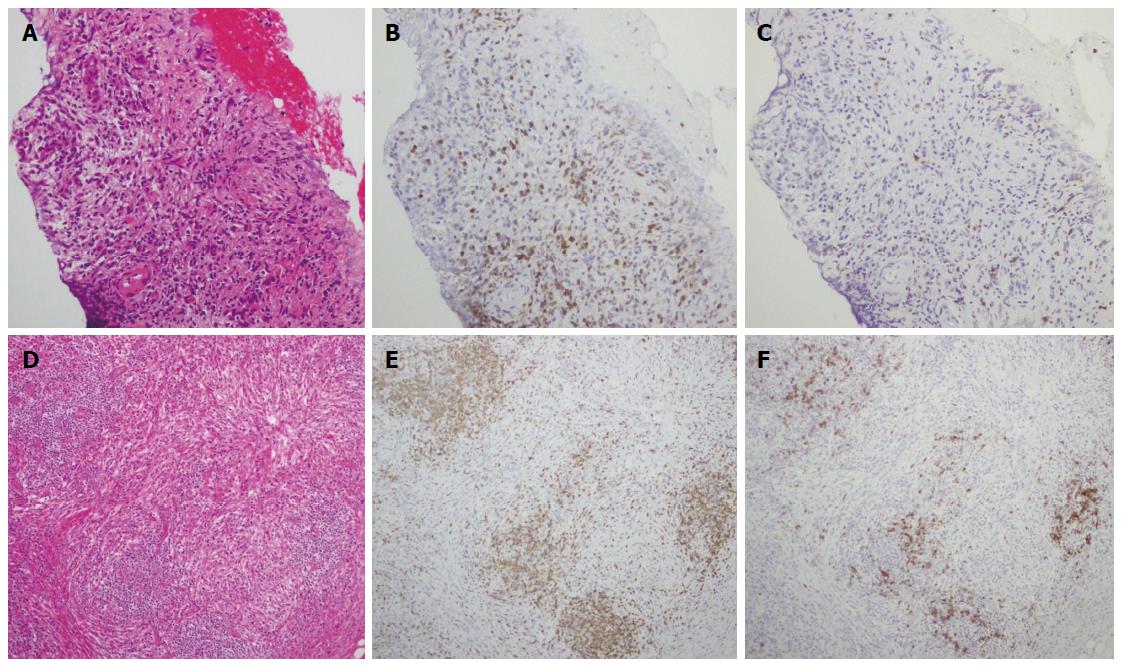
There are several possible reasons for the absence of high serum IgG4 levels and IgG4-positive plasma cell infiltration in the present case. The first possible reason is an impaired class switch from IgG to IgG4. Although the elevated serum IgG concentrations in this case indicated intact IgG production, the low proportion of IgG4 suggested an impaired class switch to IgG4. In addition, immunostaining revealed a decrease in infiltrating CD20-positive B cells compared with that in CD3-positive T cells. Greater CD20-positive B cell infiltration than CD3-positive T cell infiltration is commonly observed in IgG4-negative AIP patients[13].
We evaluated pancreas aspirates with anti-CD3 and anti-CD20 antibodies to establish whether T cells or B cells were more active. In the present case, examination of the pancreas and salivary gland tissues revealed the presence of greater CD3-positive T cell infiltration than CD20-positive B cell infiltration (Figure 8). This finding was different from that in a previous report[13]. Our finding suggests that the IgG4-negative presentation is the result of a suppressed T cell-independent pathway, or of BAFF-mediated, reduced IgG4 production. The accumulation of further cases similar to the present one is required to clarify the pathogenesis of AIP.
In conclusion, we treated a patient with AIP whose symptoms were compatible with type 1 AIP but who did not show elevated serum IgG4 or IgG4-positive plasma cells in any involved organs. This finding suggests that the pathogenesis of type 1 AIP is not always associated with a mechanism involving the overproduction of IgG4.
A 50-year-old male was referred to our hospital for the evaluation of hyperproteinemia.
The physical examination showed elastic, painless, and persistent swelling of the salivary and lacrimal glands.
Autoimmune pancreatitis, IgG4-related disease, sialadenitis, interstitial nephritis.
An elevation of hepatobiliary enzymes (386 IU/L alkaline phosphatase, 101 IU/L γ-glutamyl transpeptidase) and pancreatic enzymes (245 IU/L serum amylase, 339 IU/L serum lipase), renal dysfunction (1.36 mg/dL creatinine), and the serum levels of IgG were extremely high (6,662 mg/dL); however, serum IgG4 levels were normal (78.2 mg/dL).
Fluorodeoxyglucose (FDG)-positron emission tomography revealed high FDG uptake in the pancreas, both lacrimal glands, submandibular glands, parotid glands, bilateral pulmonary hilar lymph nodes, kidneys, and an abdominal computed tomography depicted localized swelling of the pancreatic head, mild dilation of the upstream main pancreatic duct, a thickened common bile duct wall, swelling of the bilateral kidneys.
The histological findings by endoscopic ultrasonography-guided fine-needle aspiration revealed lymphoplasmacytic sclerosing pancreatitis without IgG4-positive plasma cells, and percutaneous biopsies of the swollen salivary glands and kidneys showed remarkable lymphoplasmacytic infiltration and fibrosis.
Prednisolone was orally administered at a dose of 30 mg/d to treat autoimmune pancreatitis (AIP) and intestinal nephritis.
Serum IgG4-negative AIP has been reported in several studies. These reports described that IgG4-negative AIP presents with weak symptoms such as abdominal pain and jaundice as well as the appearance of other organ involvement (OOI) compared to IgG4-positive AIP. This patient exhibited significant OOI in several organs; however, there was no elevation of the serum IgG4 levels or IgG4-positive plasma cell infiltration. This case might differ from the IgG4-negative AIP cases reported previously.
This case exhibited significant OOI in several organs including the pancreas; however, there was no elevation of the serum IgG4 levels or IgG4-positive plasma cell infiltration in the pancreas, salivary gland, or kidneys; this case suggests that the phenotype of type 1 AIP does not require IgG4 elevation.
The authors have provided an extensive review on the various known phenotypes of AIP and their case report offers insight into a less common phenotype. This article suggests that the pathogenesis of type 1 AIP is not always associated with a mechanism involving the overproduction of IgG4.
| 1. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [PubMed] |
| 2. | Kamisawa T, Notohara K, Shimosegawa T. Two clinicopathologic subtypes of autoimmune pancreatitis: LPSP and IDCP. Gastroenterology. 2010;139:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Members of the Criteria Committee for Autoimmune Pancreatitis of the Japan Pancreas Society. Diagnostic criteria for autoimmune pancreatitis by the Japan Pancreas Society. J Jpn Pancreas (Suizou). 2002;17:585-587. |
| 4. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [PubMed] |
| 5. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 642] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 7. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1085] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 8. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387-395. [PubMed] |
| 11. | Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119-1127. [PubMed] |
| 12. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [PubMed] |
| 13. | Kamisawa T, Takuma K, Tabata T, Inaba Y, Egawa N, Tsuruta K, Hishima T, Sasaki T, Itoi T. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol. 2011;46:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Zhang MM, Zou DW, Wang Y, Zheng JM, Yang H, Jin ZD, Li ZS. Contrast enhanced ultrasonography in the diagnosis of IgG4-negative autoimmune pancreatitis: A case report. J Interv Gastroenterol. 2011;1:182-184. [PubMed] |
| 15. | Paik WH, Ryu JK, Park JM, Song BJ, Park JK, Kim YT, Lee K. Clinical and pathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J Gastroenterol. 2013;19:4031-4038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646-1653. [PubMed] |
| 17. | Friedlander J, Quiros JA, Morgan T, Zhang Z, Tian W, Kehr E, Shackleton DV, Zigman A, Stenzel P. Diagnosis of autoimmune pancreatitis vs neoplasms in children with pancreatic mass and biliary obstruction. Clin Gastroenterol Hepatol. 2012;10:1051-1055.e1. [PubMed] |
| 18. | Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538-1546. [PubMed] |
| 19. | Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, Shinozaki S, Kubo Y, Nakamura S. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Akitake R, Watanabe T, Zaima C, Uza N, Ida H, Tada S, Nishida N, Chiba T. Possible involvement of T helper type 2 responses to Toll-like receptor ligands in IgG4-related sclerosing disease. Gut. 2010;59:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Kusuda T, Uchida K, Miyoshi H, Koyabu M, Satoi S, Takaoka M, Shikata N, Uemura Y, Okazaki K. Involvement of inducible costimulator- and interleukin 10-positive regulatory T cells in the development of IgG4-related autoimmune pancreatitis. Pancreas. 2011;40:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Watanabe T, Yamashita K, Fujikawa S, Sakurai T, Kudo M, Shiokawa M, Kodama Y, Uchida K, Okazaki K, Chiba T. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012;64:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Watanabe T, Yamashita K, Sakurai T, Kudo M, Shiokawa M, Uza N, Kodama Y, Uchida K, Okazaki K, Chiba T. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. 2013;48:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abu-El-Haija M, Kamisawa T, Moon JH S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH