Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9774
Peer-review started: January 10, 2015
First decision: March 10, 2015
Revised: April 25, 2015
Accepted: June 16, 2015
Article in press: June 16, 2015
Published online: September 7, 2015
Processing time: 239 Days and 18.2 Hours
AIM: To determine the possible predisposing factors of bezoar-induced small bowel obstruction (BI-SBO) and to discuss the diagnostic value of multi-slice spiral computed tomography, particularly contrast-enhanced scanning, in this condition.
METHODS: A total of 35 BI-SBO cases treated at our hospital from January 2007 to December 2013 were retrospectively analysed. Complete clinical and computed tomography (CT) data of the patients were available and confirmed by surgery. SBO was clinically diagnosed on the basis of clinical manifestations. Of the 35 patients, 18 underwent abdominal and pelvic CT planar scanning with GE 64-slice spiral CT and 17 underwent abdominal and pelvic CT planar scanning with GE 64-slice spiral CT combined with contrast-enhanced examination. Original images were processed using a GE ADW4.3 workstation to obtain MPR, CPR, MIP and CTA images. The images of all patients were evaluated by two abdominal imaging experts. The main analytical contents of planar scanning included intestinal bezoar conditions, changes in the intestinal wall and changes in peri-intestinal conditions. Vascular hyperaemia and arterial blood supply conditions at a specific obstruction site and the distal end of the obstruction site were evaluated through contrast-enhanced examination.
RESULTS: The proportion of males to females among the 35 cases was 1:1.69 (13:22); median age was 63.3 years. The following cases were observed: 29 (82.8%) cases occurred in autumn and winter and showed a history of consuming high amounts of persimmon and hawthorn; 19 (54.3%) cases revealed a history of gastrointestinal surgery; 19 exhibited incomplete dentition, with missing partial or whole posterior teeth; 26 suffered from obstruction at the ileum. A total of 51 bezoars were found in these patients, of whom 16 (45.7%) had multiple bezoars. CT planar scanning of bezoars showed lumps with mottled gas inside the intestinal cavity. Furthermore, 9 cases of bezoars had envelopes and 11 cases were accompanied with thickening of the distal wall of the obstructed bowel. Scanning of 17 cases was enhanced; the results revealed that the mesenteric blood vessels at the obstruction site and the proximal site were dilated, and a total of 7 cases were accompanied with distal vascular dilation and intestinal wall thickening.
CONCLUSION: BI-SBO exhibits regional and seasonal characteristics. CT planar and contrast-enhanced scanning can be applied to diagnose and observe vascular conditions in obstructed zones.
Core tip: Bezoar-induced small bowel obstruction (BI-SBO) is a relatively rare clinical condition. BI-SBO exhibits regional and seasonal characteristics. This condition is associated with various predisposing factors. Multi-slice spiral computed tomography planar scanning can reveal intestinal bezoar conditions and changes in the intestinal wall and peri-intestinal conditions. Contrast-enhanced examination can further reveal vascular conditions, such as vascular hyperaemia and arterial blood supply conditions, in obstructed zones.
- Citation: Wang PY, Wang X, Zhang L, Li HF, Chen L, Wang X, Wang B. Bezoar-induced small bowel obstruction: Clinical characteristics and diagnostic value of multi-slice spiral computed tomography. World J Gastroenterol 2015; 21(33): 9774-9784
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9774
Small bowel obstruction (SBO) is a common clinical disorder caused by various conditions. Common causes include postoperative adhesions (60%-80%), volvulus, intussusceptions, hernia, and tumours; bezoar-induced SBO (BI-SBO) is clinically rare, accounting for approximately 4% of all SBO cases[1-3]. Bezoars are solidified substances formed by mixing indigestible exogenous substances with other substances in the gastrointestinal tract; bezoars are commonly found inside the stomach but can enter the small intestine via the pylorus. Bezoar impaction in the small intestine likely causes SBO. BI-SBO cases are caused by small intestine-originated bezoars; in general, the small intestine of patients manifests common conditions, such as stenosis, diverticulum or tumorigenesis[4,5]. Bezoar formation is related to various factors, such as gastric motility disorders and gastrointestinal surgery. However, clinical manifestations of BI-SBO are not easily distinguished from intestinal obstruction caused by other factors[2]. As such, early surgical treatment is an essential and feasible treatment option; delayed treatment may significantly increase the incidence of complications and mortality[6].
Imaging examination plays an important role in the diagnosis of BI-SBO; for instance, multi-slice spiral computed tomography (MSCT) can be applied not only to accurately reveal causes, obstruction sites and obstruction degree but also to determine co-existing intestinal ischaemia or potential intestinal diseases[5,6]. Computed tomography (CT) images can also be used by clinicians as reference to develop rational treatment programs. With awareness of clinicians on BI-SBO and timely MSCT examination, this condition can be diagnosed in early stages and effectively treated. However, contrast-enhanced MSCT scanning has been rarely applied to diagnose BI-SBO. This study aimed to identify the predisposing factors affecting bezoar formation and to evaluate the diagnostic value of MSCT scanning, particularly contrast-enhanced examination, in BI-SBO; thus, clinical imaging and understanding of this disease can be enhanced.
A total of 35 BI-SBO cases treated at our hospital (Affiliated Hospital of Binzhou Medical College) from January 2007 to December 2013 were retrospectively analysed. Complete clinical and CT data of patients were available. Of the 35 patients, 13 were male and 22 were female; the age of these patients ranged from 45 to 83 years, with a mean age of 63.3 ± 10.4 years. SBO was clinically diagnosed on the basis of clinical manifestations (vomiting, abdominal pain, bloating, blocked defecation and exsufflation). All of the patients were diagnosed with BI-SBO through CT examination and confirmed through surgical procedures, including laparotomy and gastric or small intestinal endoscopy-broken suction operations.
CT examination was performed using a GE 64-slice spiral CT scanner (GE Lightspeed 64 VCT, United States). Of the 35 patients, 18 underwent abdominal and pelvic CT planar scanning and 17 patients underwent abdominal and pelvic CT planar scanning combined with contrast-enhanced examination. None of the patients underwent intestinal CT visual examination. In enhanced examination, a high pressure syringe was used to inject 100 mL of a non-ionic contrast agent (300 mg I Omnipaque; GE Healthcare, Shanghai, China) via the cubital vein at an injection rate of 3.5 mL/s. After the contrast agent was injected, scanning was performed at 26 and 60 s; afterwards, arterial and portal venous phase images were obtained, respectively. The following CT scan parameters were set: collimation, 5 mm; thickness, 5 mm; layer interval, 2.5 mm; reconstructed layer thickness, 0.625 mm; 120 kVp; and 250 mAs. Clinical data, original images and images processed in a GE ADW4.3 workstation (MPR, CPR, MIP and CTA) were recorded.
The images of all patients were evaluated by two abdominal imaging experts; the following main analytical contents were considered: (1) intestinal bezoar conditions, such as location, number, shape, presence of an envelope and co-existence of a bezoar; (2) changes in the intestinal wall based on intestinal wall thickening, intestinal dilation and intestinal ischaemia or infarction, among others; (3) changes in peri-intestinal conditions, including mesenteric fuzzy (i.e., mesenteric fat density increased) and ascites; (4) contrast-enhanced examination mainly evaluated vascular hyperaemia (i.e., whether the number of mesenteric vessels increased or decreased and whether or not lumen expansion occurred) and arterial blood supply conditions at a particular obstruction site and the distal end of the obstruction site. If the two experts disagreed with a diagnostic opinion, a consensus was obtained after further consultations were performed.
The patients’ clinical data are shown in Table 1. The proportion of males to females among the 35 patients was 1:1.69 (13:22); median age was 63.3 years (with a range of 45 years to 83 years). Clinical manifestations included all of the symptoms of acute intestinal obstruction, particularly abdominal distension (100%) and abdominal pain (65.7%); the most common sign was the presence of an abdominal bulge (82.9%). Three patients with acute obstruction suffered from fever, tenderness at specific abdominal site and rebound tenderness during physical examination. The patients were subjected to emergent surgery after they underwent abdominal and pelvic CT planar scanning. Thirty-two patients received conservative treatments, such as gastrointestinal decompression for 1 d to 7 d. After the corresponding examinations were completed, 28 patients underwent open surgery, and 4 patients underwent gastrointestinal endoscopic bezoar-broken suction operations. The patients were followed for three months; no recurrence was found.
| Predisposing factor | Case |
| Sex | |
| Male | 13 (37.1) |
| Female | 22 (62.9) |
| Age (yr) | |
| < 63 | 15 (42.9) |
| ≥ 63 | 20 (57.1) |
| Symptom | |
| Abdominal pain | 23 (65.7) |
| Abdominal distension | 35 (100) |
| Vomiting | 15 (42.9) |
| Defecation and exhaust stopping | 12 (34.3) |
| Physical sign | |
| Fever | 7 (20) |
| Abdominal protuberance | 29 (82.9) |
| Abdominal tenderness | 9 (25.7) |
| Tenderness and rebound Tenderness | 9 (25.7) |
| Cords | 7 (20) |
| Food intake before the onset | |
| Persimmon, hawthorn, jujube | 29 (82.9) |
| Uncertainty | 6 (17.1) |
| Previous history | |
| Stomach stone or bezoar treatment history | 3 (8.6) |
| Gastroduodenal operation history | 19 (54.3) |
| Other history of abdominal operation | 6 (8.6) |
| Dental status | |
| Teeth sound (partial denture) | 9 (25.7) |
| Partial or total loss of posterior teeth | 26 (74.3) |
A total of 25 patients exhibited a history of abdominal surgery. Of these 25 patients, 19 underwent gastroduodenal surgery (3 cases of vagotomy and pyloroplasty; 16 cases of gastrectomy and gastrointestinal anastomosis, including 5 cases of gastroduodenal anastomosis and 11 cases of gastrojejunostomy), 3 underwent appendectomy, 2 underwent subtotal hysterectomy, and 1 underwent post-traumatic splenectomy. Other medical histories included 9 cases of diabetes (6 cases were subjected to abdominal surgery and 3 other cases did not undergo surgery) and 1 case of secondary hypothyroidism after this patient was subjected to hyperthyroid surgery.
The following patients manifested dental conditions. A total of 9 cases showed complete dentition (25.7%); of these cases, 2 retained all of their teeth, 4 had whole dentures and 3 exhibited partial dentures. A total of 19 cases exhibited incomplete dentition with missing partial or whole posterior teeth.
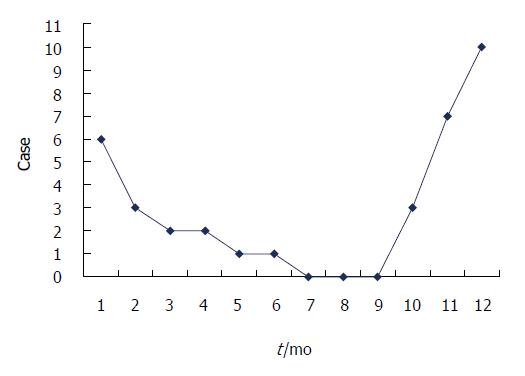
BI-SBO may occur throughout a year, but this condition is common from October to February, which coincides with Chinese autumn and winter (29/35, 82.9%). Figure 1 shows the incidence of BI-SBO in each month. Before the onset of BI-SBO, 29 patients exhibited a history of consuming high amounts of persimmon (15 cases), hawthorn (13 cases) and jujube (1 case) under fasting conditions. Of these patients, 3 revealed a history of gastrolith treatment on 3 d to 9 d before the condition was manifested (gastroscopic broken suction operation) and 6 did not experience food intake-related conditions.
A total of 51 bezoars were found in the 35 BI-SBO patients, of whom 19 contained only one bezoar and 16 revealed multiple bezoars (45.7%, Table 2). The obstruction sites were the duodenum (1 case), jejunum (8 cases) and ileum (26 cases). Co-existing bezoars were located in the stomach (5 bezoars), jejunum (3 bezoars) and ileum (8 cases). A total of 35 bezoars (100%) were accurately diagnosed through CT examination, whereas 2 co-existing gastroliths among the 16 co-existing bezoars were misdiagnosed.
| Impaction site(n = 35) | Concomitant site | Case, n | CT confirmed, n | |
| Impaction bezoars | Concomitant bezoars | |||
| Duodenum | No | 1 | 1 | 0 |
| Jejunum | No | 6 | 6 | 0 |
| Stomach | 2 | 2 | 2 | |
| Ileum | 1 | 1 | 1 | |
| Ileum | No | 12 | 12 | 0 |
| Stomach | 3 | 3 | 1 | |
| Jejunum | 3 | 3 | 3 | |
| Ileum | 7 | 7 | 7 | |
| Total | 35 | 35 | 14 | |
CT planar scanning revealed that the obstructed bezoars appeared as lumps with clear edges inside the intestinal cavity. The density of these bezoars revealed varied results with mottled gas shadow inside the cavity. In 3 cases of hawthorn bezoar, scattered high-density seeds were obtained. In terms of morphological characteristics, 26 were oval, 5 were tubular and 4 were round. The performance of coexisting bezoars was similar to the obstructed bezoars. Only 6 (17.1%) cases of single obstructed bezoars and 3 cases of co-existing gastroliths contained an envelope (Table 3). CT images revealed that the 2 misdiagnosed gastrolith cases were also covered by an envelope, whereas the remaining bezoars were not covered by an envelope. In terms of obstruction sites, 34 cases exhibited proximal bowel dilation and 1 case occurred at the bottom of the site subjected to surgical anastomosis without evident proximal bowel dilation. All of the patients showed a gas-liquid surface in the obstructed proximal dilated intestine or stomach. A total of 34 cases manifested thickening in the obstructed zone and proximal intestinal wall (97.1%) and 11 cases showed thickening of the obstructed distal intestinal wall. A total of 32 cases exhibited mesenteric fuzzy (91.4%) and 13 cases contained a small amount of ascites (37.1%).
| Signs | Case | Percentage | |
| Impaction bezoars | 35 | ||
| Well delineated mass in the intestinal lumen, with a mixed density and containing air | With encapsulating wall | 6 | 17.1 |
| Without encapsulating wall | 29 | 82.9 | |
| Bowel and perienteric changes | |||
| Bowel wall thickening | Obstruction and proximal | 34 | 97.1 |
| With distal to the obstruction | 11 | 31.4 | |
| Lumen dilatation proximal to the obstruction | 34 | 97.1 | |
| Intestinal infarction | 0 | 0 | |
| Mesenteric haziness | 32 | 91.4 | |
| Ascites | 13 | 37.1 | |
| CTA changes | 17 | ||
| Mesenteric vascular engorgement | Obstruction and proximal | 17 | 100 |
| With distal to the obstruction | 7 | 41.2 | |
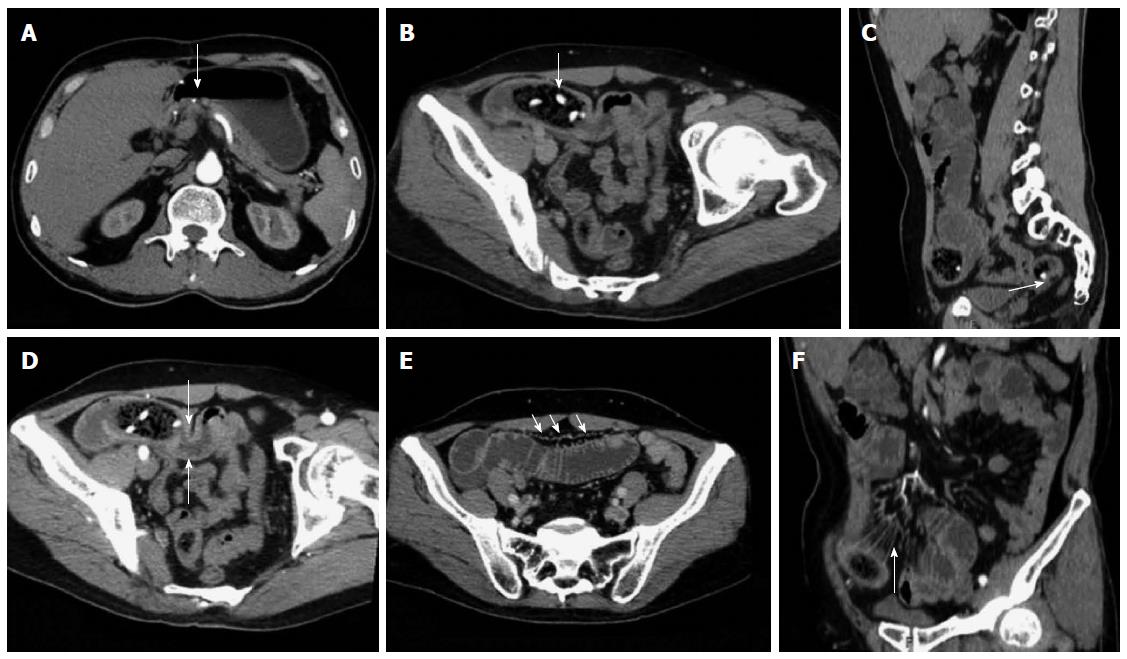
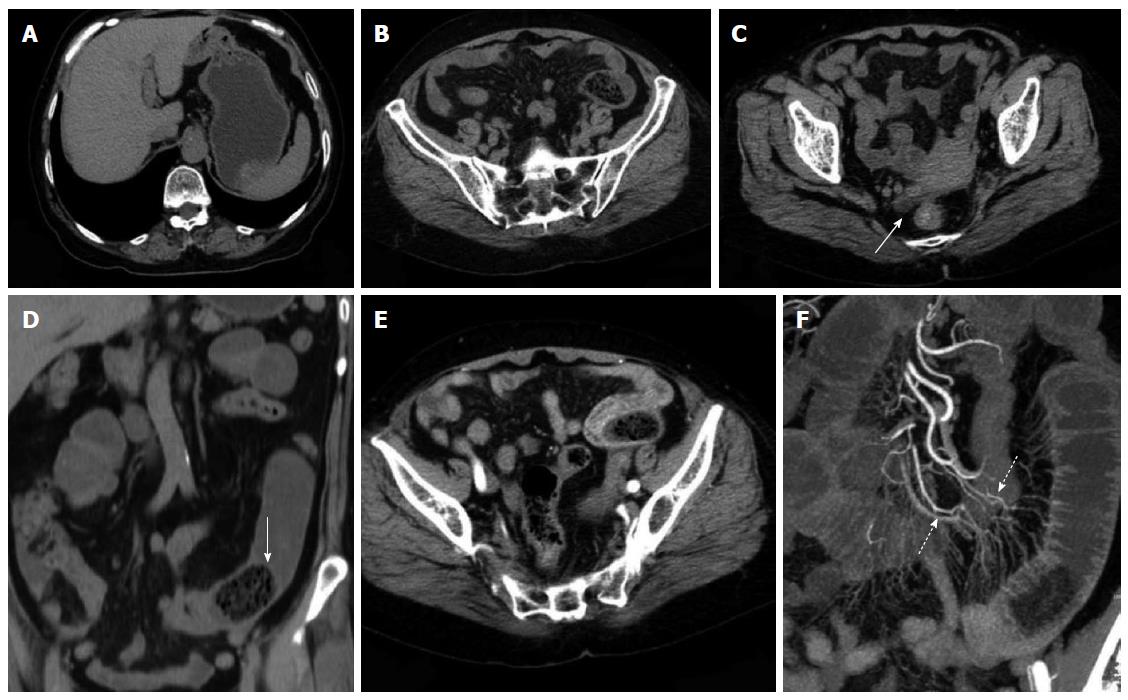
Enhanced scanning revealed 17 cases of mesenteric vascular congestion and dilation in the obstructed zone and obstructed proximal end; of these 17 patients, 7 were accompanied with hyperaemia and thickening of the obstructed distal vessel wall. The obstruction sites and distal end of the obstruction sites of these 7 patients were supplied with blood via one mesenteric arterial branch (Table 3, Figures 2, 3, 4, 5).
A bezoar refers to the coagulum formed inside the digestive tract when indigestible exogenous substances are mixed with other substances; this condition is affected by several factors. Bezoars can be classified into different groups according to component sources: phytobezoar, trichobezoar, drug bezoar and lactic acid bacterium bezoar. Among these groups, phytobezoar is the most common type[7]. Phytobezoar is commonly found inside the stomach; partial phytobezoars may enter the small intestine, thereby causing impaction and complete SBO. BI-SBO is clinically rare, accounting for approximately 4% of all SBO cases[1-3,8]. Bezoar formation is not directly correlated with gender and age[1,9-11]; furthermore, bezoar formation may be affected by several factors, such as diet, anatomical features, organic composition of the gastrointestinal tract and functional disorders[7,8,12-16].
In terms of diet, phytobezoar is mainly caused by consuming high amounts of vegetables or fruits, such as persimmon, hawthorn, date, prune, grape skin, celery, mango, banana, cactus fruit, fig and mushroom, which contain indigestible plant fibres[12,17,18]. These fruits and vegetables are rich in fibres, tannic acid and lignins. When consumed at high amounts, tannic acid is transformed from a monomeric form into a tannin-cellulose-protein complex via the actions of gastric acid. This complex exhibits a concrete-like viscosity and easily adhered to the gastrointestinal wall; a viscous mass is formed and may be incarcerated inside the gastrointestinal lumen, thereby causing obstruction[12,19,20]. Furthermore, seeds of multi-seed fruits, such as cactus fruit and fig, are prone to accumulate inside the small intestine and form obstructive lumps inside the intestinal cavity; as a result, intestinal obstruction occurs[12,13]. BI-SBO can affect individuals worldwide, although regional and seasonal characteristics can be observed. Conditions, such as persimmon and hawthorn stones, are more common in subtropical and temperate regions than in other regions; the onset of these conditions usually occurs in autumn and winter. In this study, all of the patients were located in the temperate zone; of these patients, 82.9% were detected in autumn and winter (31/35), and this finding is consistent with that described in previous studies[12,17,21].
In addition to the intake of large amounts of cellulose materials, other possible risk factors include history of gastrointestinal surgeries (such as vagotomy and pyloroplasty, gastrectomy and gastrojejunostomy or gastroduodenal anastomosis, and gastrointestinal stoma anastomosis), gastric motility disorders caused by various factors and poor dentition[2,5,22,23].
Vagotomy and partial gastrectomy can reduce gastric acid secretion, resulting in a weakly acidic environment and decrease in gastric motility; thus, removal of undigested solids from the stomach is likely delayed, thereby causing high amounts of viscous contents to form inside the stomach, which is prone to bezoar formation[12]. Gastroenterostomy, gastrojejunostomy or pyloroplasty can expand the outlet of the stomach; as such, undigested vegetable or fruit fibre lumps inside the stomach easily enter and form obstructive lumps in the small intestine, thereby causing incarceration and obstruction[12,16,19,24,25]. After gastroenterostomia occurs, the stricture of anastomotic stoma is a possible cause of bezoar formation[12,16,25]. In this study, 19 patients, including 11 cases of major gastrectomy plus gastrojejunostomy and 5 cases of major gastrectomy plus gastroduodenal anastomosis, revealed a history of gastrointestinal surgery. This high incidence of gastroenterostomia is similar to that recorded by Bedioui et al[12]. Ho et al[19] and Ulusan et al[24] also summarised the surgical histories of patients with BI-SBO, but relationships between different surgical methods and BI-SBO have not been reported.
The hypodontia of posterior dentition or dentural malocclusion likely affects chewing functions; as a result, chewing and digestion disorders related to fibrous food occur; thus, lumps in the gastrointestinal tract can be easily formed and the probability of SBO occurrence possibly increases[15,22]. In this study, patients with partial or complete hypodontia of posterior dentition exhibited a significantly higher incidence of SBO than those with complete dentition; this result indicated that dentition status affects the incidence of BI-SBO. Similar to other studies, our study showed that diabetes, hypothyroidism, pernicious anaemia, kidney failure, postoperative bowel adhesions and other diseases can indirectly affect gastrointestinal motility and emptying; thus, BI-SBO occurs[17,25,26].
BI-SBO usually occurs at the narrow part of the small intestine, in particular, the distal end of the ileum is the most common site, especially at 50 cm to 70 cm away from the ileocaecal valve. This condition commonly occurs at this site because the lumen of this small intestinal segment is narrow. Thus, bowel movements are slowed down, and a large amount of water inside bezoars is absorbed and motility is reduced. The jejunum is the second most common site[27,28]. In this study, 9 cases of BI-SBO were found in the jejunum and 26 cases were detected in the ileum; this finding is consistent with that described in previous studies. Considering patients’ histories of gastrointestinal surgery, we found that the obstructive sites of BI-SBO may be related to the size of gastrointestinal anastomosis. On one hand, a large anastomotic size (diameter ≥ 30 mm) may easily cause a high incidence of SBO. This condition is possible because a large gastrolith mat easily enter the small intestine via the anastomotic stoma and cause incarceration in the corresponding luminal stenotic site; in particular, a bezoar with a diameter greater than that of the small intestine may easily form incarceration at a region closer to the anastomotic site. On the other hand, a small anastomotic stoma likely results in a low incidence of SBO. This condition is possible because a gastrolith larger than anastomotic stoma cannot easily enter the small intestine via the anastomotic stoma; thus, gastrolith enlarges in the stomach. By contrast, a small gastrolith easily enters the small intestine and gradually forms a large bezoar with slow intestinal movements; for instance, patients with reduced gastrointestinal motor functions exhibit reduced bezoar movement. Thus, obstruction sites are often found away from anastomotic sites. In this study, the patient with gastroduodenal anastomosis exhibited the largest SBO site. This patient revealed a wide anastomotic stoma (diameter = 40 mm). The obstruction site was located beneath the anastomotic stoma and above the duodenal papilla. Patients with BI-SBO exhibited co-existing bezoars in other parts, in addition to the obstructive bezoar; co-existing bezoars could be found in any part of the stomach and the small intestine. Approximately one-third of the patients also have gastroliths; these residual co-existing bezoars may be the main reason for recurrence after patients undergo surgery to remove bezoars from the small intestine[5,10]. In this study, 16 (45.7%) patients showed co-existing bezoars, of whom 5 manifested co-existing gastroliths. This ratio was lower than that documented in a previous study. Only 3 patients underwent gastroscopic bezoar-broken suction operation 3 d to 9 d before disease onset; thus, SBO may be caused by bezoar residue or debris that formed after surgery is completed.
The clinical manifestations of BI-SBO may vary according to different bezoar sizes, obstruction sites and degrees; the most common manifestations are complete mechanical intestinal obstructions, such as abdominal pain, bloating, nausea and vomiting. The diagnosis of BI-SBO based on clinical symptoms may delay surgical treatment and increase morbidity and mortality[2,22]. If BI-SBO patients revealed histories of gastrointestinal surgery or laparotomy, clinicians may initially consider adhesive intestinal obstruction and implement conservative treatments, which may adversely affect illness. Therefore, appropriate examination methods should be selected to diagnose BI-SBO in early stages and clinical treatments should be promptly administered[19,22,24].
Imaging examination is the main basis of SBO diagnosis. Abdominal X-ray, barium enema, abdominal ultrasound and endoscopy have been applied to diagnose SBO, but these examinations exhibit poor diagnostic accuracies and limitations. As such, the clinical use of these techniques is limited[9,10,19,22]. MSCT is characterized by several advantages, including fast scanning speed, non-invasive procedure and volume scanning. MSCT has become the preferred examination method to diagnose SBO. CT yields a diagnostic sensitivity for acute SBO of 73% to 95%[29] and an accuracy of 83%[30]. For BI-SBO, abdominal and pelvic CT scanning can be applied to locate bezoar causing SBO and obstructive degree, as well as co-existing multiple bezoars, intestinal ischaemia, strangulation, perforation or other potential intestinal diseases. In this procedure, acute abdominal symptoms caused by other causes are excluded; thus, right treatment options are selected and most efficient surgical approaches are determined[5,6,10,29-31].
In CT planar scanning, bezoar appears as round, oval or tubular masses with clear boundaries located inside the intestinal lumen, although densities varied and mottled gas density can be observed inside the lumen. Some bezoars may be covered with ring envelopes[5,9,31]. Altintoprak et al[13] reported that bezoars contain a large amount of gas and show wide intervals, as well as scattered high-density seeds characterizing hawthorn bezoars. Kim et al[5] suggested that gastrolith surface contains one coating layer of gastric mucosal secretions, which form a gelatinous membrane and cover bezoar surface; this membrane may be the encapsulating wall found in CT images. In CT planar scanning, air and fat can be identified by adjusting to a suitable window (WW-500HU, WL-50HU); the processed and obtained MPR image can then be used to observe intestinal bezoar, wall thickness, dilation extension, changes in intestinal perimeter and co-existence of bezoars[10,32]. Intestinal oedema and bleeding caused by bezoar incarceration can be used as “target signs”. CT target signs likely prevent the passage of obstructive bezoar through the intestines. As such, conservative treatments may not be suitable or may eventually fail, and emergent surgery is thus needed[5]. In this study, 35 cases manifested typical signs, and accurate diagnosis was performed through CT planar scanning. However, 2 cases of co-existing gastrolith were misdiagnosed possibly because of radiologist’s insufficient understanding of co-existing bezoars; observation was also not sufficiently comprehensive. The results also showed that 3 cases of hawthorn bezoar presented shadows of high-density seeds, whereas 1 case of diospyrobezoar did not show these high-density shadows. Furthermore, 5 cases of co-existing gastroliths were covered with an envelope, but the obstructive bezoars of the corresponding cases did not show definite capsules. This finding was explained by Kim et al[5]. All of the patients in our study showed no definite target signs.
Multi-slice contrast-enhanced spiral CT examination (particularly in the arterial phase) combined with post-processing technologies (MPR, CPR, MIP and CTA) can help identify lesion segments of the small intestine, vascular anatomical and morphological changes and intestinal ischaemia or infarction; the combined technology can also be applied to observe postoperative changes, including surgical methods, anastomotic stoma and input and output loops; thus, inductive factors, such as postoperative changes, adhesions or diverticulitis, can be determined before surgery is performed[21,24]. Geffroy et al[33] suggested that a dilated ansa is not significantly enhanced compared with an adjacent normal intestinal ansa of patients with SBO; as such, this condition is a specific sign of intestinal ischaemia. Geffroy et al[33] also demonstrated that CT planar scanning results may provide a false indication that the strengthening of lesion intestinal walls is normal when contrast-enhanced images show that the density of a dilated intestinal wall is increased (sign of ischaemia). As a consequence, intestinal ischaemia is misdiagnosed or diagnosed in late stages. In this study, the CTA images of 17 enhanced examination cases showed blood supply from mesenteric arterial branches and anastomotic branches in the lesion regions. The images also accurately revealed the obstruction site, thickening and tortuosity of anastomotic blood vessels of proximal dilated intestines, mesenteric perivascular exudation, oedema and ascites of the distal end of obstructive intestines; intestinal ischaemia or necrosis signs, such as enhancement reduction, were not found.
Kim et al[5] considered that the thickening of the distal small intestinal wall in the obstruction site is a significant CT sign to determine the onset of basic intestinal diseases in patients with phytobezoars. However, this hypothesis should be further investigated. In our study, 11 patients with thickened distal small intestinal wall in the obstruction site did not suffer from basic intestinal diseases; this finding is not consistent with that obtained by Kim et al[5]. Analysing the CTA images, we found that the thickening of distal small intestinal wall in the obstruction site may be related to the blood supply of the mesenteric branches; in other words, they are formed in proximity to the obstruction site. Furthermore, intestinal swelling, mesenteric vascular tortuosity and thickening and peri-intestinal secretion become evident when the distal end of the obstruction site and the transitional segment (located in the obstruction site between the dilated intestine and the collapsed intestine) supply blood for the same artery. By contrast, the inflammation degree of the distal end of the obstructive intestine likely decreases or swelling unlikely occurs when the distal end of the obstruction site and the transitional segment separately supply blood for the arterial branch (or the transitional segment located at the junction of two blood-supplying arteries); mesenteric vascular tortuosity and thickening, as well as peri-intestinal and perivascular exudation, are also unlikely evident.
In CT diagnosis, BI-SBO should be distinguished from intestinal faeces- and gallstone-induced SBO. Bezoar and faeces related to SBO have been described; for instance, lumps should be considered as faeces if lumps inside the dilated intestines exhibit several characteristics, such as long longitudinal diameter, insufficient density in texture, no capsule, found in the proximal end of obstruction sites and floating-like properties. Lumps may be considered as bezoars if intestinal masses are located in the obstructive transitional zone, characterised by a clear edge, and bubble-like mottled behaviour and covered with an envelope[5,10,30,32]. The performance of gallstone ileus is similar to that of bezoar and can be accurately diagnosed on the basis of Rigler’s image triad (SBO, pneumobilia and positive small intestinal ectopic gallstone) and biliary-enteric fistula[31,34-36]. Few bezoars may exhibit non-gas, solid soft tissue-density lumps. With CT planar scanning, small intestinal tumours or intussusceptions can be hardly identified; with enhanced CT scanning or contrast-enhanced examination, diagnosis can be confirmed.
The formation of bezoars may be related to various predisposing factors; the onset of this condition in patients is often simultaneously affected by many factors. BI-SBO exhibits regional and seasonal characteristics; thus, clinicians should understand this feature to perform efficiently. BI-SBO also shows characteristic CT signs; thus, diagnosis through CT planar scanning is relatively easy based on the observed performance. Abdominal and pelvic contrast-enhanced CT examination can also efficiently reveal intestinal lesions, peri-intestinal changes and blood-supplying arteries; thus, this technique can help clinicians administer early appropriate interventions and improve patient outcomes.
Small bowel obstruction (SBO) is a common clinical condition caused by various factors, including postoperative adhesions, volvulus, intussusceptions, hernia or tumours. Bezoar impaction-induced SBO (BI-SBO) is a clinically rare condition. Multi-slice spiral computed tomography (MSCT) plays an important role in the diagnosis of BI-SBO.
The use of MSCT scanning can help diagnose SBO, reveal causes, obstruction site and obstruction degree and accurately determine co-existing intestinal ischaemia or potential intestinal diseases.
BI-SBO exhibits regional and seasonal characteristics. This condition is associated with various predisposing factors, such as gastric motility disorders and gastrointestinal surgery. MSCT is a valuable technology to diagnose BI-SBO. The major advantage of contrast-enhanced examination is the ability to observe vascular situations in obstructed zones, including vascular hyperaemia and arterial blood supply conditions.
Predisposing factors can trigger susceptible groups to develop BI-SBO. Contrast-enhanced MSCT examination can be applied to routinely observe vascular conditions of patients with SBO. Further research should be conducted on SBO to promote the widespread use of MSCT.
A bezoar refers to the coagulum formed inside the digestive tract when indigestible exogenous substances are mixed with other substances. A bezoar can be classified into different groups according to component sources: phytobezoar, trichobezoar, drug bezoar or lactic acid bacterium bezoar.
This study is an original research that highlights the application of MSCT to diagnose BI-SBO and simultaneously analyse predisposing factors.
| 1. | Erzurumlu K, Malazgirt Z, Bektas A, Dervisoglu A, Polat C, Senyurek G, Yetim I, Ozkan K. Gastrointestinal bezoars: a retrospective analysis of 34 cases. World J Gastroenterol. 2005;11:1813-1817. [PubMed] |
| 2. | Ko S, Lee T, Ng S. Small bowel obstruction due to phytobezoar: CT diagnosis. Abdom Imaging. 1997;22:471-473. [PubMed] |
| 3. | Licht M, Gold BM, Katz DS. Obstructing small-bowel bezoar: diagnosis using CT. AJR Am J Roentgenol. 1999;173:500-501. [PubMed] |
| 4. | Kim JH, Chang JH, Nam SM, Lee MJ, Maeng IH, Park JY, Im YS, Kim TH, Park IY, Han SW. Duodenal obstruction following acute pancreatitis caused by a large duodenal diverticular bezoar. World J Gastroenterol. 2012;18:5485-5488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Ha HK, Sohn MJ, Kim AY, Kim TK, Kim PN, Lee MG, Myung SJ, Yang SK, Jung HY. CT findings of phytobezoar associated with small bowel obstruction. Eur Radiol. 2003;13:299-304. [PubMed] |
| 6. | Akcakaya A, Sahin M, Coskun A, Demiray S. Comparison of mechanical bowel obstruction cases of intra-abdominal tumor and non-tumoral origin. World J Surg. 2006;30:1295-1299. [PubMed] |
| 7. | Yakan S, Sirinocak A, Telciler KE, Tekeli MT, Deneçli AG. A rare cause of acute abdomen: small bowel obstruction due to phytobezoar. Ulus Travma Acil Cerrahi Derg. 2010;16:459-463. [PubMed] |
| 8. | Teng H, Nawawi O, Ng K, Yik Y. Phytobezoar: an unusual cause of intestinal obstruction. Biomed Imaging Interv J. 2005;1:e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Yildirim T, Yildirim S, Barutcu O, Oguzkurt L, Noyan T. Small bowel obstruction due to phytobezoar: CT diagnosis. Eur Radiol. 2002;12:2659-2661. [PubMed] |
| 10. | Ripollés T, García-Aguayo J, Martínez MJ, Gil P. Gastrointestinal bezoars: sonographic and CT characteristics. AJR Am J Roentgenol. 2001;177:65-69. [PubMed] |
| 11. | Pujar K A, Pai A S, Hiremath V B. Phytobezoar: a rare cause of small bowel obstruction. J Clin Diagn Res. 2013;7:2298-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Bedioui H, Daghfous A, Ayadi M, Noomen R, Chebbi F, Rebai W, Makni A, Fteriche F, Ksantini R, Ammous A. A report of 15 cases of small-bowel obstruction secondary to phytobezoars: predisposing factors and diagnostic difficulties. Gastroenterol Clin Biol. 2008;32:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Altintoprak F, Degirmenci B, Dikicier E, Cakmak G, Kivilcim T, Akbulut G, Dilek ON, Gunduz Y. CT findings of patients with small bowel obstruction due to bezoar: a descriptive study. ScientificWorldJournal. 2013;2013:298392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Oh SH, Namgung H, Park MH, Park DG. Bezoar-induced Small Bowel Obstruction. J Korean Soc Coloproctol. 2012;28:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Salemis NS, Panagiotopoulos N, Sdoukos N, Niakas E. Acute surgical abdomen due to phytobezoar-induced ileal obstruction. J Emerg Med. 2013;44:e21-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Roy M, Fendrich I, Li J, Szomstein S, Rosenthal RJ. Treatment option in patient presenting with small bowel obstruction from phytobezoar at the jejunojejunal anastomosis after Roux-en-Y gastric bypass. Surg Laparosc Endosc Percutan Tech. 2012;22:e243-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Stein CM, Gelfand M. Gastro-intestinal phytobezoars in Zimbabwean Africans. Trans R Soc Trop Med Hyg. 1985;79:508-509. [PubMed] |
| 18. | Ho MP, Chou AH, Cheung WK. Small bowel obstruction secondary to a mushroom bezoar. J Am Geriatr Soc. 2013;61:1041-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Ho TW, Koh DC. Small-bowel obstruction secondary to bezoar impaction: a diagnostic dilemma. World J Surg. 2007;31:1072-1078; discussion 1079-1080. [PubMed] |
| 20. | Ladas SD, Kamberoglou D, Karamanolis G, Vlachogiannakos J, Zouboulis-Vafiadis I. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment Pharmacol Ther. 2013;37:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Tayeb M, Khan FM, Rauf F, Khan MM. Phytobezoar in a jejunal diverticulum as a cause of small bowel obstruction: a case report. J Med Case Rep. 2011;5:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Escamilla C, Robles-Campos R, Parrilla-Paricio P, Lujan-Mompean J, Liron-Ruiz R, Torralba-Martinez JA. Intestinal obstruction and bezoars. J Am Coll Surg. 1994;179:285-288. [PubMed] |
| 23. | Liou CH, Yu CY, Lin CC, Chao YC, Liou YC, Juan CJ, Chen CY. CT diagnosis of small bowel obstruction due to phytobezoar. J Formos Med Assoc. 2003;102:620-624. [PubMed] |
| 24. | Ulusan S, Koç Z, Törer N. Small bowel obstructions secondary to bezoars. Ulus Travma Acil Cerrahi Derg. 2007;13:217-221. [PubMed] |
| 25. | Sarhan M, Shyamali B, Fakulujo A, Ahmed L. Jejunal Bezoar causing obstruction after laparoscopic Roux-en-Y gastric bypass. JSLS. 2010;14:592-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | de Menezes Ettinger JE, Silva Reis JM, de Souza EL, Filho Ede M, Gãlvao do Amaral PC, Ettinger E, Fahel E. Laparoscopic management of intestinal obstruction due to phytobezoar. JSLS. 2007;11:168-171. [PubMed] |
| 27. | de Toledo AP, Rodrigues FH, Rodrigues MR, Sato DT, Nonose R, Nascimento EF, Martinez CA. Diospyrobezoar as a cause of small bowel obstruction. Case Rep Gastroenterol. 2012;6:596-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Quintana JF, Walker RN, McGeehan A. Child with small bowel obstruction and perforation secondary to ileal bezoar. Pediatr Emerg Care. 2008;24:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Burkill GJ, Bell JR, Healy JC. The utility of computed tomography in acute small bowel obstruction. Clin Radiol. 2001;56:350-359. [PubMed] |
| 30. | Zissin R, Osadchy A, Gutman V, Rathaus V, Shapiro-Feinberg M, Gayer G. CT findings in patients with small bowel obstruction due to phytobezoar. Emerg Radiol. 2004;10:197-200. [PubMed] |
| 31. | Billaud Y, Pilleul F, Valette PJ. [Mechanical small bowel obstruction due to bezoars: correlation between CT and surgical findings]. J Radiol. 2002;83:641-646. [PubMed] |
| 32. | Delabrousse E, Lubrano J, Sailley N, Aubry S, Mantion GA, Kastler BA. Small-bowel bezoar versus small-bowel feces: CT evaluation. AJR Am J Roentgenol. 2008;191:1465-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Geffroy Y, Boulay-Coletta I, Jullès MC, Nakache S, Taourel P, Zins M. Increased unenhanced bowel-wall attenuation at multidetector CT is highly specific of ischemia complicating small-bowel obstruction. Radiology. 2014;270:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Lassandro F, Romano S, Ragozzino A, Rossi G, Valente T, Ferrara I, Romano L, Grassi R. Role of helical CT in diagnosis of gallstone ileus and related conditions. AJR Am J Roentgenol. 2005;185:1159-1165. [PubMed] |
| 35. | Kim Y, Park BJ, Kim MJ, Sung DJ, Kim DS, Yu YD, Lee JH. Biliary phytobezoar resulting in intestinal obstruction. World J Gastroenterol. 2013;19:133-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Kim TO, Lee SH, Kim GH, Heo J, Kang DH, Song GA, Lee JW, Cho M. Common bile duct stone caused by a phytobezoar. Gastrointest Endosc. 2006;63:324; discussion 324. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kasano Y, Ugezu C, Munjal K S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM