Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9741
Peer-review started: February 27, 2015
First decision: March 26, 2015
Revised: April 23, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: September 7, 2015
Processing time: 192 Days and 14.2 Hours
AIM: To evaluate the possibility of treatment effect monitoring using hepatic fat quantification magnetic resonance (MR) in pediatric nonalcoholic steatohepatitis (NASH).
METHODS: We retrospectively reviewed the medical records of patients who received educational recommendations and vitamin E for NASH and underwent hepatic fat quantification MR from 2011 to 2013. Hepatic fat fraction (%) was measured using dual- and triple-echo gradient-recalled-echo sequences at 3T. The compliant and non-compliant groups were compared clinically, biochemically, and radiologically.
RESULTS: Twenty seven patients (M:F = 24:3; mean age: 12 ± 2.3 years) were included (compliant group = 22, non-compliant = 5). None of the baseline findings differed between the 2 groups, except for triglyceride level (compliant vs non-compliant, 167.7 mg/dL vs 74.2 mg/dL, P = 0.001). In the compliant group, high-density lipoprotein increased and all other parameters decreased after 1-year follow-up. However, there were various changes in the non-compliant group. Dual-echo fat fraction (-19.2% vs 4.6, P < 0.001), triple-echo fat fraction (-13.4% vs 3.5, P < 0.001), alanine aminotransferase (-110.7 IU/L vs -10.6 IU/L, P = 0.047), total cholesterol (-18.1 mg/dL vs 3.8 mg/dL, P = 0.016), and triglyceride levels (-61.3 mg/dL vs 11.2 mg/dL, P = 0.013) were significantly decreased only in the compliant group. The change in body mass index and dual-echo fat fraction showed a positive correlation (ρ = 0.418, P = 0.030).
CONCLUSION: Hepatic fat quantification MR can be a non-invasive, quantitative and useful tool for monitoring treatment effects in pediatric NASH.
Core tip: Few noninvasive methods have been evaluated to accurately assess and monitor the progression of nonalcoholic steatohepatitis (NASH) in children. In this study, we used hepatic fat quantification magnetic resonance (MR) and compared the compliant and non-compliant groups for treatment of pediatric NASH. The compliant group showed not only laboratory improvement but also a decrease in the fat fraction in both dual- and triple-echo sequences after follow-up. Therefore, hepatic fat quantification MR can be a non-invasive, quantitative and useful tool for monitoring treatment effects in pediatric NASH.
- Citation: Koh H, Kim S, Kim MJ, Kim HG, Shin HJ, Lee MJ. Hepatic fat quantification magnetic resonance for monitoring treatment response in pediatric nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21(33): 9741-9748
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9741
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children and adolescents[1-3]. It varies in severity from simple fatty liver to nonalcoholic steatohepatitis (NASH), which may induce hepatic fibrosis, cirrhosis, and hepatocellular carcinoma[4,5]. Cirrhosis due to NAFLD has already been reported in 2 boys aged 10 and 14 years[6]. Therefore, prompt diagnosis and suitable treatment are mandatory. Proper diet, exercise, and vitamin E may help improve steatosis in liver histology[7-10].
Liver biopsy is the modality of choice to accurately diagnose NAFLD and to monitor disease progression during treatment, even in children and adolescents. However, this procedure is invasive and may result in life-threatening complications[11,12]. In addition, it has many drawbacks, including sampling error and inter- and intra-observer variability in interpretation. Liver biopsy can also be associated with complications such as abdominal pain, hypotension, hemobilia, and intraperitoneal hemorrhage, the last of which has an associated mortality rate of up to 0.5%. Furthermore, liver biopsy is not generally accepted by patients and especially not by children and adolescents[13-15]. Therefore, there is a need to develop noninvasive methods to accurately assess and monitor the progression of NAFLD in children.
Various imaging studies can be used for the noninvasive assessment of hepatic fat including ultrasonography, computed tomography (CT), and magnetic resonance (MR) imaging. Ultrasonography can detect fatty liver as increased liver parenchymal echogenicity. There is no risk of radiation exposure using this method. However, its ability to grade the fatty liver in children is limited[5], despite an attempt to quantitatively analyze hepatic fat with ultrasonography[16]. Moreover, positive ultrasonographic results in severely obese adolescents cannot be used to accurately predict the presence and severity of hepatic steatosis[17].
CT can also detect hepatic fat infiltration by analyzing hepatic parenchymal attenuation[18]. However, hepatic attenuation can also be affected by other conditions such as hemosiderin deposition[19]. Moreover, the risk of radiation exposure is a major disadvantage, especially in children. No studies have yet compared the CT assessment of hepatic steatosis with histologic grades of fatty infiltration in children[5].
Both MR imaging and MR spectroscopy can detect hepatic fat infiltration simply and accurately without radiation exposure[20], even though MR spectroscopy needs a long examination time for a small volume of the liver. When using MR imaging, quantitative measurement of the fat fraction using a chemical shift technique, which distinguishes resonant frequencies between fat and water, can easily evaluate fat in the liver. In addition, it can be obtained using the dual- or triple-echo gradient recalled-echo sequences, which can be used easily and quickly in children[21]. However, there is limited information regarding the utility of hepatic fat quantification MR in children with NASH, and no studies have used this technique to monitor treatment effects in these patients. Therefore, the purpose of this study was to evaluate the possibility of treatment monitoring with hepatic fat quantification MR as a non-invasive and quantitative method in pediatric NASH.
This retrospective study was approved by the Institutional Review Board of our hospital (1-2014-0007) and the acquisition of informed consent for review of patients’ images and medical records was waived. We declare that none of the authors of the present study has any competing commercial, personal, political, intellectual or religious interest with respect to the present study. We reviewed the medical records of 27 pediatric patients referred for NASH between January 2011 and December 2013. Diet and a physical exercise education program for the reduction of body weight were provided carefully and continuously to all patients. A low-calorie diet (25-30 kcal/kg per day) composed of specified quantities of fat (25%-30%), carbohydrate (50%-60%) and protein (15%-20%) was recommended. Clinical nutritionists in our hospital guided the diet planning and a moderate exercise program (1 h/d at least 5 d a week). Vitamin E (daily dose 800 IU) was also recommended to all patients to improve liver histology[22]. Compliance with the diet and exercise treatment regime was checked at every clinic visit from daily records about feeding, exercise, and medication history written by parents and caregivers. Treatments other than diet, physical exercise education, and vitamin E were not provided. Sex, age at the time of diagnosis, body weight, body mass index (BMI), and laboratory data were collected. Hepatic fat quantification MR was performed at the initial referral and 1 year later for follow-up. We reviewed body weight, BMI, and laboratory results at the time of MR acquisition. Laboratory results included aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, alkaline phosphatase (ALP), cholesterol, triglycerides, high density lipoprotein (HDL), and low density lipoprotein (LDL) levels.
We retrospectively reviewed hepatic fat quantification MR of NASH patients. The hepatic fat fraction (%) was measured using dual- and triple-echo gradient-recalled-echo sequences on a 3T MR system (Tim Trio; Siemens Medical Solutions, Erlangen, Germany) with a phased body array coil. For dual-echo chemical-shift gradient-echo MR, we obtained axial images of the liver using gradient echo T1-weighted, dual-echo, in-phase, and opposed-phase sequences [TR 226 ms; TE 1.23 (opposed-phase) and 2.46 (in-phase) ms; flip angle 65°; section thickness 6 mm; matrix size 192 × 256; and field of view 300 cm × 400 cm]. For triple-echo MR, the imaging protocol included a breath-hold low-flip-angle T1-weighted, triple-echo, spoiled gradient-echo sequence [TR 226 ms; TE 2.46 (in-phase 1), 3.69 (opposed-phase), and 4.92 (in-phase 2) ms; flip angle 20°; section thickness 6 mm; matrix size 256 × 192; and field of view 315 cm × 420 cm].
One radiologist (Lee MJ), who had 10 years’ experience in pediatric radiology, randomly drew 2 regions of interest (ROIs) that avoided hepatic vessels in the homogenous parenchyma of the right hepatic lobe on a picture archiving and communication system (Centricity, General Electric Corporation, Milwaukee, WI, United States). The reviewer obtained signal intensity at the same level of in-phase (IP) and opposed-phase (OP) images. The hepatic fat fraction in dual-echo MR was calculated from the following equation: Dual-echo fat fraction (%) = [(IP-OP)/(2 × IP)] × 100[23].
Theoretically, three ROIs should be obtained with the same method used to measure the triple-echo fat fraction. The signal intensities can be obtained at the same level IP1, OP, and IP2 images. The hepatic fat fraction in triple-echo MR can be calculated from the equation as follows: Triple-echo fat fraction (%) ≤ [(IP1 + IP2)/2 - OP]/(IP1 + IP2) > × 100[24].
In our workstation, the triple-echo fat fraction was automatically calculated and we obtained a triple-echo fat map for direct fat fraction measurement in a single ROI.
Patients who followed the educational recommendations and showed marked changes in BMI, AST, and ALT over 1 year under observation were defined as the compliant group. Patients who did not follow the recommendations well were defined as the non-compliant group. Statistical analysis was performed using SPSS version 20.0.0 (IBM Corp., Armonk, NY, United States). We compared clinical, biochemical, and radiological parameters between the compliant group and the non-compliant group. Continuous variables, including age, body weight, BMI, laboratory results, and hepatic fat fractions on MR, were analyzed with the Mann-Whitney U test. The difference in sex between the 2 groups was analyzed using Fisher’s exact test. To compare the results of the initial study with 1 year follow-up, the Wilcoxon signed-rank test was used. Spearman correlation analysis between body weight or BMI change and fat fraction change measured on MR was also performed. P values < 0.05 were considered statistically significant. The statistical methods of this study were reviewed by Ha Yan Kim from Biostatistics Collaboration Unit, Yonsei University College of Medicine.
Twenty seven children with NASH underwent fat quantification MR during the study period. All children underwent both a pre-treatment and a follow-up MR and were included in this study. There were 24 boys and 3 girls, with a mean age of 12.0 ± 2.3 years (range, 9-19 years). There were 22 patients (81.5%) in the compliant group and 5 (18.5%) in the non-compliant group. Table 1 demonstrates the baseline findings in all children. There were no significant differences between the groups except for the triglyceride level, which was higher in the compliant group (167.7 mg/dL) than in the non-compliant group (74.2 mg/dL) (P = 0.001).
| All children (n = 27) | Compliant group (n = 22) | Non-compliant group (n = 5) | P value1 | ||
| Demographics | Male, n (%) | 24 (88.9) | 20 (90.9) | 4 (80.0) | NS |
| Age (yr) | 12.0 ± 2.3 | 11.8 ± 1.9 | 12.8 ± 3.7 | NS | |
| B.wt (kg) | 66.2 ± 16.0 | 65.4 ± 16.5 | 70.0 ± 14.5 | NS | |
| BMI (kg/m2) | 26.6 ± 3.4 | 28.8 ± 3.6 | 25.9 ± 2.5 | NS | |
| Laboratory findings | AST (IU/L) | 79.9 ± 79.4 | 83.0 ± 85.8 | 66.0 ± 44.0 | NS |
| ALT (IU/L) | 138.7 ± 112.0 | 148.6 ± 119.2 | 95.2 ± 63.7 | NS | |
| T.Bil (mg/dL) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | NS | |
| Albumin (g/dL) | 4.6 ± 0.2 | 4.6 ± 0.2 | 4.5 ± 0.2 | NS | |
| ALP (IU/L) | 208.6 ± 77.3 | 210.5 ± 73.8 | 200.4 ± 100.8 | NS | |
| Cholesterol (mg/dL) | 187.9 ± 30.0 | 192.6 ± 30.0 | 167.0 ± 21.0 | NS | |
| TG (mg/dL) | 150.4 ± 74.1 | 167.7 ± 71.1 | 74.2 ± 16.4 | 0.001 | |
| HDL (mg/dL) | 45.0 ± 8.3 | 44.9 ± 9.0 | 45.2 ± 4.7 | NS | |
| LDL (mg/dL) | 120.5 ± 30.3 | 123.3 ± 32.0 | 108.0 ± 18.5 | NS | |
| Hepatic fat fraction on MR | Dual-echo sequence (%) | 34.2 ± 8.5 | 34.9 ± 8.2 | 31.3 ± 9.8 | NS |
| Triple-echo sequence (%) | 22.8 ± 7.3 | 23.6 ± 7.2 | 19.5 ± 7.4 | NS |
Table 2 shows the results of the initial and follow-up findings in the 2 groups. Body weight did not change significantly in either group. However, BMI decreased only in the compliant group (from 28.8 kg/m2 to 26.3 kg/m2, P = 0.016). Almost all laboratory results except albumin and ALP changed significantly during follow-up in the compliant group. AST, ALT, cholesterol, triglycerides, and LDL decreased, and HDL increased in the compliant group, which reflected the treatment effect. However, no laboratory results changed during follow-up in the non-compliant group.
| Compliant group (n = 22) | Non-compliant group (n = 5) | |||||
| Initial | After 1 yr | P-value1 | Initial | After 1 yr | P value1 | |
| Male, n (%) | 20 (90.9) | 4 (80.0) | ||||
| Age (yr) | 11.8 ± 1.9 | 12.8 ± 3.7 | ||||
| B.wt (kg) | 65.4 ± 16.5 | 67.3 ± 14.7 | NS | 70.0 ± 14.5 | 72.3 ± 15.0 | NS |
| BMI (kg/m2) | 28.8 ± 3.6 | 26.3 ± 3.4 | 0.016 | 25.9 ± 2.5 | 26.4 ± 2.7 | NS |
| AST (IU/L) | 83.0 ± 85.8 | 29.2 ± 20.0 | 0.002 | 66.0 ± 44.0 | 50.0 ± 37.5 | NS |
| ALT (IU/L) | 148.6 ± 119.2 | 37.9 ± 41.8 | < 0.001 | 95.2 ± 63.7 | 84.6 ± 63.5 | NS |
| T.Bil (mg/dL) | 0.6 ± 0.3 | 0.7 ± 0.4 | 0.015 | 0.6 ± 0.3 | 0.70 ± 0.37 | NS |
| Albumin (g/dL) | 4.6 ± 0.2 | 4.6 ± 0.3 | NS | 4.5 ± 0.2 | 4.70 ± 0.30 | NS |
| ALP (IU/L) | 210.5 ± 73.8 | 202.1 ± 93.5 | NS | 200.4 ± 100.8 | 153.6 ± 71.6 | NS |
| Cholesterol (mg/dL) | 192.6 ± 30.0 | 174.5 ± 28.2 | 0.001 | 167.0 ± 21.0 | 170.8 ± 22.5 | NS |
| TG (mg/dL) | 167.7 ± 71.1 | 106.4 ± 39.8 | < 0.001 | 74.2 ± 16.4 | 85.4 ± 20.1 | NS |
| HDL (mg/dL) | 44.9 ± 9.0 | 48.4 ± 9.6 | < 0.001 | 45.2 ± 4.7 | 46.0 ± 4.5 | NS |
| LDL (mg/dL) | 123.3 ± 32.0 | 100.3 ± 26.3 | < 0.001 | 108.0 ± 18.5 | 105.0 ± 30.8 | NS |
| Dual-echo sequence (%) | 34.9 ± 8.2 | 15.8 ± 8.2 | < 0.001 | 31.3 ± 9.8 | 35.9 ± 8.9 | NS |
| Triple-echo sequence (%) | 23.6 ± 7.2 | 10.1 ± 4.6 | < 0.001 | 19.5 ± 7.4 | 22.9 ± 5.6 | NS |
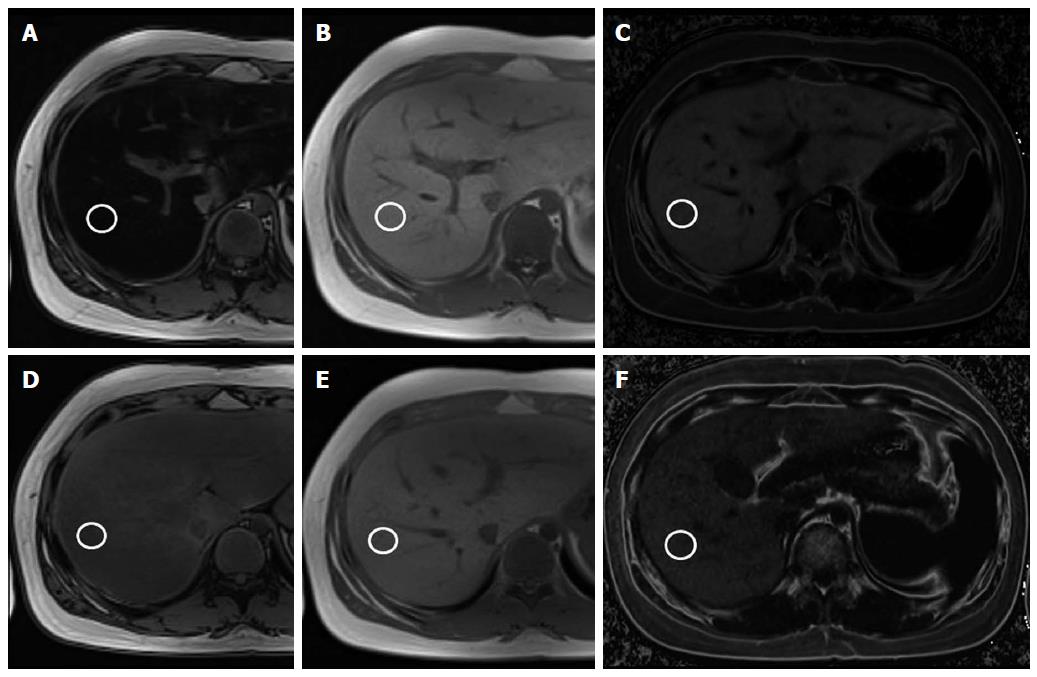
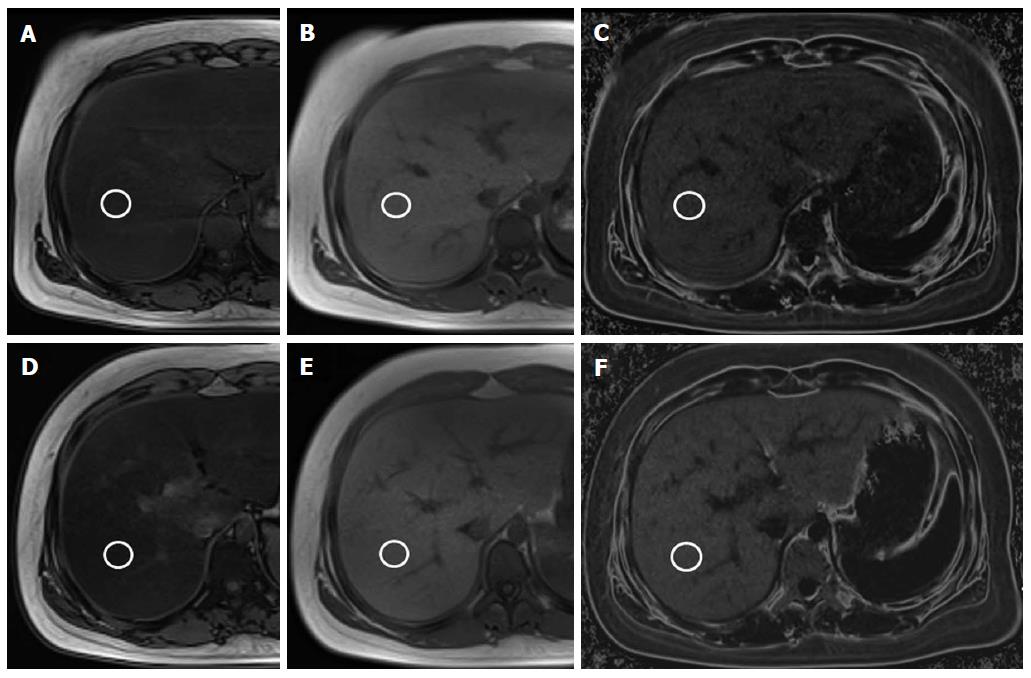
On hepatic fat quantification MR, both dual- and triple-echo fat fractions were decreased in the compliant group (P < 0.001) (Figure 1). The mean initial hepatic fat fraction was 34.9% on dual-echo MR and 23.6% on triple-echo MR, and it decreased to 15.8% on dual-echo MR and to 10.1% on triple-echo MR in the compliant group. The follow-up MR showed increased mean fat fraction in the non-compliant group on both dual-echo MR (from 31.3% to 35.9%, P > 0.05) and triple-echo MR (from 19.5% to 22.9%, P > 0.05) (Figure 2).
The 2 groups differed in both dual-echo fat fraction (-19.2% vs 4.6%, P < 0.001) and triple-echo fat fraction (-13.4% vs 3.5%, P < 0.001), with a decreased fat fraction observed only in the compliant group. Among the laboratory results, ALT (-110.7 IU/L vs -10.6 IU/L, P = 0.047), cholesterol (-18.1 mg/dL vs 3.8 mg/dL, P = 0.016), and triglycerides (-61.3 mg/dL vs 11.2 mg/dL, P = 0.013) differed between the 2 groups.
In the correlation analysis which included all patients, the BMI change and the dual-echo fat fraction change showed a positive correlation (ρ = 0.418, P = 0.030). However, the BMI change and triple-echo fat fraction change was not correlated (ρ = 0.316, P = 0.109). The body weight change and fat fraction change also did not show a significant correlation in both dual-echo (ρ = 0.213, P = 0.285) and triple-echo (ρ = 0.135, P = 0.501) sequences.
Dual- and triple-echo gradient recalled-echo sequences can easily perform noninvasive assessment and quantitative measurement of hepatic fat, even in children. Our study demonstrated that not only clinical and laboratory results but also fat fraction on MR improved in children with good treatment compliance. These results suggest the possible usefulness of fat quantification MR as a noninvasive and quantitative tool for monitoring treatment effects in children with NASH.
Ultrasonography is the favored method for evaluating hepatic disease in children because results are easily obtainable and it is noninvasive. Disturbed propagation of ultrasound waves can occur when there are lipid droplets within hepatocytes[5]. This causes the scattering of the waves, which creates more returning echoes to the transducer. Therefore, we can qualitatively determine fatty infiltration in the liver when the liver parenchymal echogenicity is increased when compared with the kidney. Shannon et al[16] performed a cohort study of pediatric patients with biopsy-proven NAFLD and demonstrated excellent correlation between the ultrasonographic steatosis score and the histologic grade of steatosis. However, Bohte et al[17] showed the limitations of ultrasonography in severely obese adolescents when evaluating the severity of hepatic steatosis. In addition, a recent systemic review for imaging liver fat in children concluded that ultrasonography had a low positive predictive value of 47%-62% for the diagnosis of fatty liver[5].
MR imaging is one of the most sensitive tools used to evaluate fat infiltration in the liver. Both MR imaging and MR spectroscopy can accurately and reproducibly measure hepatic fat[25]. However, MR spectroscopy evaluates only a small portion of the liver and requires a long examination time and expertise for data acquisition and analysis[23,25]. When using MR imaging, the degree of fat infiltration can be estimated by using chemical shift imaging[20]. The term chemical shift refers to the difference in precessional (or resonance) frequency between 2 proton MR signals, expressed in parts per million of the resonance frequency of the static magnetic field B0. This technique uses differences in the resonance frequencies of water and lipids to differentiate tissues containing only water from those containing both water and lipids[5]. The characteristic resonance frequencies of fat and water and the detection of a fat-specific frequency allows for quantitative measurement of hepatic steatosis. Therefore, while normal liver parenchyma exhibits similar signal intensity on IP and OP images, the fatty liver shows diminished signal intensity on OP images, as seen in our study.
The dual-echo technique is available on most clinical imagers with a field strength of 1.0T or higher. We can obtain both IP and OP images after a single radiofrequency excitation using this technique, allowing rapid acquisition of images during a single breath hold[23]. There are many reports about the utility of this technique in evaluating hepatic steatosis in adults[26]. However, there is only one report of the dual-echo technique being performed in children. In 2011, Pacifico et al[21] demonstrated a strong correlation between the dual-echo MR fat fraction and the histologic grade of hepatic steatosis among 25 children with NAFLD. No studies have yet evaluated the utility of this technique for monitoring treatment effects in children with NASH. This is the first study detecting longitudinal changes in hepatic fat in pediatric NASH, even though we did not perform histologic correlation as biopsy is not considered a proper method for treatment monitoring, especially in children. We demonstrated that not only clinical and laboratory results but also fat fraction on MR improved in children with good treatment compliance. We also showed that the BMI change and the dual-echo fat fraction change had a positive correlation. Therefore, the dual-echo technique can be a useful monitoring tool in children with fatty liver disease.
Triple-echo MR imaging is somewhat similar to dual-echo MR imaging. However, the triple-echo technique acquires a second IP image in addition to the first OP and IP images. The signal intensities of the first OP and IP images are corrected for the T2* effect using the T2* time estimated from the signal decay between the first and second IP images[25]. The dual-echo technique cannot correct for potentially confounding T2* relaxation effects, which should considered in cases with elevated liver iron content, such as cirrhosis and hemochromatosis[23,27]. Qayyum et al[28] demonstrated that fat quantification determined with chemical shift imaging correlated better with the histopathologically determined percentage of fat in non-cirrhotic patients compared with cirrhotic patients.
Several recent reports evaluated the triple-echo technique for quantification of hepatic steatosis in adults[29,30]. Hwang et al[29] demonstrated a correlation between both triple-echo MR imaging and MR spectroscopy with macrovesicular steatosis. They suggested cutoff values of 4.93% and 5.79% for the detection of substantial macrovesicular steatosis for both techniques, respectively, without a significant difference. Wu et al[30] compared dual-echo MR, triple-echo MR, and MR spectroscopy for the evaluation of hepatic steatosis and found that triple-echo MR imaging had the highest correlation. However, no studies evaluated triple-echo MR imaging in children with NASH. Our study demonstrated that both the dual-echo and triple-echo techniques showed a significant decrease in hepatic fat fraction after treatment. The mean fat fraction decreased from 34.9% to 15.8% with the dual-echo sequence and from 23.6% to 10.1% with the triple-echo sequence in the compliant group. However, we could not evaluate the effect of hepatic iron content or fibrosis between the 2 sequences in these patients. Further study is required to compare the accuracy of these sequences in children with NAFLD.
Our study has several limitations. First, diagnosis and improvement during NASH follow-up were not proven pathologically. We could not directly correlate or compare the MR fat fraction with histologic grades of fatty infiltration. We also could not evaluate or consider hepatic fibrosis in our patients. However, liver biopsy is not a routine examination in children with NASH and is not appropriate for treatment monitoring. The second limitation was the small number of patients with a short-term follow-up period. We included only children with NASH who underwent both a pre-treatment and a 1-year follow-up MR for hepatic fat quantification during the study period. Even though all children in the compliant group showed decreased hepatic fat fraction on both sequences in this short period of follow-up, long-term monitoring is mandatory in these patients. Additional prospective studies with a large number of children and long-term follow-up are needed to validate our results. The third limitation was that chemical shift MR imaging cannot differentiate the water dominant and fat dominant liver[23]. In fact, this technique cannot evaluate a fatty liver with more than a 50% fat component. However, a hepatic fat fraction of more than 50% is rare; in a study of over 2000 patients by Szczepaniak et al[31], no patients had a fat fraction exceeding 50%. Therefore, this may be only a theoretical concern.
In conclusion, hepatic fat quantification MR can be a useful tool to non-invasively and quantitatively monitor treatment effects in pediatric NASH. Furthermore, it can also help reduce the number of unnecessary biopsies in these patients.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children. Prompt diagnosis and suitable treatment are necessary to prevent hepatic fibrosis and cirrhosis. In this study, we evaluated the possibility of treatment monitoring with hepatic fat quantification magnetic resonance (MR) as a non-invasive and quantitative method in pediatric nonalcoholic steatohepatitis (NASH).
Even though liver biopsy is the modality of choice for NAFLD diagnosis, it is invasive and not favored especially in children. Therefore, there is a need to develop noninvasive methods to accurately assess and monitor the progression of NAFLD for children.
There is debate about the modality of choice for the diagnosis and monitoring of the NAFLD in pediatric patients. Non-invasive imaging studies such as hepatic fat quantification MR could be used to quantitatively monitor treatment effects in pediatric NAFLD. However, there is limited information regarding the utility of hepatic fat quantification MR in children with NAFLD.
Quantitative measurement of the fat fraction using a chemical shift technique on MR can be an accurate and easy method to evaluate hepatic fat, even in children. We need to validate the use of this technique not only for NAFLD diagnosis but also for treatment monitoring.
Fat quantification MR using a chemical shift technique can evaluate fat in the liver by distinguishing resonant frequencies between fat and water. Both dual- and triple-echo gradient recalled-echo sequences can be used for this purpose. The dual-echo sequence can be obtained with a single radiofrequency excitation, allowing rapid acquisition of images during a single breath hold. The triple-echo sequence can correct for potentially confounding T2* relaxation effects, which should considered in cases with elevated liver iron content, such as cirrhosis and hemochromatosis.
This is an interesting research study which evaluated the utility of hepatic fat quantification MR as a non-invasive and quantitative method for treatment monitoring in pediatric NASH. This study compared the compliant and non-compliant groups and demonstrated fat fraction and laboratory findings change only in the compliant group. These results suggest that hepatic fat quantification MR can be used in the treatment monitoring in pediatric NASH.
| 1. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 872] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1071] [Article Influence: 53.6] [Reference Citation Analysis (1)] |
| 4. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 664] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 5. | Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 506] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 9. | Kawanaka M, Nishino K, Nakamura J, Suehiro M, Goto D, Urata N, Oka T, Kawamoto H, Nakamura H, Yodoi J. Treatment of nonalcoholic steatohepatitis with vitamins E and C: a pilot study. Hepat Med. 2013;5:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Bell LN, Wang J, Muralidharan S, Chalasani S, Fullenkamp AM, Wilson LA, Sanyal AJ, Kowdley KV, Neuschwander-Tetri BA, Brunt EM. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Castéra L, Nègre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 734] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Kim SY, Seok JY, Han SJ, Koh H. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr. 2010;51:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 15. | Bonny C, Rayssiguier R, Ughetto S, Aublet-Cuvelier B, Baranger J, Blanchet G, Delteil J, Hautefeuille P, Lapalus F, Montanier P. [Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region]. Gastroenterol Clin Biol. 2003;27:1021-1025. [PubMed] |
| 16. | Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, Feldstein AE, Nobili V. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr. 2011;53:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Bohte AE, Koot BG, van der Baan-Slootweg OH, van Werven JR, Bipat S, Nederveen AJ, Jansen PL, Benninga MA, Stoker J. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology. 2012;262:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, Lee KH, Yu ES, Hwang S, Lee SG. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, Saab S, Lu DS. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Ma X, Holalkere NS, Kambadakone R A, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253-1277. [PubMed] |
| 21. | Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012-3019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Pearlman M, Loomba R. State of the art: treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2014;30:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, Hamilton G, Chavez AD, Schwimmer JB, Sirlin CB. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231-260. [PubMed] |
| 24. | Guiu B, Loffroy R, Petit JM, Aho S, Ben Salem D, Masson D, Hillon P, Cercueil JP, Krause D. Mapping of liver fat with triple-echo gradient echo imaging: validation against 3.0-T proton MR spectroscopy. Eur Radiol. 2009;19:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392-7402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (2)] |
| 26. | Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, Brenner D, Sirlin C, Loomba R. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Westphalen AC, Qayyum A, Yeh BM, Merriman RB, Lee JA, Lamba A, Lu Y, Coakley FV. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience. Radiology. 2005;237:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 29. | Hwang I, Lee JM, Lee KB, Yoon JH, Kiefer B, Han JK, Choi BI. Hepatic steatosis in living liver donor candidates: preoperative assessment by using breath-hold triple-echo MR imaging and 1H MR spectroscopy. Radiology. 2014;271:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Wu CH, Ho MC, Jeng YM, Hsu CY, Liang PC, Hu RH, Lai HS, Shih TT. Quantification of hepatic steatosis: a comparison of the accuracy among multiple magnetic resonance techniques. J Gastroenterol Hepatol. 2014;29:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Akarsu M, Chuang WL, Savopoulos CG S- Editor: Wang JL L- Editor: Cant MR E- Editor: Zhang DN