Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9717
Peer-review started: April 26, 2015
First decision: May 18, 2015
Revised: June 5, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 7, 2015
Processing time: 135 Days and 17.3 Hours
Cancer-associated fibroblasts (CAFs) are important components of various types of tumors, including gastric cancer (GC). During tumorigenesis and progression, CAFs play critical roles in tumor invasion and metastasis via a series of functions including extracellular matrix deposition, angiogenesis, metabolism reprogramming and chemoresistance. However, the mechanism of the interaction between gastric cancer cells and CAFs remains largely unknown. MicroRNAs (miRNAs) are a class of non-coding small RNA molecules, and their expression in CAFs not only regulates the expression of a number of target genes but also plays an essential role in the communication between tumor cells and CAFs. In this review, we provide an overview of recent studies on CAF miRNAs in GC and the relevant signaling pathways in gastrointestinal tumors. Focusing the attention on these signaling pathways may help us better understand their role in tumor invasion and metastasis and identify new molecular targets for therapeutic strategies.
Core tip: Gastric cancer (GC) is one of the most common cancers worldwide. GC usually metastasizes to distant organs in advanced stages. Cancer-associated fibroblasts (CAFs) play an important role in GC invasion and metastasis. Therefore, a better understanding of the special interaction between GC cells and CAFs may be useful for identifying the underlying mechanisms of tumor progression.
- Citation: Yan Y, Wang LF, Wang RF. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J Gastroenterol 2015; 21(33): 9717-9726
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9717
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer deaths worldwide[1]. More than 70% of GC cases occur in Asia, of which half are in China[2]. Although a recent study has reported that the death and adverse event rates for GC patients under surgical care have decreased[3], the morbidity of GC remains high in Asia, and it is the third most common cancer after breast and lung cancers[4].
Patients with precancerous lesions and early GC typically have no obvious symptoms. Many GC individuals are diagnosed at an advanced stage. Due to a lack of appropriate diagnostic biomarkers and personalized anti-cancer treatment, the survival rate of GC patients is poor[2]. In advanced stages of GC, tumor cells have invaded into the blood or lymphatic vessels and metastasized to distant organs. Multiple steps and factors are involved in the progression towards advanced stages of GC. One of the most crucial factors is the bidirectional interaction between tumor cells and their microenvironment[5].
It is well recognized that the tumor microenvironment (TME) plays an important role in tumor progression. The TME is a complex tissue environment, composed of the extracellular matrix (ECM) and various types of stromal cells, such as cancer-associated fibroblasts (CAFs), macrophages, inflammatory cells, and mesenchymal stem cells[6]. All of these factors, but especially CAFs, make tremendous contributions to tumor growth and metastasis. In this review, we elaborate some novel and valuable results of recent studies regarding CAFs in GC and also highlight possible research directions for future studies.
CAFs, which are characterized by multiple specific markers, are most frequently reported to overexpress α-smooth muscle actin (α-SMA) and fibroblast-activated protein (FAP), whereas caveolin-1 (Cav-1) typically shows reduced expression[7-9]. CAFs have been extracted from different types of human carcinomas, including pancreatic and gastric[9,10]. CAFs are the most prominent components of the TME in tumor tissue and play an essential role in tumor-stromal interactions[5].
CAFs are spindle-shaped, blast-like cells, and a number of reports have stated that they originate from cells through a variety of different mechanisms[11]. Several experimental studies have reported that mesenchymal stem cells (MSCs) are a significant source of CAFs. Zhu et al[12] found that GC-MSCs-primed neutrophils could induce MSCs to gradually differentiate into CAFs in vitro. Gu also discovered that GC cells activate human umbilical cord-derived MSCs (hucMSCs) and induce them to differentiate into CAFs by stimulating TGF-β/Smad signaling[9]. Recent studies also have demonstrated that CAFs are generated during the epithelial mesenchymal transition (EMT) via tumor-associated endothelial cells, which delaminate from blood vessels to generate mesenchymal cells with multiple-differentiation potential[6]. Moreover, increased levels of TGF-β in the microenvironment can induce resident tissue fibroblasts to acquire a CAF phenotype, which is associated with the up-regulation of α-SMA and the down-regulation of CD34[7,13]. Our previous study demonstrated that fibroblasts from the gastric cancer invasive front (interface zone fibroblasts, INFs) have a strongly positive FAP expression[14].
In general, the CAF phenotype is distinct from normal fibroblasts (NFs). CAFs can overexpress a wide range of factors, such as cytokines, growth factors and chemokines (MMPs, TGF-β, MCT4, VEGF, HGF, IL-22) that are critical to induce the deposition of ECM, promote angiogenesis and EMT, regulate metabolic reprogramming, and enhance proliferation and chemotherapy resistance[11,15,16]. These CAF functions play important roles in cancer progression and promote tumor cell invasion and metastasis.
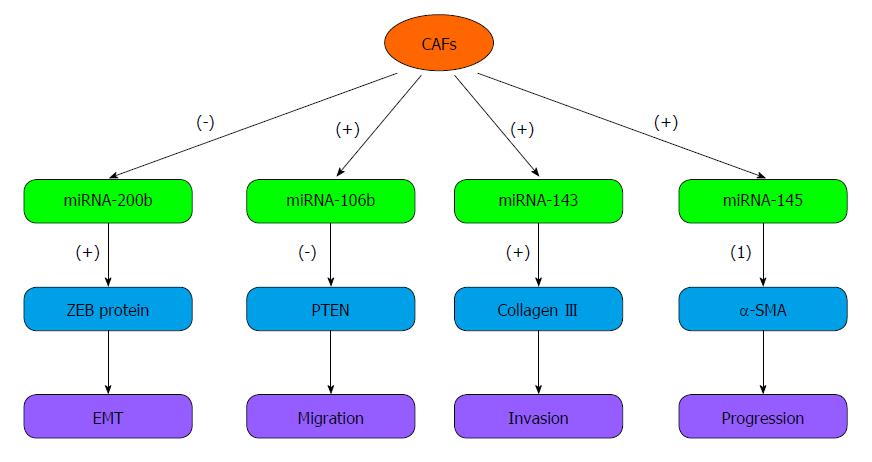
MicroRNAs (miRNAs) are a class of non-coding small RNA molecules that play a key role in regulating the expression of target genes at the post-transcriptional level[17]. In recent decades, miRNAs have become a topic of interest in oncology. Many studies have suggested that miRNAs affect tumor growth, invasion and metastasis. Some miRNAs have been reported as novel diagnostic biomarkers and as new therapeutic targets for tumors such as gastric cancer, breast cancer and others[18,19]. Many studies have demonstrated that miRNAs, such as miR-20b, miR-20a, miR-17, and miR-382, are expressed by tumor cells in various types of tumors[20,21]. Recently, miRNAs related to CAFs have aroused increased attention. Several reports have shown that miRNAs also play critical roles in the CAFs of various human cancers. miRNAs can not only orchestrate the expression of target genes, but also promote tumor invasion and migration. Next, we summarize the recent research on the miRNAs in CAFs that are involved in GC invasion and metastasis (Figure 1) and discuss future prospects.
miRNA-106b is a member of the miRNA-106b~25 cluster that plays oncogenic roles in tumors via impacting tumor cell proliferation, apoptosis, and the cell cycle in vitro and tumorigenesis in vivo[22-24]. Prasad et al[24] showed that in melanoma cells, miRNA-106b is markedly up-regulated and acts as an oncogene to enhance cell proliferation. Exposure of melanoma cells to grape seed proanthocyanidins (GSPs), an inhibitor of miRNA-106b, can down-regulate miRNA-106b levels and inhibit cell proliferation by blocking the cell cycle. In a large-scale analysis, plasma concentration of miRNA-106b was notably higher in GC patients than in controls and dramatically decreased in post-operative samples compared with pre-operative samples[25].
In addition, miRNA-106b can also be overexpressed in CAFs. Yang et al[19] showed that miRNA-106b levels are increased in CAFs compared with NFs established from patients with GC. A decrease in miRNA-106b expression in CAFs notably inhibited gastric tumor cell migration and invasion by increasing the expression of PTEN.PTEN is regarded as a tumor suppressor gene that influences the pathogenesis, invasion and metastasis of carcinomas possibly through modulating the balance between apoptosis and proliferation[26]. Lost or decreased expression of PTEN protein occurs commonly in GC tumorigenesis and progression[27].
Twist is a helix-loop-helix transcription factor that includes two twist-like proteins, Twist-1 and Twist-2[28]. Accumulating evidence indicates that Twist-1 is a key regulator in the process of tumor cell invasion and metastasis by inducing EMT[29]. In gastric CAFs, Twist-1 expression is up-regulated, and its expression facilitates GC progression and poor clinical outcomes[30]. Woo et al[31] demonstrated that Twist-1 expression is induced by the IL6/STAT3 axis. Furthermore, the authors also revealed that Twist-1 is an important regulator in suppressing the senescence of CAFs and NFs. Knockdown of Twist-1 gene expression in the rat choroid plexus epithelial cell line Z310 markedly reduced tumor cell proliferation and invasion[32].
However, it has been reported that miRNA-106b could down-regulate Twist-1 expression via its direct interaction with Twist-1 mRNA at the 3′-untranslated region (3’-UTR) to suppress EMT-associated endometrial tumor cell invasion[33]. This result is in accordance with miRNA-106b expression in renal cell carcinoma, where it is over-expressed in tumor tissue but significantly lower in the tumors of patients with metastasis[34]. Therefore, these results suggest that miRNA-106b may be a more complex miRNA and have multiple effects in different types of tumor cells.
It is well known that miRNA-143 serve as a tumor suppressor. In GC cell lines, miRNA-143 expression is significantly decreased. Increasing miRNA-143 expression can suppress cancer cell growth and induce apoptosis by targeting cycloxygenase-2 (COX-2)[35]. COX-2 and VEGF interact together to induce local angiogenesis and promote tumorigenesis and metastasis[36]. Down-regulation of COX-2 expression can significantly promote apoptosis and inhibit proliferation, migration and invasion of human gastric cancer cells[37]. Naito et al[38] also discovered that DNA methylation might cause the transcriptional inactivation of miRNA-143 to suppress its expression in GC cells. Using 5-aza-2-deoxycytidine to treat GC cell lines can restore miRNA-143 expression and inhibit cancer cell invasion.
Nevertheless, several studies have suggested that miRNA-143 plays a dual role in cancer, and its expression in tumor stromal cells may support tumor progression. Naito discovered that miRNA-143 is overexpressed in CAFs derived from diffuse type GC compared with NFs. The authors found that miRNA-143 promoted gastric cancer cell invasion by regulating the expression of collagen type III in CAFs[38]. Collagen type III is an extracellular matrix protein in the soft tissue tumor that significantly increases tumor cell migration and invasion in a dose-dependent manner[39]. Collagen III and fibronectin are up-regulated by the TGF-β/Smad pathway in mesothelial cells. This effect can increase GC cell adhesion to mesothelial cells and promote GC cell peritoneal metastasis[40]. Transfection of an miRNA-143 inhibitor can down-regulate miRNA-143 expression significantly, and the induction of collagen type III in fibroblasts is suppressed[38].
miRNA-145, another well-known non-coding small RNA located on chromosome 5, is suggested to be co-transcribed with miRNA-143[41]. These molecules can work together to target a group of transcription factors, such as Kruppel-like factor 4(KLF4), myocardin and ELK-1, to induce differentiation and repress proliferation of smooth muscle cells[42]. Similar to miRNA-143, a low level of miRNA-145 in cancer cells induces cell proliferation through interacting with SENPI[43]. However, miRNA-145 is up-regulated by TGF-β and mainly localized in stromal fibroblasts but not in cancer cells. In addition, high expression of miRNA-145 in activated fibroblasts is viewed as a potential prognostic factor of diffuse type GC and is associated with a more advanced tumor stage and histological classification[44].
CAF, a type of complex stroma cell in TME, not only contributes to cancer cell malignant progression and metastatic dissemination by expressing miRNAs themselves, but they can also modulate the expression of miRNAs in the surrounding tumor cells.
miRNA-200b is a member of microRNA-200 family that plays a critical role in suppressing tumor invasion and regulating EMT in some types of human cancer, such as lung adenocarcinoma and breast cancer[45-47]. Its expression decreased docetaxel chemoresistance of lung adenocarcinoma cells via directly targeting E2F3. Attenuated miRNA-200b levels were demonstrated to be associated with high chemoresistance and poor prognosis[45]. Current research suggests that CAFs can down-regulate the expression of miRNA-200b, which can up-regulate ZEB expression and down-regulate CDH1 expression in epithelial cells to induce tumor cell invasion and peritoneal dissemination in GC[48]. ZEB could lead to EMT and tumor metastasis through the TGF-β-miRNA-200-ZEB network. In hepatocellular carcinoma (HCC), the lncRNA activated by TGF-β could increase ZEB1 and ZEB2 expression by binding the miRNA-200 family, including miRNA-200b. Therefore, the overexpression of ZEB1/2 induces EMT and promotes tumor invasion and metastasis[49].
Because miRNA-200b has been suggested to play a pivotal role in tumor progression, most researchers have focused on miRNA-200b in tumor cell metastasis. However, there is no information regarding its role in CAFs. At present, the effect of miRNA-200b in CAFs is still unclear. It has been reported that miRNA-143 and miRNA-145 have different expression patterns in tumor cells and CAFs. The expression of miRNA-143 and miRNA-145 is up-regulated in CAFs but down-regulated in tumor cells. Whether the miRNA-200b has a similar behavior is unclear. Further studies are needed to clarify this question.
There are various factors and steps involved in cancer progression. Multiple cytokines and intracellular signaling pathways are involved in each step of tumor progression[5]. Extracellular or intracellular factors induce target gene mutation and abnormal expression through signaling transduction pathways to influence tumor initiation and progression. Elucidating these complicated signaling pathways is essential to understanding various biological tumor behaviors[50].
It is common knowledge that TGF-β/Smad signaling is a major signaling pathway in various types of tumor cells. This signaling pathway is closely related to the malignant progression of tumors. TGF-β, as the main regulator of TGF-β/Smad signaling, can induce nuclear localization and the transcriptional activity of Smads when activated[51]. Smad, a complex protein, plays essential roles in tumor progression. Liu et al[52] demonstrated that miRNA-130a/301a/454 share the same 3’-UTR binding seed sequence, and these molecules reduce the expression level of Smad4 protein, which directly correlates to the development of colon cancer.
When TGF-β/Smad signaling is activated, the downstream factors, such as MMPs, plasminogen activator inhibitor (PAI)-1 and TGF-β1 itself, are largely overexpressed[53]. TGF-β1 is closely related to tumor invasion and metastasis and can modulate its downstream transcription factor KLF8 to induce EMT in gastric cancer cells[54]. Moreover, KLF8 increases anti-apoptotic Bcl-2 and decreases pro-apoptotic Bax and caspase-3 expression in the SEC7901 cell line[55]. Treatment of tumor cells with the Chinese herbs Scutellariabaicalensis and Fritillariacirrhosa markedly block cancer cell proliferation and invasion through inhibiting the TGF-β/Smad pathway, which is accompanied by the down-regulation of the expression of Snail, Slug, and MMPs[56].
The Ras signaling pathway plays an important role in human cancers and is now considered a potential target for tumor treatment[57]. The activated Ras stimulates downstream signaling cascades to complete the link between the cell surface and the nucleus[58]. Some experimental data have indicated that K-Ras, the most frequently mutated Ras isoform, is a key factor in cell growth, angiogenesis, tumorigenesis and progression[59]. A recent study found that YAP1 and K-Ras converged specifically on the transcription factor FOS and then coordinately activated the EMT program[60]. Moreover, ZNF312b could promote the transcriptional activation of the K-Ras gene and accelerate GC cell proliferation by binding ZNF312b in the ZNF-binding region of K-Ras promoter[61]. Therefore, inhibition of K-Ras activation is considered a significant approach in anticancer research. In colon cancer, the p38γ inhibitor pirfenidone preferentially reduced mutated K-Ras protein expression in tumor tissues and restrained the xenograft growth of K-Ras-dependent colon cancers in nude mice[62].
The JAK/STAT signaling pathway conveys information from the membrane to the nucleus to orchestrate target gene expression[63]. Cytokines bind to a specific receptor on the cell surface and recruit JAKs. The JAK molecules are recruited and activated by cytokine receptors, leading to the phosphorylation of the downstream STAT proteins. Once STAT proteins are activated, they dissociate from the receptor and rapidly translocate from the cytoplasm into the nucleus. Then, phosphorylated STAT proteins increase or decrease the expression of target genes through recognizing and binding to specific DNA sequences[64,65].
Accumulating evidence has indicated that numerous cytokines are involved in the JAK/STAT signaling pathway, such as IL-3, IL-6, IL-21, and IL-22[12,64,66]. Their receptors are divided into four primary families: the IL-2R family; the IL-3R family; the IL-6R family; and the INF-R family[64]. IL-6 and IL-22 activate STAT3 and contribute to colorectal cancer cell (CRC) proliferation and growth. Anti-IL-6 reduces p-STAT3 Y705 expression and leads to the growth inhibition of CRC cells[67]. Furthermore, IL-6 might promote tumor cell genetic alteration through interfering hMSH3 nuclear localization and DNA repair[68].
The NF-κB transcription factor is a heterodimeric protein, which was first identified based on its interaction with the immunoglobulin light-chain enhancer in B cells[69]. In recent years, NF-κB has been considered a key link between inflammation and cancer. NF-κB activation is mainly driven by inflammatory cytokines within the TME, such as IL-6 and TNF-α, or survival genes, such as Bcl-X (L)[70]. Moreover, NF-κB cooperates with the JAK/STAT pathway to promote tumor proliferation and progression via controlling distinct or overlapping groups of downstream genes[69,71].
NF-κB is a significant transcription factor in the regulation of MMP expression. In HCC, IL-17A induced MMP2 and MMP9 expression to promote tumor invasion and metastasis via NF-κB activation[72]. NF-κB also induces EMT in mammary epithelium via ROS activation[73]. Inhibiting the NF-κB pathway can restrain tumor growth and the expression of relevant inflammatory cytokines. miRNA-181c negatively regulates the inflammatory response via inhibiting NF-κB pathway activation and down-regulating the production of proinflammatory mediators, such as IL-1β and iNOS[74]. Gallotannin suppresses the activity of the NF-κB pathway through the inhibition of IĸBα phosphorylation and degradation, which is accompanied with the low expression of NF-κB-regulated inflammatory cytokines (IL-8, TNF-α, IL-1α) and cell cycle arrest in HT-29 and HCT-166 cell lines[75].
The critical signaling pathways in gastrointestinal tumors have been reported. However, what is the most important signaling pathway involved in CAFs in GC? Recently, hypoxia signaling has been extensively reported.
Hypoxia is a universal phenomenon in solid tumors compared with normal tissues, as well as gastrointestinal cancers[76]. Hypoxia not only leads to the expression of multiple target genes but also participates in various signaling pathways. Under hypoxic conditions, HIF-1α activates the EGFR/STAT and TGF-β/Smad signaling pathways and increases the levels of their downstream targets, and this effect enhances cell proliferation and promotes EMT[77]. Therefore, hypoxia signaling has been universally acknowledged as a noteworthy pathway in tumorigenesis and progression. The foremost hypoxia-responsive protein is hypoxia-inducible factor (HIF)[76,78]. HIF is a heterodimer consisting of an oxygen-sensitive α-subunit (HIF-1α, HIF-2α or HIF-3α) and a constitutively expressed β-subunit[78,79]. In normoxia, HIF-1α is ubiquitylated and degraded by interaction with prolyl hydroxylases (PHDs) and the tumor suppressor von Hippel-Lindau (VHL) protein. However, this process is suppressed in hypoxia, resulting in HIF-1α accumulation[80]. In most tumor types, such as pancreatic cancer and nasopharyngeal cancer, HIF-1α overexpression reduces overall survival and results in a poor patient prognosis[81,82]. In addition, HIF-1α-positive expression could be a useful prognostic marker for GC[83].
A growing body of evidence has suggested that the hypoxia signaling pathway plays a critical role in tumorigenesis through inducing EMT, angiogenesis, energy metabolism and chemotherapy resistance. Survivin is an inhibitor of apoptosis protein family that is scarcely expressed in normal tissues but is overexpressed in most human cancers. HIF-1α can up-regulate survivin levels, which will cause cisplatin resistance in GC cells[84]. In addition, HIF-1α induces chemoresistance by activating the MDR1 gene. MDR1 is a tumor promoter gene that encodes for P-gp, which can reduce the intracellular concentration of chemotherapeutic drugs[55,85].
With tumor progression, the tumor gradually exhibits a hypoxic and under-nourished niche. To sustain growth, the formation of a large number of new blood vessels plays a pivotal role in providing sufficient nutrients and oxygen concentration for tumor cells. However, a high density of blood vessel is related to tumor metastasis[86].
Under hypoxic conditions, CAFs increase the expression levels of genes related to angiogenesis, such as VEGF and angiopoietin[87,88]. VEGF is the most important factor to induce tumor vessel formation, and VEGF overexpression promotes tumor growth[89]. Down-regulated expression of HIF-1α and VEGF can suppress tumor angiogenesis. A member of p53 family, Tap73, is a tumor suppressor that decreases HIF-1 activity via promoting HIF-1α polyubiquitination and consequent proteasomal degradation in an oxygen-independent manner. Consequently, the expression of HIF-1α downstream factors, such as VAGF-A and VAGF-R2, is decreased[90]. In contrast, human rhomboid family-1 (RHBDF1) can protect HIF-1α protein stability and activity by diminishing RACK1-HIF-1α interaction, thus decreasing HIF-1α proteasomal degradation and shifting HIF-1α protein binding to HSP90[91].
Furthermore, recent evidence has also suggested that hypoxia is a key regulatory factor of energy metabolic reprogramming of CAFs[16]. HIF-1α induces CAFs to up-regulate the expression of monocarboxylate transporter-4 (MCT4) and reduces Cav-1 expression, releasing a mass of energy metabolites (such as L-lactate and ketones) to “feed” the tumor cells[16,92]. Subsequently, the tumor cells use the energy metabolites via mitochondrial tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS), thereby producing efficient adenosine triphosphate (ATP) production and facilitating tumor growth, invasion and metastasis[16,93].
From the above, we can observe that hypoxia signaling is a fatal pathway both in tumor cells and CAFs. It appears reasonable that the relevant genes of the hypoxia signaling pathway, such as HIF-1α, may be therapeutic targets for cancer. Therefore, it is strongly suggested that hypoxia signaling may serve as the most important pathway in CAFs and will be a potential cancer therapeutic target.
Previous studies have shown that adriamycin induces chemoresistance and increases tumor cell invasion[94]. Pantoprazole, a proton pump inhibitor, suppresses adriamycin-resistant GC cell invasion via inhibiting the induction of EMT and the activation of the canonical Wnt/β-catenin signaling pathway in SGC7901/ADR cells[95].
Tumor vascularization indicates that nutrition has been reestablished. Therefore, inhibiting the formation of tumor blood vessels is an effective method to treat tumors. In recent years, VEGF has become a research topic of interest and an important therapeutic target in GC. Certain VEGF pathway target agents are used in preclinical and clinical treatment of GC[96]. For example, ramucirumab, a new monoclonal antibody VEGFR-2 antagonist, was shown to prolong survival in patients with advanced GC in a phase III clinicaltrial. In a phase 3 study, 355 patients were assigned to receive ramucirumab (n = 238) or placebo (n = 117).The median overall survival was 5.2 mo (IQR: 2.3-9.9) in patients in the ramucirumab group and higher than 3.8 mo (1.7-7.1) in those in the placebo group. However, the rate of hypertension in the ramucirumab group was higher than in the placebo group [38 (16%) vs 9 (8%)][97].
Recent clinical trials with conventional chemotherapeutic agents have shown encouraging results in GC. However, chemoresistance is a main obstacle for GC treatment with the wide application of chemotherapy agents. 5-Fluorouracil (5-Fu) is one of the most common antineoplastic agents for GC. This drug can lead to cell damage and death by influencing mRNA translation and DNA synthesis[98]. Current research has found that 5-Fu induces residual cells to differentiate into CD133+, CD326+ and CD44+CD24-subpopulation, which is associated with properties of cancer stem cells and chemoresistance. Furthermore, 5-Fu-resistant cells have enhanced BMI1 expression, which correlates with decreased recurrence-free survival compared with BMI1-negative GC patients[99].
The interaction between tumor cells and their microenvironment is regarded as a key factor in tumor invasion and metastasis. CAFs, one of the foremost components of TME, exist in all types of human cancer. Compared with tumor cells, the genotypes of CAFs are stabilized. This characteristic of CAFs may make them effective treatment targets in antitumor therapy.
For example, several FAP-related drugs have been discovered in tumor therapy by targeting CAFs, such as FAP activity inhibitors and anti-FAP antibodies[15]. In tumor-bearing mice that were vaccinated against FAP, tumor growth is significantly retarded[100]. Furthermore, Ohshio et al[101] demonstrated that inhibition of CAF function improved the antitumor immune responses in tumor tissues of tumor-bearing mouse models. The populations of the suppressor immune cells CD4+CD25+Foxp3+Tregs and CD11b+Gr-1+MDSCs are decreased in the anti-CAF therapy group compared with those in controls. Furthermore, the levels of SDF-1, PEG2 and TGF-β1 expression in tumor tissues were also dramatically reduced. However, tumor growth suppression was not observed. This result implies that the antitumor effects are complicated in CAF-targeted techniques.
In this review, we describe the role of CAFs and the relevant signaling pathways in gastric carcinoma. Various cytokines, growth factors and chemokines secreted by CAFs constitute a favorable environment that can induce tumor growth, invasion and metastasis. At present, miRNA- and signaling pathway-related studies are becoming hotspots in the oncology field. However, our current understanding of these factors in CAFs is limited. The function of miRNAs is still controversial. Therefore, it is necessary to develop several specific animal models focusing on the mechanisms of miRNAs and signaling pathways in CAFs in further research. Looking forward, perhaps some specific targets will be identified in our future studies by the accumulating successful research. Inhibition of these targets in CAFs will provide a new therapeutic direction for tumor treatment.
| 1. | Montori G, Coccolini F, Ceresoli M, Catena F, Colaianni N, Poletti E, Ansaloni L. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol. 2014;2014:912418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 3. | Young JA, Shimi SM, Kerr L, McPhillips G, Thompson AM. Reduction in gastric cancer surgical mortality over 10 years: An adverse events analysis. Ann Med Surg (Lond). 2014;3:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483-4490. [PubMed] [DOI] [Full Text] |
| 5. | Chung HW, Lim JB. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J Gastroenterol. 2014;20:1667-1680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 6. | Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4060] [Cited by in RCA: 6080] [Article Influence: 506.7] [Reference Citation Analysis (0)] |
| 7. | Calon A, Tauriello DV, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol. 2014;25:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 8. | Wang RF, Zhang LH, Shan LH, Sun WG, Chai CC, Wu HM, Ibla JC, Wang LF, Liu JR. Effects of the fibroblast activation protein on the invasion and migration of gastric cancer. Exp Mol Pathol. 2013;95:350-356. [PubMed] |
| 9. | Zhao X, He Y, Gao J, Fan L, Li Z, Yang G, Chen H. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8:e59102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 10. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 2027] [Article Influence: 168.9] [Reference Citation Analysis (1)] |
| 11. | Valcz G, Sipos F, Tulassay Z, Molnar B, Yagi Y. Importance of carcinoma-associated fibroblast-derived proteins in clinical oncology. J Clin Pathol. 2014;67:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, Mao F, Wang M, Zhu W, Qian H. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Terai S, Fushida S, Tsukada T, Kinoshita J, Oyama K, Okamoto K, Makino I, Tajima H, Ninomiya I, Fujimura T. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer. 2015;18:306-313. [PubMed] |
| 14. | Shan LH, Sun WG, Han W, Qi L, Yang C, Chai CC, Yao K, Zhou QF, Wu HM, Wang LF. Roles of fibroblasts from the interface zone in invasion, migration, proliferation and apoptosis of gastric adenocarcinoma. J Clin Pathol. 2012;65:888-895. [PubMed] |
| 15. | Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol Med. 2013;19:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 16. | Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279-289. [PubMed] |
| 18. | Gyparaki MT, Basdra EK, Papavassiliou AG. MicroRNAs as regulatory elements in triple negative breast cancer. Cancer Lett. 2014;354:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Yang TS, Yang XH, Chen X, Wang XD, Hua J, Zhou DL, Zhou B, Song ZS. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (9)] |
| 21. | Seok JK, Lee SH, Kim MJ, Lee YM. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42:8062-8072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Li F, Liu J, Li S. MicorRNA 106b~25 cluster and gastric cancer. Surg Oncol. 2013;22:e7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Li B, Shi XB, Nori D, Chao CK, Chen AM, Valicenti R, White Rde V. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate. 2011;71:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Prasad R, Katiyar SK. Down-regulation of miRNA-106b inhibits growth of melanoma cells by promoting G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1 protein. Oncotarget. 2014;5:10636-10649. [PubMed] |
| 25. | Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 514] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 26. | Li XH, Zheng HC, Takahashi H, Masuda S, Yang XH, Takano Y. PTEN expression and mutation in colorectal carcinomas. Oncol Rep. 2009;22:757-764. [PubMed] |
| 27. | Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35-39. [PubMed] |
| 28. | Wushou A, Hou J, Zhao YJ, Shao ZM. Twist-1 up-regulation in carcinoma correlates to poor survival. Int J Mol Sci. 2014;15:21621-21630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Nuti SV, Mor G, Li P, Yin G. TWIST and ovarian cancer stem cells: implications for chemoresistance and metastasis. Oncotarget. 2014;5:7260-7271. [PubMed] |
| 30. | Sung CO, Lee KW, Han S, Kim SH. Twist1 is up-regulated in gastric cancer-associated fibroblasts with poor clinical outcomes. Am J Pathol. 2011;179:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Lee KW, Yeo SY, Sung CO, Kim SH. Twist1 is a key regulator of cancer-associated fibroblasts. Cancer Res. 2015;75:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Hasselblatt M, Mertsch S, Koos B, Riesmeier B, Stegemann H, Jeibmann A, Tomm M, Schmitz N, Wrede B, Wolff JE. TWIST-1 is overexpressed in neoplastic choroid plexus epithelial cells and promotes proliferation and invasion. Cancer Res. 2009;69:2219-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Dong P, Kaneuchi M, Watari H, Sudo S, Sakuragi N. MicroRNA-106b modulates epithelial-mesenchymal transition by targeting TWIST1 in invasive endometrial cancer cell lines. Mol Carcinog. 2014;53:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Slaby O, Jancovicova J, Lakomy R, Svoboda M, Poprach A, Fabian P, Kren L, Michalek J, Vyzula R. Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J Exp Clin Cancer Res. 2010;29:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q, Dan ZL, Tian DA, Zhang P. MicroRNA-143 suppresses gastric cancer cell growth and induces apoptosis by targeting COX-2. World J Gastroenterol. 2013;19:7758-7765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Han X, Li H, Su L, Zhu W, Xu W, Li K, Zhao Q, Yang H, Liu H. Effect of celecoxib plus standard chemotherapy on serum levels of vascular endothelial growth factor and cyclooxygenase-2 in patients with gastric cancer. Biomed Rep. 2014;2:183-187. [PubMed] |
| 37. | Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang K, Li X, Sun W. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine. 2014;21:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Naito Y, Sakamoto N, Oue N, Yashiro M, Sentani K, Yanagihara K, Hirakawa K, Yasui W. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci. 2014;105:228-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Chintala SK, Sawaya R, Gokaslan ZL, Rao JS. The effect of type III collagen on migration and invasion of human glioblastoma cell lines in vitro. Cancer Lett. 1996;102:57-63. [PubMed] |
| 40. | Lv ZD, Na D, Liu FN, Du ZM, Sun Z, Li Z, Ma XY, Wang ZN, Xu HM. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res. 2010;29:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Cui SY, Wang R, Chen LB. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med. 2014;18:1913-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1310] [Cited by in RCA: 1329] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 43. | Wang C, Tao W, Ni S, Chen Q, Zhao Z, Ma L, Fu Y, Jiao Z. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci. 2015;106:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Naito Y, Yasuno K, Tagawa H, Sakamoto N, Oue N, Yashiro M, Sentani K, Goto K, Shinmei S, Oo HZ. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol Rep. 2014;32:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Zhang X, Zhang B, Gao J, Wang X, Liu Z. Regulation of the microRNA 200b (miRNA-200b) by transcriptional regulators PEA3 and ELK-1 protein affects expression of Pin1 protein to control anoikis. J Biol Chem. 2013;288:32742-32752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Li X, Roslan S, Johnstone CN, Wright JA, Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene. 2014;33:4077-4088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Kurashige J, Mima K, Sawada G, Takahashi Y, Eguchi H, Sugimachi K, Mori M, Yanagihara K, Yashiro M, Hirakawa K. Epigenetic modulation and repression of miR-200b by cancer-associated fibroblasts contribute to cancer invasion and peritoneal dissemination in gastric cancer. Carcinogenesis. 2015;36:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 49. | Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1283] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 50. | Chowdhury S, Sarkar RR. Comparison of human cell signaling pathway databases--evolution, drawbacks and challenges. Database (Oxford). 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 810] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 52. | Liu L, Nie J, Chen L, Dong G, Du X, Wu X, Tang Y, Han W. The oncogenic role of microRNA-130a/301a/454 in human colorectal cancer via targeting Smad4 expression. PLoS One. 2013;8:e55532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JH, Mesker W, ten Dijke P. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu C, Xiao X, Wu K, Nie Y, Zhang H. KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J Cancer Res Clin Oncol. 2013;139:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Zhang H, Sun L, Xiao X, Xie R, Liu C, Wang Y, Wei Y, Zhang H, Liu L. Krüppel-like factor 8 contributes to hypoxia-induced MDR in gastric cancer cells. Cancer Sci. 2014;105:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Bokhari AA, Syed V. Inhibition of Transforming Growth Factor-β (TGF-β) Signaling by Scutellaria baicalensis and Fritillaria cirrhosa Extracts in Endometrial Cancer. J Cell Biochem. 2015;116:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Ward AF, Braun BS, Shannon KM. Targeting oncogenic Ras signaling in hematologic malignancies. Blood. 2012;120:3397-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol. 2000;10:147-154. [PubMed] |
| 59. | Friday BB, Adjei AA. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127-144. [PubMed] |
| 60. | Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 648] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 61. | Song IS, Oh NS, Kim HT, Ha GH, Jeong SY, Kim JM, Kim DI, Yoo HS, Kim CH, Kim NS. Human ZNF312b promotes the progression of gastric cancer by transcriptional activation of the K-ras gene. Cancer Res. 2009;69:3131-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Qi X, Xie C, Hou S, Li G, Yin N, Dong L, Lepp A, Chesnik MA, Mirza SP, Szabo A. Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer. Oncotarget. 2014;5:4269-4282. [PubMed] |
| 63. | O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 913] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 64. | Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 65. | Quintás-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 2013;19:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 66. | Ma J, Ma D, Ji C. The role of IL-21 in hematological malignancies. Cytokine. 2011;56:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 68. | Tseng-Rogenski SS, Hamaya Y, Choi DY, Carethers JM. Interleukin 6 alters localization of hMSH3, leading to DNA mismatch repair defects in colorectal cancer cells. Gastroenterology. 2015;148:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 69. | He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 1000] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 70. | Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 647] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 71. | Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 932] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 72. | Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 73. | Cichon MA, Radisky DC. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail. Oncotarget. 2014;5:2827-2838. [PubMed] |
| 74. | Zhang L, Li YJ, Wu XY, Hong Z, Wei WS. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J Neurochem. 2015;132:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Al-Halabi R, Bou Chedid M, Abou Merhi R, El-Hajj H, Zahr H, Schneider-Stock R, Bazarbachi A, Gali-Muhtasib H. Gallotannin inhibits NFĸB signaling and growth of human colon cancer xenografts. Cancer Biol Ther. 2011;12:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM. Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer. 2005;41:2792-2805. [PubMed] |
| 77. | Kannan A, Krishnan A, Ali M, Subramaniam S, Halagowder D, Sivasithamparam ND. Caveolin-1 promotes gastric cancer progression by up-regulating epithelial to mesenchymal transition by crosstalk of signalling mechanisms under hypoxic condition. Eur J Cancer. 2014;50:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437-443. [PubMed] |
| 79. | Nauta TD, van Hinsbergh VW, Koolwijk P. Hypoxic signaling during tissue repair and regenerative medicine. Int J Mol Sci. 2014;15:19791-19815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 80. | Koh MY, Powis G. HAF : the new player in oxygen-independent HIF-1alpha degradation. Cell Cycle. 2009;8:1359-1366. [PubMed] |
| 81. | Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD, Liang TB. Hypoxia-inducible factor 1α expression and its clinical significance in pancreatic cancer: a meta-analysis. Pancreatology. 2014;14:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L, Li Z, Yang D. Expression of HIF-1α and CAIX in nasopharyngeal carcinoma and their correlation with patients’ prognosis. Med Oncol. 2014;31:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, Cai XJ. Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1α as a prognostic role in gastric cancer. World J Gastroenterol. 2014;20:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, Sun B, Zhang Q, Wang X, Jiang H. Up-regulation of survivin by AKT and hypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer. FEBS J. 2014;281:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 86. | Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679-1684. [PubMed] |
| 87. | Rupp C, Scherzer M, Rudisch A, Unger C, Haslinger C, Schweifer N, Artaker M, Nivarthi H, Moriggl R, Hengstschläger M. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene. 2015;34:815-825. [PubMed] |
| 88. | De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013;15:R64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 89. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 90. | Amelio I, Inoue S, Markert EK, Levine AJ, Knight RA, Mak TW, Melino G. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1α degradation. Proc Natl Acad Sci USA. 2015;112:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Zhou Z, Liu F, Zhang ZS, Shu F, Zheng Y, Fu L, Li LY. Human rhomboid family-1 suppresses oxygen-independent degradation of hypoxia-inducible factor-1α in breast cancer. Cancer Res. 2014;74:2719-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB, Casimiro MC, Wang C, Pestell RG. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 93. | Suh DH, Kim HS, Kim B, Song YS. Metabolic orchestration between cancer cells and tumor microenvironment as a co-evolutionary source of chemoresistance in ovarian cancer: a therapeutic implication. Biochem Pharmacol. 2014;92:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Shi Y, Zhang Y, Zhao Y, Hong L, Liu N, Jin X, Pan Y, Fan D. Overexpression of ZNRD1 promotes multidrug-resistant phenotype of gastric cancer cells through upregulation of P-glycoprotein. Cancer Biol Ther. 2004;3:377-381. [PubMed] |
| 95. | Zhang B, Yang Y, Shi X, Liao W, Chen M, Cheng AS, Yan H, Fang C, Zhang S, Xu G. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transition. Cancer Lett. 2015;356:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Abdel-Rahman O. Targeting vascular endothelial growth factor (VEGF) pathway in gastric cancer: preclinical and clinical aspects. Crit Rev Oncol Hematol. 2015;93:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1625] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 98. | Álvarez P, Marchal JA, Boulaiz H, Carrillo E, Vélez C, Rodríguez-Serrano F, Melguizo C, Prados J, Madeddu R, Aranega A. 5-Fluorouracil derivatives: a patent review. Expert Opin Ther Pat. 2012;22:107-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 99. | Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu PF, Cheng XD. 5-Fluorouracil chemotherapy of gastric cancer generates residual cells with properties of cancer stem cells. Int J Biol Sci. 2015;11:284-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156-11163. [PubMed] |
| 101. | Ohshio Y, Teramoto K, Hanaoka J, Tezuka N, Itoh Y, Asai T, Daigo Y, Ogasawara K. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci. 2015;106:134-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fujiwara T, Shi C S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S