Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9387
Peer-review started: January 23, 2015
First decision: March 10, 2015
Revised: March 30, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: August 21, 2015
Processing time: 210 Days and 3 Hours
AIM: To compare the outcomes of endoscopic mucosal resection with a cap (EMR-C) with those of endoscopic submucosal dissection (ESD) for the resection of rectal neuroendocrine tumors.
METHODS: One hundred and sixteen lesions in 114 patients with rectal neuroendocrine tumor (NET) resected with EMR-C or ESD were included in the study. This study was performed at Pusan National University Yangsan Hospital between July 2009 and August 2014. We analyzed endoscopic complete resection rate, pathologic complete resection rate, procedure time, and adverse events in the EMR-C (n = 65) and ESD (n = 51) groups. We also performed a subgroup analysis by tumor size.
RESULTS: Mean tumor size was 4.62 ± 1.66 mm in the EMR-C group and 7.73 ± 3.14 mm in the ESD group (P < 0.001). Endoscopic complete resection rate was 100% in both groups. Histologic complete resection rate was significantly greater in the EMR-C group (92.3%) than in the ESD group (78.4%) (P = 0.042). Mean procedure time was significantly longer in the ESD group (14.43 ± 7.26 min) than in the EMR-C group (3.83 ± 1.17 min) (P < 0.001). Rates of histologic complete resection without complication were similar for tumor diameter ≤ 5 mm (EMR-C, 96%; ESD, 100%, P = 0.472) as well as in cases of 5 mm < tumor diameter ≤ 10 mm (EMR-C, 80%; ESD, 71.0%, P = 0.524).
CONCLUSION: EMR-C may be simple, faster, and more effective than ESD in removing rectal NETs and may be preferable for resection of small rectal NETs.
Core tip: This study suggests that rates of endoscopic and histologic complete resection without adverse events were high in endoscopic mucosal resection with a cap (EMR-C) for treating rectal neuroendocrine tumors (NETs). EMR-C seems to be a safe, easy, and effective method for the resection of rectal NETs because it is technically easier and less time-consuming than endoscopic submucosal dissection.
- Citation: Park SB, Kim HW, Kang DH, Choi CW, Kim SJ, Nam HS. Advantage of endoscopic mucosal resection with a cap for rectal neuroendocrine tumors. World J Gastroenterol 2015; 21(31): 9387-9393
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9387
Rectal neuroendocrine tumor (NET) is a slow growing tumor that originates in the cells of the neuroendocrine system. Rectal NETs comprise 8% to 30% of all gastro-intestinal (GI) NETs[1]. The frequency of rectal NETs is higher in Asian countries compared to Western countries[2]. The incidence of rectal NETs has recently increased more in South Korea; this is related to the increasing number of screening colonoscopies, improved detection through endoscopic developments, and a deeper understanding of rectal NETs. Rectal NETs usually appear as sessile submucosal lesions covered with yellowish mucosa[3,4]. Clinical course of tumors less than 10 mm in diameter is relatively good. The risk of metastases has been reported to be 0%-10% for tumors < 10 mm and 4%-30%, for tumors 10-19 mm in diameter[5], and 57%-80% for tumors ≥ 20 mm in diameter[6]. Standard treatment for rectal NETs < 10 mm is endoscopic resection, and the appropriate therapies for rectal NETs 10-19 mm in diameter are endoscopic resection, transanal resection [or transanal endoscopic microsurgery (TEM)], or radical rectal resection[7]. Endoscopic treatment is acceptable for small rectal NETs, but there is still controversy regarding which type of endoscopic resection is best. Numerous treatment strategies for rectal NETs have been reported. Conventional endoscopic mucosal resection (EMR) is simple, but there is some risk of incomplete resection. Endoscopic submucosal dissection (ESD) has several advantages over conventional EMR, including a higher en bloc resection rate, lower local recurrence rate, and more accurate pathological evaluation[8]. However, ESD requires great skill and experience and more time, and carries a risk of adverse events such as perforation and bleeding[9]. EMR with a cap (EMR-C) is an endoscopic procedure for cutting the submucosal layer by lifting the mucosa with saline injection, followed by aspirating the lesion into a transparent cap. EMR-C has advantages over both EMR and ESD. An advantage of EMR-C is that it is simpler and less time-consuming. Previous studies comparing EMR-C with ESD showed inconsistent results; Zhou et al[9] reported a complete-resection rate of only 52.5% for EMR-C, but Zhao et al[10] reported the rate to be 100%. This study compares the efficacy of EMR-C with that of ESD for the resection of rectal NETs.
Between July 2009 and August 2014, EMR-C and ESD were performed for 116 lesions in 114 patients with rectal NETs at Pusan National University Yangsan Hospital. Sixty-five lesions were resected with EMR-C and 51 with ESD. We performed endoscopic ultrasonography (EUS) using a UM-DP20-25R, 20-MHz (Olympus Medical Systems Corp., Tokyo, Japan) to estimate the size and the depth of invasion of rectal NETs in all patients before resection. Tumors invading the muscularis propria layer were treated surgically, tumors measuring 10-19 mm on EUS were treated with ESD, and EMR-C and ESD were alternated, if possible, for tumors ≤ 10 mm in diameter. The procedures were performed by three endoscopists (authors KHW, CCW, and PSB). Data were obtained retrospectively from a database. All resection specimens were measured and vertical and lateral resection margins evaluated. We analyzed the rate of endoscopic and histologic complete resection, procedure time, and adverse events. We also performed a subgroup analysis of EMR-C and ESD outcomes by tumor size. Tumors ≤ 10 mm in diameter were divided into two groups, ≤ 5 mm and 5-10 mm. Procedure time was defined as the time from submucosal injection to completion of resection, and adverse events were defined as bleeding (including immediate and delayed bleeding) and perforation (including microperforation and frank perforation).
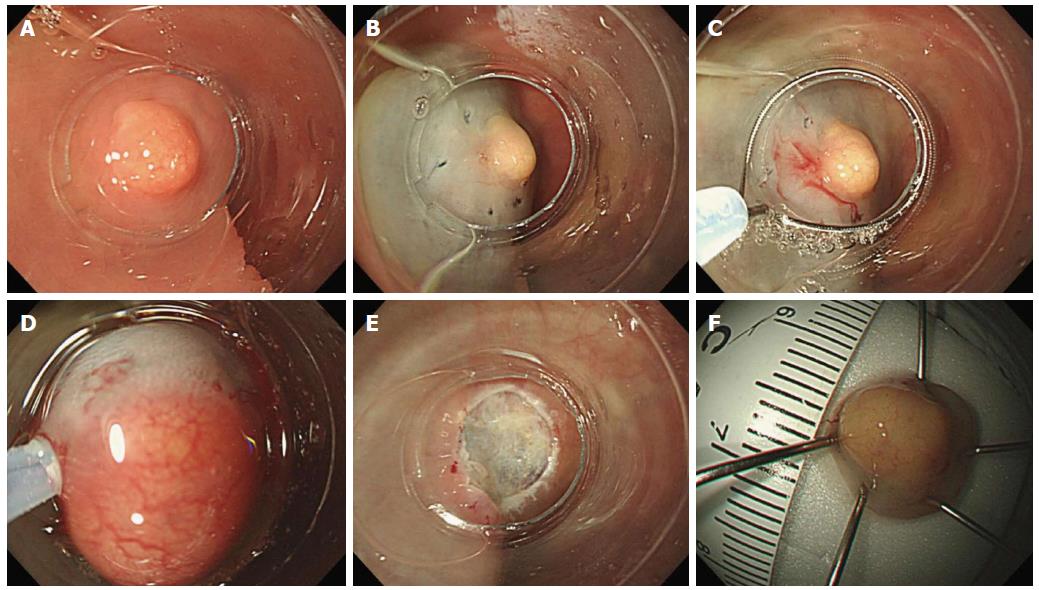
EMR-C: We used a single-channel scope (GIF-H260, Olympus Medical Systems Corp.) with an oblique distal attachment (MAJ-290, Olympus Medical Systems Corp.) and a 25-mm single-use crescent electrosurgical snare (SD-221L-25, Olympus Medical Systems Corp.). First, submucosal injection was used to lift the tumor. After attaching the oblique cap to the distal end of the endoscope, the crescent-shaped snare was positioned on the internal circumferential ridge at the tip of the cap. The lesion was drawn into the cap using the suction function, then snared and resected using an electrosurgical unit (ERBE VIO 300 D, ERBE Elektromedizin GmbH, Tübingen, Germany) (Figure 1).
ESD: ESD was carried out with a single-channel scope (GIF-H260, Olympus Medical Systems Corp.) and an electrosurgical unit (ERBE VIO 300 D, ERBE Elektromedizin GmbH). After lifting the tumor with submucosal injection, a circumferential incision was made around the lesion and submucosal dissection carried out with a DualKnife Electrosurgical Knife (Olympus Medical Systems Corp.).
Histopathologic evaluation: Each resected rectal NET was measured. All specimens were fixed in 10% formalin and stained with hematoxylin-eosin. Depth of invasion and margin infiltration were evaluated. Histologic complete resection (R0) was defined as histopathologically tumor-free lateral and vertical margins. Indeterminate margin (Rx) was defined as margin that could not be evaluated for reasons such as electrocautery artifact and inappropriate orientation.
Statistical analysis was performed using PASW for Windows, Version 18.0 (SPSS Inc., Chicago, IL, United States). Quantitative results are expressed as medians and ranges. Statistical comparisons between the two groups were performed using the χ2 statistic and Fisher’s exact test. A P value < 0.05 was considered to be significant.
Of 116 lesions in 114 patients, 65 lesions were treated with EMR-C and 51 with ESD. The baseline characteristics and clinical outcomes of the EMR-C and ESD groups are shown in Tables 1 and 2, respectively.
| EMR-C(n = 65) | ESD(n = 51) | P value | |
| Age (yr), mean ± SD | 52.31 ± 9.83 | 48.47 ± 12.23 | 0.063 |
| Male gender | 43 (66.2) | 33 (64.7) | 0.872 |
| Follow up period (d), mean ± SD | 689.58 ± 468.94 | 760.84 ± 458.91 | 0.414 |
| Specimen size (mm), | 10.15 ± 2.21 | 13.10 ± 3.99 | < 0.001 |
| mean ± SD (range) | (6.0-15.0) | (8.0-25.0) | |
| Tumor size (mm), | 4.62 ± 1.66 | 7.73 ± 3.14 | < 0.001 |
| mean ± SD (range) | (1.0-10.0) | (3.0-18.0) | |
| EUS measured size (mm), | 4.72 ± 1.51 | 7.27 ± 2.54 | < 0.001 |
| mean ± SD (range) | (1.0-8.0) | (2.7-17.0) | |
| Tumor size (mm) | |||
| 0 < tumor size ≤ 5 | 50 | 13 | |
| 5 < tumor size ≤ 10 | 15 | 31 | |
| > 10 | 0 | 7 |
| EMR-C(n = 65) | ESD(n = 51) | P value | |
| Procedure time (min), mean ± SD, | 3.83 ± 1.17 | 14.43 ± 7.26 | < 0.001 |
| Complication | 0 (0.0) | 4 (7.8) | 0.044 |
| Bleeding | 0 | 4 (7.8) | |
| Perforation | 0 | 0 | |
| Endoscopic complete resection | 65/65 (100) | 51/51 (100) | |
| Histologic complete resection | 60/65 (92.3) | 40/51 (78.4) | 0.042 |
| Vertical margin involvement | 1 (1.5) | 1 (2.0) | 0.864 |
| Lateral margin involvement | 1 (1.5) | 2 (3.9) | 0.710 |
| Vertical and Lateral margin involvement | 0 (0.0) | 2 (3.9) | 0.159 |
| Indeterminate margin | 3 (4.6) | 6 (11.8) | 0.178 |
| Vertical:Lateral:Vertical and Lateral, n | 2:1:0 | 1:3:2 | |
| Lymphovascular invasion | 0 | 1 | 0.322 |
As seen in Table 1, mean patient age did not differ significantly between the EMR-C and ESD groups (P = 0.063). The size of the resected specimen was significantly larger in the ESD group than in the EMR-C group (P < 0.001). The size of the tumor was also significantly larger in the ESD group (P < 0.001). Table 2 shows that resection time was significantly longer in the ESD group (P < 0.001). Endoscopic complete resection rates were 100% in both groups, and histologic complete resection rate was significantly higher in the EMR-C group than in the ESD group (P = 0.042). Vertical, lateral, and vertical and lateral resection margin involvement did not differ between groups (P = 0.864, P = 0.710, and P = 0.159, respectively; Table 2). Indeterminate margin resection rate did not differ between groups (P = 0.178). No local recurrence or distant metastasis occurred during the follow-up periods of cases of incomplete resection. Rates of adverse events are shown in Table 2. All bleeding was successfully controlled using coagulation. There were significantly more adverse events in the ESD group than in the EMR-C group (P = 0.044). Lymphovascular invasion occurred in one case in the ESD group.
A subgroup analysis was performed for patients who had small rectal NETs (≤ 10 mm in diameter). Of 116 lesions, 109 were ≤ 10 mm in diameter. Tumors ≤ 10 mm in diameter were further divided into two groups: ≤ 5 mm and 5-10 mm in diameter.
Table 3 presents the results of subgroup analysis of baseline characteristics for both groups by tumor size ≤ 5 mm. Mean diameters were similar between groups (P = 0.158), but resection time was significantly longer in the ESD group than in the EMR-C group (P < 0.001). Rates of endoscopic complete resection were 100% in both groups. Histologic complete resection rate did not differ significantly between groups (P = 0.472). Margin involvement for both groups is also given in Table 3. Adverse events occurred more frequently in the ESD group, but there was not a significant difference between groups [two cases of bleeding (15.4%) in the ESD group; no adverse events in the EMR-C group (P = 0.165)].
| NET size | Size ≤5 mm (n = 63) | 5 mm < size ≤10 mm (n = 46) | ||||
| EMR-C(n = 50) | ESD(n = 13) | P value | EMR-C(n = 15) | ESD(n = 31) | P value | |
| Age (yr), mean ± SD | 50.78 ± 9.45 | 45.85 ± 15.46 | 0.151 | 57.4 ± 9.63 | 48.48 ± 11.04 | 0.011 |
| Male gender | 33 (66.0) | 7 (53.8) | 0.417 | 10 (66.7) | 21 (67.7) | 0.942 |
| Follow up period (d), mean ± SD | 714.1 | 806.31 | 0.567 | 607.87 | 710.9 | 0.419 |
| ± 489.29 | ± 604.66 | ± 397.56 | ± 403.00 | |||
| Specimen size (mm), | 10.05 | 10.83 | 0.287 | 10.47 | 13.14 | 0.004 |
| mean ± SD | ± 2.21 | ± 2.78 | ± 2.26 | ± 3.68 | ||
| Tumor size (mm), | 3.9 | 4.31 | 0.158 | 7.00 | 7.87 | 0.051 |
| mean ± SD | ± 0.95 | ± 0.75 | ± 1.25 | ± 1.43 | ||
| EUS measured size (mm), | 4.44 | 5.77 | 0.005 | 5.80 | 7.03 | 0.019 |
| mean ± SD | ± 1.31 | ± 2.01 | ± 1.47 | ± 5.80 | ||
Results of subgroup analysis of clinical outcomes for both groups by tumor size > 5 mm and ≤ 10 mm are shown in Table 4. Patients in the EMR-C group were significantly older than those in the ESD group (P = 0.011). Although the size of the resected specimen was significantly larger in the ESD group (P = 0.004), tumor size was similar between groups (P = 0.051). Resection time was significantly longer in the ESD group (P < 0.001), and rates of endoscopic complete resection were 100% in both groups. There was not a significant difference in histologic complete resection rate between groups (P = 0.524). Margin involvement for both groups for this range of tumor sizes is shown in Table 4. Vertical margin involvement was 0% for both groups. Between-group differences in lateral, vertical and lateral, and indeterminate margin involvement were not significant (P = 0.979, P = 0.161 and P = 0.810, respectively; Table 4). Adverse events occurred more frequently in the ESD group, but there was not a significant difference between groups [two cases of bleeding (6.5%) in the ESD group; no adverse events in the EMR-C group (P = 0.161)].
| size ≤5 mm (n = 63) | 5 mm < size ≤10 mm (n = 46) | |||||
| EMR-C(n = 50) | ESD(n = 13) | P value | EMR-C(n = 15) | ESD(n = 31) | P value | |
| Procedure time (min), mean ± SD | 3.94 ± 1.27 | 12.52 ± 3.42 | < 0.001 | 3.45 ± 0.60 | 14.96 ± 8.85 | < 0.001 |
| Complication | 0 | 2 (15.4) | 0.165 | 0 | 2 (6.5) | 0.161 |
| Bleeding | 0 | 2 (15.4) | 0 | 2 (6.5) | ||
| Perforation | 0 | 0 | 0 | 0 | ||
| Endoscopic complete resection | 50 (100) | 13 (100) | 15 (100) | 31 (100) | ||
| Histologic complete resection | 48 (96.0) | 13 (100) | 0.472 | 12 (80.0) | 22 (71.0) | 0.524 |
| Vertical margin involvement | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Lateral margin involvement | 0 (0.0) | 0 (0.0) | 1 (6.7) | 2 (6.5) | 0.979 | |
| Vertical and Lateral margin involvement | 1 (2.0) | 0 (0.0) | 0.614 | 0 | 2 (6.5) | 0.161 |
| Indeterminate margin | 1 (2.0) | 0 (0.0) | 0.614 | 2 (13.3) | 5 (16.1) | 0.810 |
| Vertical:lateral: Vertical and Lateral | 1:0:0 | 0:0:0 | 1:1:0 | 1:2:2 | ||
The incidence of rectal NETs is increasing with the number of screening colonoscopies, advances in endoscope technology, and deeper understanding of rectal NETs. Rectal NETs commonly appear as submucosal lesions with yellowish mucosa[3,4]. There is still controversy about the treatment of rectal NETs, and numerous treatment strategies have been reported[7]. The standard treatment for rectal NETs < 10 mm in diameter is endoscopic resection; appropriate therapies for lesions 10-19 mm in diameter are endoscopic local resection, transanal resection, or radical rectal resection[7]. The European Neuroendocrine Tumors Society has published consensus guidelines for the management of patients with colorectal neuroendocrine neoplasms[11]. EUS can be used to evaluate tumor size and depth of invasion, and its accuracy in determination of depth of invasion is high (91%-100%)[12-14].
EMR has been widely used because of its simplicity and low rate of adverse events[15-17]. However, rectal NETs are located in the deep mucosal layer toward the submucosal layer, so conventional EMR can result in incomplete resection or local recurrence after EMR[18,19]. Compared with conventional EMR, the advantage of EMR-C is its low rate of positive tumor resection margin[20,21]. ESD has advantages of higher en bloc resection and more accurate pathologic evaluation, but ESD requires great skill and experience and longer time, and carries a risk of adverse events such as perforation and bleeding[9]. Compared with ESD, the advantage of EMR-C is that it is simpler and less time-consuming. EMR-C has several advantages over both EMR and ESD. The tumor is elevated into the cap using suction, and the procedure can be safely and easily performed in a short time and does not require special endoscopic skill. This study demonstrated that EMR-C was a simple and less time consuming endoscopic procedure than ESD.
Previous studies of EMR-C reported complete resection rates from 52.5% to 100%, but those studies contained small numbers of patients[9,10,20-25]. In our study, EMR-C was performed on 65 lesions, with a complete resection rate of 92.3%. Interestingly, the histologic complete resection rate was significantly higher in the EMR-C group (92.3%) than in the ESD group (78.4%) (P = 0.042). The rate of indeterminate margin was higher in the ESD group (six cases, 11.8%) than in the EMR-C group (three cases, 4.6%). Positive resection margins do not necessarily indicate remnant tumor; rather, they may indicate cautery damage during endoscopic resection[12,26]. The circular resected margin of a tumor obtained using EMR-C can remain undamaged, but during ESD, the use of electrocautery during incision and dissection may damage the tumor margins and explains the high rate of pathologic incomplete resection in the ESD group. More important is the endoscopic complete resection rate. We followed up patients every six months for two years, and then yearly thereafter. We checked general condition, computed tomography scans and colonoscopy (or sigmoidoscopy) results. There was no tumor recurrence in either group, including in patients who had positive or indeterminate margins, during a mean follow-up period of 720.91 d.
Lymphovascular invasion was found in one patient with a 6-mm tumor treated with ESD. Although metastatic involvement of tumors < 10 mm occurs in about 0%-10% of cases[1,5,6,26,27], endoscopic treatment is acceptable for small rectal NETs. Konishi et al[28] reported an incidence of lymph node metastasis as high as 7% in rectal NETs ≤ 10 mm treated with radical resection. Our study demonstrated a rate of metastasis of 0.92% (one of 109 patients). This patient with lymphovascular invasion required additional surgical therapy.
In cases of tumors ≤ 5 mm in size, endoscopic and histologic complete resections were 100% and 96.0%, respectively, for the EMR-C group, and there were no adverse events. In cases of 5 < tumor diameter ≤ 10 mm, endoscopic and histologic complete resections were 100% and 80.0%, respectively, for the EMR-C group, without adverse events. These results are similar to those of ESD, suggesting that EMR-C is not inferior to ESD for resecting small NETs. There is a theoretical possibility that perforation due to insufficient submucosal injection or too much suction could cause adverse events in EMR-C. However, because appropriate suction was used in our study, no perforation occurred.
This study has some limitations. Firstly, this is a retrospective, single-center study. Secondly, the study was not randomized. EUS was performed before resection, so the procedure was performed according to the endoscopist’s preference. Large prospective, randomized, controlled studies will be necessary to validate our results.
In conclusion, EMR-C may be feasible for the resection of rectal NETs because it is technically easier and less time-consuming than ESD. This study also suggests that rates of endoscopic and histologic complete resection without adverse events were high in EMR-C for treating rectal NETs.
Endoscopic mucosal resection with a cap (EMR-C) and endoscopic submucosal dissection (ESD) have been used to treat neuroendocrine tumors (NETs) for decades and the reported results have been variable. Until now, there has been no consensus about which modality is superior. The advantages of ESD include more accurate pathological evaluation, but the authors experienced some indeterminate margin pathologic results after ESD. So, they compared the efficacy of EMR-C with that of ESD for the resection of rectal NETs.
Many previous studies have evaluated the outcomes of endoscopic procedures for rectal NETs. The authors evaluated a somewhat larger number of patients undergoing EMR-C and performed a subgroup analysis by tumor size.
Endoscopic complete resection rate was 100% in both groups. Histologic complete resection rate was significantly greater in the EMR-C group than in the ESD group. The study demonstrates the rate of histologic complete resection of EMR-C to be superior to that of ESD because the circumferential tumor-resection margin treated in EMR-C will be undamaged.
EMR-C is feasible for the resection of rectal NETs because it is technically easier and less time-consuming than ESD. These results will provide important information to select a treatment strategy for patients with small rectal NETs.
EMR-C is an endoscopic procedure for cutting the submucosal layer by lifting the mucosa with saline injection, followed by aspirating the lesion into a transparent cap.
The authors concluded that EMR-C may be simple, faster, and more effective than ESD in removing rectal NETs and may be preferable for resection of small rectal NETs. The paper is well written and brings forward some new information. The experience the authors shared in this manuscript is beneficial for further study in this field.
| 1. | Gleeson FC, Levy MJ, Dozois EJ, Larson DW, Wong Kee Song LM, Boardman LA. Endoscopically identified well-differentiated rectal carcinoid tumors: impact of tumor size on the natural history and outcomes. Gastrointest Endosc. 2014;80:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Choi HH, Kim JS, Cheung DY, Cho YS. Which endoscopic treatment is the best for small rectal carcinoid tumors? World J Gastrointest Endosc. 2013;5:487-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Jetmore AB, Ray JE, Gathright JB, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum. 1992;35:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949-1953. [PubMed] |
| 5. | Fahy BN, Tang LH, Klimstra D, Wong WD, Guillem JG, Paty PB, Temple LK, Shia J, Weiser MR. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy. 2010;42:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, Chen WF, Ma LL, Zhang YQ, Qin WZ, Cai MY. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010;24:2607-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zhao ZF, Zhang N, Ma SR, Yang Z, Han X, Zhao YF, Gao F, Gong ZJ, Yang L. A comparative study on endoscopy treatment in rectal carcinoid tumors. Surg Laparosc Endosc Percutan Tech. 2012;22:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Ishii N, Horiki N, Itoh T, Maruyama M, Matsuda M, Setoyama T, Suzuki S, Uchida S, Uemura M, Iizuka Y. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Abe T, Kakemura T, Fujinuma S, Maetani I. Successful outcomes of EMR-L with 3D-EUS for rectal carcinoids compared with historical controls. World J Gastroenterol. 2008;14:4054-4058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, Antillon MR. Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol. 2009;15:4273-4277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Ichikawa J, Tanabe S, Koizumi W, Kida Y, Imaizumi H, Kida M, Saigenji K, Mitomi H. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003;35:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kiesslich R, Neurath MF. Endoscopic mucosal resection: an evolving therapeutic strategy for non-polypoid colorectal neoplasia. Gut. 2004;53:1222-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 19. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 20. | Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, Kitano A, Arakawa T. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res. 2000;28:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kim YJ, Lee SK, Cheon JH, Kim TI, Lee YC, Kim WH, Chung JB, Yi SW, Park S. [Efficacy of endoscopic resection for small rectal carcinoid: a retrospective study]. Korean J Gastroenterol. 2008;51:174-180. [PubMed] |
| 22. | Sohn DK, Han KS, Hong CW, Chang HJ, Jeong SY, Park JG. Selection of cap size in endoscopic submucosal resection with cap aspiration for rectal carcinoid tumors. J Laparoendosc Adv Surg Tech A. 2008;18:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Celebi Kobak A, Zeybel M, Ayhan S, Kara E, Ellidokuz E. Endoscopic submucosal resection of a rectal carcinoid tumor by cap aspiration - snare resection method. Turk J Gastroenterol. 2006;17:313-315. [PubMed] |
| 24. | Imada-Shirakata Y, Sakai M, Kajiyama T, Kin G, Inoue K, Torii A, Kishimoto H, Ueda S, Okuma M. Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy. 1997;29:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, Sato R, Murakami K, Fujioka T. Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy. 2004;36:202-205. [PubMed] |
| 26. | Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008;143:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1203] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Buanes TA, Guo XZ, Lorenzo-Zuniga V, Paoluzi OA, Tomizawa M S- Editor: Ma YJ L- Editor: Logan S E- Editor: Wang CH