Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9328
Peer-review started: March 28, 2015
First decision: June 2, 2015
Revised: June 22, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: August 21, 2015
Processing time: 145 Days and 23.8 Hours
AIM: To investigate the functional consequences of rs2736100 polymorphism in telomere length and examine its link to gastric cancer risk.
METHODS: Telomere length and human telomerase reverse transcriptase (hTERT) mRNA expression were measured in 35 gastric cancer tissues and 5 cell lines and correlated to rs2736100 polymorphism. The relationship between rs2736100 polymorphism and the risk of gastric cancer were examined in 243 gastric cancer patients and 246 healthy individuals.
RESULTS: The rs2736100 A allele carrier is closely associated with reduced hTERT mRNA expression and shortened telomere length in gastric cancer tissue and cell lines. When gastric cancers were stratified by histological subtype, telomere length and hTERT mRNA levels were significantly increased in those with the C/C genotype in intestinal-type gastric cancer, but not in diffuse-type gastric cancer. Interestingly, there was no significant difference in the genotype and allele frequencies of the rs2736100 polymorphism between the patients with gastric cancer and healthy controls.
CONCLUSION: The rs2736100 polymorphism of the hTERT gene is involved in the regulation of hTERT expression and telomere length, but not in the risk of gastric cancer.
Core tip: The rs2736100 polymorphism is closely associated with reduced human telomerase reverse transcriptase (hTERT) mRNA expression and shortened telomere length in gastric cancer tissue and cell lines. Additionally, telomere length and hTERT mRNA levels were significantly increased in those with the C/C genotype in intestinal-type gastric cancer. Notably, there was no significant difference in the genotype and allele frequencies of the rs2736100 polymorphism between the patients with gastric cancer and healthy controls. These results suggest that the rs2736100 polymorphism of the hTERT gene is involved in the regulation of hTERT expression and telomere length, but not in the risk of gastric cancer.
-
Citation: Choi BJ, Yoon JH, Kim O, Choi WS, Nam SW, Lee JY, Park WS. Influence of the
hTERT rs2736100 polymorphism on telomere length in gastric cancer. World J Gastroenterol 2015; 21(31): 9328-9336 - URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9328
Gastric cancer remains one of the malignancies with the highest incidence and mortality rates worldwide, accounting for approximately 10% of all newly diagnosed cancers[1-3]. Gastric mucosal inflammation is generally believed to be caused by chronic Helicobacter pylori (H. pylori) infection and atrophic gastritis, while intestinal metaplasia and dysplasia represent different stages of the gastric carcinogenesis cascade[4,5]. Thus, gastric carcinogenesis is a multistep process involving genetic and epigenetic changes in oncogenes, tumor-suppressor genes, cell adhesion molecules and DNA repair genes. There are two main types of gastric adenocarcinoma according to the Lauren classification defined as intestinal-type and diffuse-type. Of these, intestinal-type carcinomas showed obvious glandular differentiation, whereas diffuse-type carcinomas are typically poorly differentiated. The phenotype variation has led to substantial research interest on the regulation of genes. Although numerous advances in the understanding of gastric cancer have been made, the precise molecular mechanism underlying gastric carcinogenesis is not yet fully understood.
Telomere length in healthy cells is highly regulated in a tissue- and cell type-specific manner and is dependent on mitotic turnover rate, telomerase activity, and telomerase-associated factors[6]. In most normal human somatic tissues, telomerase activity is undetectable, whereas it is frequently detectable in almost all types of human cancers, suggesting the importance of telomerase in the development of cancer[7]. Defects in telomere maintenance contribute to the initiation of genomic instability during carcinogenesis, including gastric cancer[8]. The telomerase reverse transcriptase (TERT) gene is located in chromosome 5p15.33 and a variant rs2736100 localized in the second intron of the gene. The rs2736100 C allele is one of the eight variants associated with long telomeres in white blood cells[9]. We previously reported that telomere length in 35 gastric cancer tissues was shortened significantly, compared with the corresponding non-cancerous gastric mucosae, and was positively correlated with human telomerase reverse transcriptase (hTERT) expression[10]. This led us to hypothesize that the rs2736100 polymorphism is closely associated with telomere length and gastric cancer risk.
In the present study, we investigated whether the rs2736100 single-nucleotide polymorphism (SNP) of the hTERT gene is associated with hTERT mRNA expression level and telomere length, and examined the ability of rs2736100 to predict gastric cancer risk. Overall, we found that rs2736100 A allele carrier is closely associated with shortened telomere length and reduced hTERT mRNA expression in gastric cancer, especially in intestinal-type. However, genotype and allele frequencies of rs2736100 polymorphism were not associated with an increased risk for gastric cancer in this population.
In this study, archival non-cancerous gastric mucosa specimens from 243 gastric cancer patients who underwent subtotal or total gastrectomy at the Catholic University of Korea, College of Medicine in Seoul, were enrolled. All gastric cancers specimens were pathologically confirmed as gastric adenocarcinoma. None of the patients received chemotherapy or radiotherapy before surgery. The healthy control subjects (n = 246) were collected from residents of the same racial and geographic area without personal or familial history of malignancy and other major disease. To exclude the effects of ethnic differences, only Koreans were included in this study. Informed consents were obtained according to the Helsinki Declaration. This study was approved by the institutional review board of the Catholic University of Korea, College of Medicine (CUMC09U089).
AGS, MKN-1, MKN-45, SNU-638, and Kato-III gastric cancer cell lines were obtained from the American Type Culture Collection. These cells were cultured at 37 °C in 5% CO2 in RPMI-1640 medium (Lonza, Basel, Switzerland) supplemented with 10% heat-inactivated fetal bovine serum.
Non-neoplastic cells from specimens of gastric cancer patients were obtained from frozen cancer-free gastric mucosa. For healthy control population, a leukocyte cell pellet from each blood sample was obtained from the Buffy coat by centrifugation of 2 mL of whole blood. The cell pellet was used for DNA extraction. The Qiagen DNA Blood Mini Kit (Qiagen, Valencia, CA, United States) was used according to the manufacturer’s instructions to obtain genomic DNA. The DNA purity and concentration were determined by Nanodrop® ND-1000 spectrophotometer (Nanodrop technologies, Wilmingon, DE, United States).
After quantification of mRNA extracted from frozen gastric mucosae, cDNA was synthesized using the reverse transcription kit from Roche Molecular System (Roche, Mannheim, Germany), according to the manufacturer’s protocol.
Previously, we examined telomere length in 35 gastric cancer tissues by real-time quantitative polymerase chain reaction (qPCR)[10]. Briefly, after quantifying the genomic DNAs extracted from each sample, real-time SYBR Green qPCR was performed on a Stratagene Mx 3000P qPCR system (Stratagene, La Jolla, CA, United States). Sequences of the primers are described in Table 1. All samples were subjected to PCR amplification with specific oligonucleotide primers for the constitutively expressed single gene copy number (36B4) and normalized. The ratio of telomere repeat copy number to single-copy gene number (T/S ratio) was determined using the comparative Ct method. Sample T/S ratios were then divided with the T/S ratio of a reference DNA included in each plate, generating relative telomere length values. Each sample was loaded in triplicate, and all PCR-plates included a standard curve for PCR efficiency calculations.
| Gene | Primer sequences |
| hTERT rs2736100 | F: 5’-GGT GCC TCC AGA AAA GCA G-3’ |
| R: 5’-GAC ACG GAT CCA GGA CCT C-3’ | |
| Telomere standard | 5’-TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG TTA GGG-3’ |
| 36B4 standard | 5’-CAG CAA GTG GGA AGG TGT AAT CCG TCT CCA CAG ACA AGG CCA GGA CTC GTT TGT ACC CGT TGA TGA TAG AAT GGG-3’ |
| Telomere | F: 5’-CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT-3’ |
| R: 5’-GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT-3’ | |
| 36B4 | F: 5’-CAG CAA GTG GGA AGG TGT AAT CC-3’ |
| R: 5’-CCC ATT CTA TCA TCA ACG GGT ACA A-3’ | |
| hTERT | F: 5’-ATG CGA CAG TTC GTG GCT CA-3’ |
| R: 5’-ATC CCC TGG CAC TGG ACG TA-3’ | |
| GAPDH | F: 5’- AAA TCA AGT GGG GCG ATG CTG-3’ |
| R: 5’- GCA GAG ATG ATG ACC CTT TTG-3’ |
In addition, hTERT mRNA transcript expression was examined in 5 gastric cancer cell lines and 35 gastric cancer tissues by real time reverse transcription PCR. After cDNA synthesis, 50 ng cDNA was amplified using Fullvelocity SYBR Green QPCR Master Mix (Stratagene) and 20 pmol/μL each of the forward and reverse primers on the Stratagene Mx 3000P QPCR system. To ensure the fidelity of mRNA extraction and reverse transcription, all samples were subjected to PCR amplification with oligonucleotide primers specific for the constitutively expressed gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and normalized. Sequences of the primers are described in Table 1. The standard curve method was used for quantification of the relative amounts of gene expression products. All samples were tested in duplicate, and the average values were used for quantification.
Since gastrokine 1 (GKN1) induces senescence and apoptosis in gastric cancer cells by regulating telomere length and hTERT expression[10], hTERT mRNA expression were measured in 5 gastric cancer cell lines after treatment with recombinant GKN1 (10 nmol/L, A&R therapeutics, Daejeon, South Korea) and correlated to rs2736100 polymorphism. To identify the effect of GKN1 on hTERT expression, non-treated gastric cells were used as a control. The effects of GKN1 were presented as the fold changes in hTERT expression relative to control.
Genomic DNAs were amplified with primers covering the rs2736100 polymorphism in the human hTERT gene. For PCR, the primer sequences were described in Table 1. The PCR procedure was performed under standard conditions in a 20 μL reaction mixture containing 2 μL of template DNA, 0.5 μmol/L of each primer, 0.2 μmol/L of each deoxynucleotide triphosphate, 1.5 mmol/L MgCl2, 0.4 unit of Ampli Taq gold polymerase (Perkin-Elmer, Foster City, CA, United States), and 2 μL of 10 × buffer. The reaction mixture was denatured for 12 min at 95 °C and then incubated for 40 cycles (denaturing for 30 s at 95 °C, annealing for 30 s at 55.4 °C and extension for 30 s at 72 °C). A final extension step was performed for 5 min at 72 °C. After amplification, the PCR products for rs2736100 polymorphism of hTERT were digested with 5 U of restriction enzyme SfcI at 37 °C for 4 h. DNA fragments then were electrophoresed on a 3% agarose gel. To ensure the reliability of the restriction fragment length polymorphism results, 50 (20.6%) PCR products of 243 gastric cancer cases were sequenced using the fluorescent dideoxy chain termination method with on ABI 3730XL Analyzer (Applied Biosystems, Foster city, CA, United States).
We firstly examined the association of hTERT polymorphism (rs2736100) with hTERT mRNA expression and telomere length in gastric cancer cell lines and tissues by student t-test and χ2 test. Next, we conducted a two-tailed t-test to determine the differences in the percentages of genotypes and alleles between patients and healthy controls, and between the two histological types. The strength of association between allele frequencies and the stomach cancer was estimated by calculating the OR and 95%CI by logistic regression analysis using genotype or the number of allele as a regressor. An adjusted analysis was also performed by logistic regression analysis after adjustment for gender and age. A P values < 0.05 were considered to be statistically significant. The statistical methods of this study were reviewed by Professor Yong Gyu Park from The Catholic University of Korea.
A total of 243 patients and 246 healthy individuals were included in this study. The 243 cases included 174 men (71.6%) and 69 women (28.4%) with a median age of 62.5 (22-83) years at initial diagnosis. Histologically, 145 cases were of intestinal-type (59.6%) and 98 were of diffuse-type (40.4%) gastric cancers. The average age was 63 ± 12 in cancer patients and 44 ± 8 in control group. Age of 50 was used for stratification and χ2 test showed that age distribution had no statistical difference between two groups (P = 0.5721 and P = 0.6110, respectively).
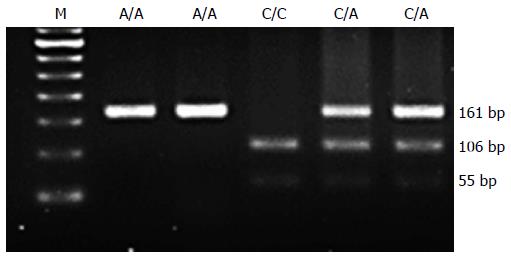
A 161-bp PCR fragment containing the hTERT SfcI polymorphic site was amplified. The A allele has no restriction site and the C allele has one restriction site. After digestion with SfcI, three genotypes were demonstrated; C/C homozygote, yielding 106- and 55-bp fragments; C/A heterozygote, yielding 161-, 106-, and 55-bp fragments; and A/A homozygote as, 161-bp fragment (Figure 1).
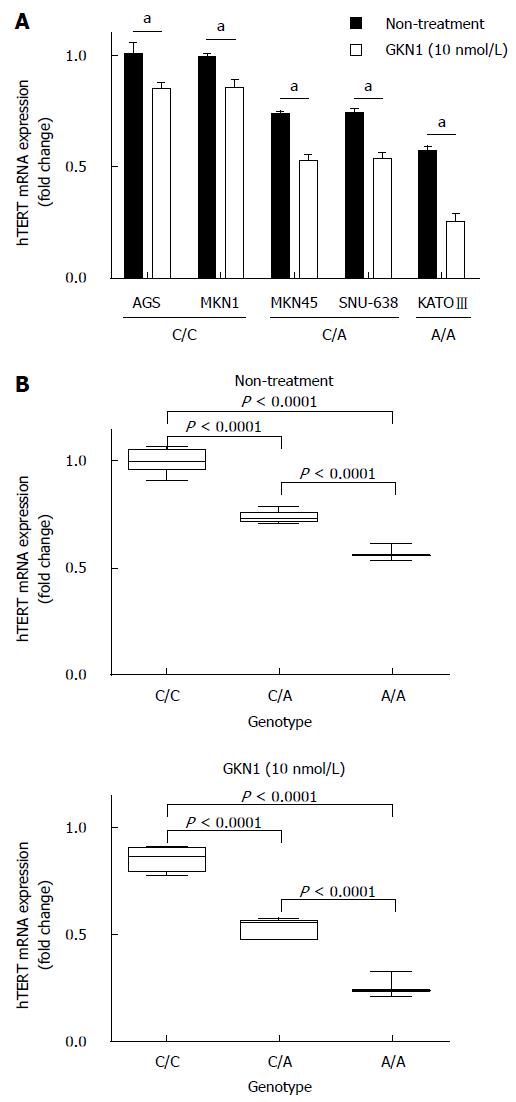
Next, to investigate whether the genotype of rs2736100 polymorphism contributes to hTERT expression, the genotypes of 5 gastric cancer cell lines were compared with hTERT mRNA expression. As shown in Figure 2A, the genotype of AGS and MKN1cells was C/C homozygote, MKN-45 and SNU-638 were heterozygote, and KATO-III was A/A homozygote. Interestingly, hTERT mRNA expression was markedly increased in AGS and MKN1 cells with C/C homozygote and reduced in KATO-III cells with A/A homozygote, compared the cells with C/A heterozygote (Figure 2B). Consistent with our previous results[10], GKN1 treatment significantly down-regulated hTERT mRNA expression in all gastric cancer cell lines, especially in MKN-45 and SNU-638 cells with C/A heterozygote and KATO-III cell with A/A homozygote (Figure 2C), suggesting that hTERT mRNA expression level is closely associated with the genotype of the rs2736100 polymorphism.
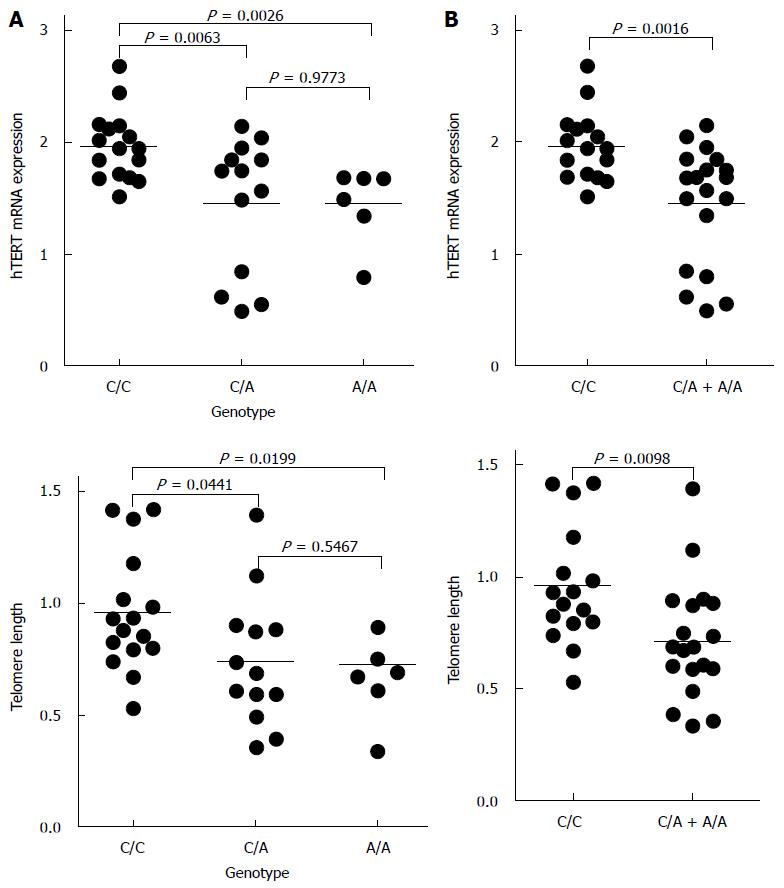
Previously, telomere length and hTERT mRNA levels in gastric cancer were measured in 35 subjects[10]. As shown in Figure 3A, the genotype of rs2736100 polymorphism of the hTERT gene was closely associated with hTERT mRNA and telomere length in gastric cancers (P = 0.0026 and P = 0.0199, respectively). When we compared the genotype of rs2736100 polymorphism with telomere length and hTERT mRNA levels, subjects carrying the A allele (A/A and C/A genotypes) showed lower hTERT mRNA levels and shortened telomere length, compared with those who had C/C homozygote genotype (P = 0.0016 and P = 0.0098, respectively) (Figure 3B).
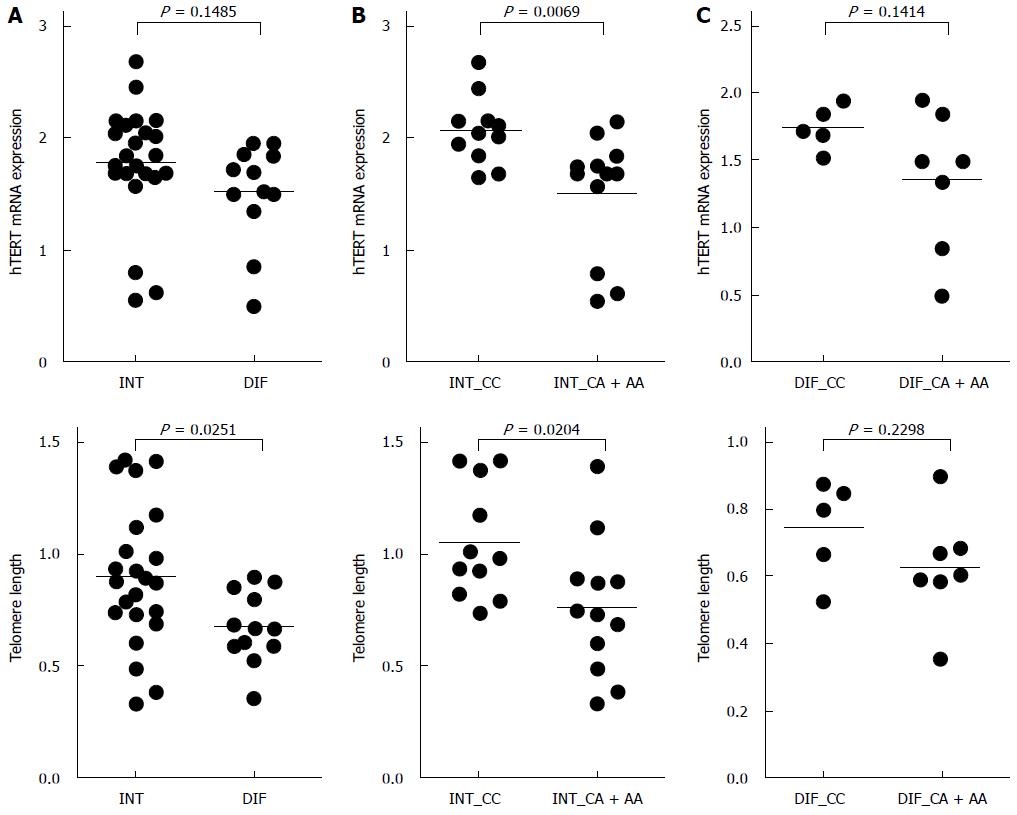
When we stratified the gastric cancers by the Lauren classification, the telomere length was shortened in diffuse-type gastric cancer (P = 0.0251). However, there was no difference in hTERT mRNA expression between intestinal- and diffuse-type gastric cancers (P = 0.1485) (Figure 4A). When we combined C/A and A/A genotypes, telomere length and hTERT mRNA levels were significantly increased in those with the C/C genotype in intestinal-type gastric cancer (P = 0.0069 and P = 0.0204, respectively) (Figure 4B). However, the genotype of rs2736100 polymorphism was not associated with telomere length and hTERT expression in those with diffuse-type gastric cancer (P = 0.1414 and P = 0.2298, respectively) (Figure 4C).
Next, to determine whether the rs2736100 polymorphism is associated with risk of gastric cancer in the Korean population, we investigated the genotype and allele frequencies of rs2736100 polymorphism in 243 gastric cancer patients and 246 healthy individuals. The distribution of genotypes in cancer group and control group was conformed to be in Hardy-Weinberg equilibrium, indicating that genotype distribution of both groups was a representative of the general population. The frequencies of the rs2736100 C/C, C/A, and A/A genotypes were 42.0% (102/243), 44.0% (107/243), and 14.0% (34/243), respectively, in gastric cancer patients and 35.0% (86/246), 49.6% (122/246) and 15.4% (38/246), respectively, in the healthy controls (Table 2). Statistically, no differences in the genotype and allele frequency of hTERT rs2736100 were observed between the healthy controls and gastric cancer patients (P = 0.2797 and P = 0.1727, respectively).
| Patients(n = 243),n (%) | Controls(n = 246),n (%) | Crude OR (95%CI) | Adjusted1 OR (95%CI) | |
| CC | 102 (42.0) | 86 (35.0) | 1.00 | 1.00 |
| CA | 107 (44.0) | 122 (49.6) | 0.739 (0.502-1.089) | 0.741 (0.447-1.228) |
| AA | 34 (14.0) | 38 (15.4) | 0.754 (0.438-1.300) | 0.811 (0.373-1.760) |
| C:A allele frequency2 | 311:175 (0.640) | 294:198 (0.598) | ||
| Trend test3 | P = 0.1501 | P = 0.3454 |
When the data were stratified according to histological subtype of gastric cancer, there was no significant difference in the risk of gastric cancer between intestinal- and diffuse-type (P = 0.5448) (Table 3). Compared with genotypes of healthy controls, no significant difference in the risk of intestinal- and diffuse-type gastric cancers was found in the carriers with an A allele (A/C or A/A genotypes) and those with the C/C genotype (P = 0.0528 and P = 0.6256) (Table 3). These findings suggest that rs2736100 polymorphism of the hTERT gene may not be associated with susceptibility to the development and differentiation of gastric cancer in Korean population.
| Patients (n = 243) | Controls (n = 246) | Adjusted1 OR (95%CI) | Adjusted1 OR (95%CI) | |||||
| CC | CA | AA | CC | CA | AA | CA vs CC | AA vs CC | |
| Age (yr) | ||||||||
| ≤ 50 | 14 | 20 | 1 | 72 | 98 | 33 | 1.053 (0.498-2.226) | 0.229 (0.039-1.351) |
| > 50 | 88 | 87 | 33 | 14 | 24 | 5 | 0.568 (0.272-1.184) | 1.029 (0.342-3.101) |
| Gender | ||||||||
| M | 72 | 77 | 25 | 49 | 63 | 23 | 0.871 (0.452-1.679) | 1.070 (0.392-2.923) |
| F | 30 | 30 | 9 | 37 | 59 | 15 | 0.582 (0.262-1.293) | 0.574 (0.162-2.031) |
| Lauren’s | ||||||||
| Diffuse | 37 | 46 | 15 | P = 0.5448 | ||||
| Intestinal | 65 | 61 | 19 | (χ2 test) | ||||
Normal cells divide for a limited number of times, whereas cancer cells usually have the ability to proliferate indefinitely[11]. In healthy cells, telomere length is highly regulated in a tissue- and cell type-specific manner and is dependent on mitotic turnover rate, telomerase activity, and telomerase-associated factors[12]. Telomerase, which adds TTAGGG sequences to the 3’ ends of telomeric DNA and promotes genomic stability, is a critical determinant of telomere length[13]. Telomeres play an important role in the maintenance of genomic stability and defects in telomere maintenance contribute to the initiation of genomic instability during carcinogenesis, including gastric cancer[8,14,15]. Interestingly, telomere shortening and dysfunction have been suggested to contribute to cancer susceptibility by increasing the risk of chromosomal aberrations caused by the breakage-fusion-bridge cycle[16]. Since cells with sufficiently shortened telomere enter an irreversible growth arrest called cellular senescence, it is necessary to elucidate mechanisms of telomere length regulation for the development of novel anti-cancer agents.
Human telomerase is composed of two subunits: telomerase RNA (hTERC) which serves as the template for telomere elongation and telomerase reverse transcriptase (hTERT) which possesses the catalytic activity to synthesize DNA from the RNA template[17-19]. The SNP rs2736100 is one of the most common variants of the hTERT gene associated with cancer risk[20-23]. In particular, it has been reported that the C allele of rs2736100 was significantly associated with longer telomere length of germ-cells[9,24]. Since telomerase activity was presently increased in most cancer cells, it is possible that the C allele is closely associated with increased hTERT expression and a higher telomerase activity, suggesting that this SNP acts on the hTERT gene encoding the reverse transcriptase of the telomerase complex, essential for maintaining the telomere length. In this study, we also found that hTERT mRNA expression level markedly increased in the gastric cancer cells with C/C homozygote and was reduced in cells with A/A homozygote, compared the cells with C/A heterozygote (Figure 2). Furthermore, the rs2736100 C allele specifically influences the cellular response to anti-mitotic agents[25]. Our previous study indicated that GKN1 may inhibit the telomere elongation by suppressing c-myc-induced hTERT expression[10]. Thus, we investigated whether the effect of GKN1 on hTERT expression is associated with the rs2736100 polymorphism in 5 gastric cancer cell lines. Expectedly, GKN1 treatment significantly down-regulated hTERT mRNA expression in MKN-45 and SNU-638 cells with C/A heterozygote and KATO-III cells with A/A homozygote (Figure 2C). Taken together, these results suggest that hTERT mRNA expression level is closely associated with rs2736100 polymorphism. However, further studies are strongly needed to identify the molecular mechanisms by which GKN1 regulates the rs2736100 polymorphism.
Next, we further confirmed the effect of rs2736100 polymorphism on hTERT mRNA expression and telomere length in gastric cancer tissues (Figure 3A and B). As shown in Figure 3B, the cases carrying the A allele (A/A and C/A genotypes) showed lower hTERT mRNA levels and shortened telomere length, compared with those who had C/C homozygote genotype (P = 0.0016 and P = 0.0098, respectively). Although the sample size (n = 35) was limited, it is likely that genotype of the rs2736100 polymorphism may be closely associated with hTERT expression and telomere length in gastric cancers.
When the gastric cancers were stratified by histologic type, the telomere length was significantly shortened in the diffuse-type (P = 0.0251), but not the intestinal-type (Figure 4A). When we combined C/A and A/A genotypes, telomere length and hTERT mRNA levels were markedly increased in intestinal-type gastric cancers with the C/C genotype (P = 0.0069 and P = 0.0204, respectively) (Figure 4B). However, these effects of the rs2736100 polymorphism on hTERT expression and telomere length were not detected in diffuse-type gastric cancers (P = 0.1414 and P = 0.2298, respectively) (Figure 4C). Therefore, our findings suggest that the rs2736100 polymorphism may be involved in telomere length maintenance of intestinal-type gastric cancer cells. In the future, to determine the significance of rs2736100 polymorphism on the differentiation of gastric cancer cells, further studies on large-scale should be performed.
To identify that rs2736100 polymorphism contribute to the risk of gastric cancer in the Korean population, we investigated the genotype and allele frequencies of rs2736100 polymorphism in tissue specimens from 243 gastric cancer patients and 246 healthy individuals. Statistically, no differences in the genotype and allele frequencies of hTERT rs2736100 polymorphism were observed between the healthy controls and gastric cancer patients (P = 0.2797 and P = 0.1727, respectively) (Table 2). When gastric cancers were stratified according to histological subtype, there was no significant difference in the risk of intestinal- and diffuse-type gastric cancer between the carriers with an A allele (A/C or A/A genotypes) and those with the C/C genotype (P = 0.0528 and P = 0.6256) (Table 3), compared with genotypes of healthy controls. Thus, we conclude that rs2736100 polymorphism of the hTERT gene may not be associated with susceptibility to the development and differentiation of gastric cancer in Korean population.
Although our study had limited statistical power probably due to their small sample size, while limited, we showed that the rs2736100 polymorphism of hTERT significantly affect telomere length and hTERT mRNA expression in gastric cancer cell lines and tissues. Unexpectedly, genotype and allele frequencies of the polymorphism were not associated with susceptibility to the development and the differentiation of gastric cancer in the Korean population. Further studies on large population are strongly necessary to clarify the biological significances and the exact effects of these polymorphisms in regulating hTERT expression.
Telomere length is dependent on mitotic turnover rate, telomerase activity, and telomerase-associated factors. Cells with sufficiently shortened telomere enter an irreversible growth arrest called cellular senescence. Telomerase activity is frequently detected in almost all types of human cancers, suggesting the importance of telomerase in cancer development. Single nucleotide polymorphism (SNP) in the telomerase reverse transcriptase (TERT) gene may influence hTERT expression and telomere length, thereby modulating the susceptibility to some cancers.
It is necessary to elucidate mechanisms of telomere length regulation for the development of novel anti-cancer agents. SNP in the second intron of the hTERT gene, rs2736100, may affect either the expression or telomerase activity and therefore may be associated with gastric cancer risk. It has been reported that telomere length in gastric cancers is positively correlated with hTERT expression and the rs2736100 C allele is associated with long telomere. The current research hotspot is to identify the significance of the rs2736100 polymorphism in telomere length regulation and genetic susceptibility of an individual to gastric cancer.
Telomerase activity was frequently increased in most cancer cells. The rs2736100 polymorphism was closely associated with the hTERT expression and telomere length in gastric cancer cells and tissues. Treatment with Gastrokine 1 (GKN1), an inhibitor of telomere elongation, significantly decreased hTERT mRNA expression. However, rs2736100 polymorphism of the hTERT gene was not associated with susceptibility to the development and differentiation of gastric cancer in the Korean population.
These results suggest that the rs2736100 polymorphism of hTERT can be used as a potential biomarker for the development of anti-cancer agents regulating telomere length in gastric cancer cells.
This is a good study in which the authors analyzed the influence of rs2736100 polymorphism on telomere length and gastric cancer risk in the Koreans. The results are interesting and suggest that the rs2736100 polymorphism of hTERT could be used as a potential biomarker of telomere length and hTERT mRNA expression in gastric cancer cells.
P- Reviewer: Boardman LA, Silva AE, G Shen GM, Yang SM S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Iacobuzio-Donahue CA, Maitra A, Ashfaq R, Hustinx S, Heitmiller RE. Claudin-4, mitogen-activated protein kinase kinase 4, and stratifin are markers of gastric adenocarcinoma precursor lesions. Cancer Epidemiol Biomarkers Prev. 2006;15:281-287. [PubMed] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25598] [Article Influence: 1706.5] [Reference Citation Analysis (10)] |
| 3. | Jung KH, Kim SM, Choi MG, Lee JH, Noh JH, Sohn TS, Bae JM, Kim S. Preoperative smoking cessation can reduce postoperative complications in gastric cancer surgery. Gastric Cancer. 2014;Epub ahead of print. [PubMed] |
| 4. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] |
| 5. | Stemmermann GN. Intestinal metaplasia of the stomach. A status report. Cancer. 1994;74:556-564. [PubMed] |
| 6. | Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:76-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Dhaene K, Van Marck E, Parwaresch R. Telomeres, telomerase and cancer: an up-date. Virchows Arch. 2000;437:1-16. [PubMed] |
| 8. | Yoo J, Park SY, Kang SJ, Kim BK, Shim SI, Kang CS. Expression of telomerase activity, human telomerase RNA, and telomerase reverse transcriptase in gastric adenocarcinomas. Mod Pathol. 2003;16:700-707. [PubMed] |
| 9. | Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hägg S, Matthews MK, Palmen J, Norata GD, O’Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, de Boer RA, Böhringer S, Braund PS, Burton PR, de Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, van Leeuwen EM, Madden PA, Mägi R, Magnusson PK, Männistö S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Viñuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, van Gilst WH, Zhu H, Consortium C, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, van der Harst P, Samani NJ. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422-427, 427e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 769] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 10. | Yoon JH, Seo HS, Choi WS, Kim O, Nam SW, Lee JY, Park WS. Gastrokine 1 induces senescence and apoptosis through regulating telomere length in gastric cancer. Oncotarget. 2014;5:11695-11708. [PubMed] |
| 11. | Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677-685. [PubMed] |
| 12. | Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223-233. [PubMed] |
| 13. | Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133-1138. [PubMed] |
| 14. | Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522-526. [PubMed] |
| 15. | Omori Y, Nakayama F, Li D, Kanemitsu K, Semba S, Ito A, Yokozaki H. Alternative lengthening of telomeres frequently occurs in mismatch repair system-deficient gastric carcinoma. Cancer Sci. 2009;100:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Newbold RF. The significance of telomerase activation and cellular immortalization in human cancer. Mutagenesis. 2002;17:539-550. [PubMed] |
| 18. | Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867-874. [PubMed] |
| 19. | Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 20. | Zou P, Gu A, Ji G, Zhao L, Zhao P, Lu A. The TERT rs2736100 polymorphism and cancer risk: a meta-analysis based on 25 case-control studies. BMC Cancer. 2012;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Mocellin S, Verdi D, Pooley KA, Landi MT, Egan KM, Baird DM, Prescott J, De Vivo I, Nitti D. Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2012;104:840-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Kinnersley B, Migliorini G, Broderick P, Whiffin N, Dobbins SE, Casey G, Hopper J, Sieber O, Lipton L, Kerr DJ. The TERT variant rs2736100 is associated with colorectal cancer risk. Br J Cancer. 2012;107:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, Yang L, Dai J, Hu L, Tan W. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011;43:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 24. | Melin BS, Nordfjäll K, Andersson U, Roos G. hTERT cancer risk genotypes are associated with telomere length. Genet Epidemiol. 2012;36:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Kim J, Jones-Hall YL, Wei R, Myers J, Qi Y, Knipp GT, Liu W. Association between hTERT rs2736100 polymorphism and sensitivity to anti-cancer agents. Front Genet. 2013;4:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |