Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.8994
Peer-review started: February 28, 2015
First decision: April 13, 2015
Revised: April 28, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: August 14, 2015
Processing time: 170 Days and 12.3 Hours
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer and represents the third leading cause of cancer-related death worldwide. Current available therapeutic approaches are poorly effective, especially for the advanced forms of the disease. In the last year, short double stranded RNA molecules termed small interfering RNAs (siRNAs) and micro interfering RNAs (miRNA), emerged as interesting molecules with potential therapeutic value for HCC. The practical use of these molecules is however limited by the identification of optimal molecular targets and especially by the lack of effective and targeted HCC delivery systems. Here we focus our discussion on the most recent advances in the identification of siRNAs/miRNAs molecular targets and on the development of suitable siRNA/miRNAs delivery systems.
Core tip: Available therapeutic approaches for hepatocellular carcinoma (HCC) are poorly effective, especially for the advanced forms of the disease. In the last year, short double stranded RNA molecules termed small interfering RNAs (siRNAs) and micro interfering RNAs (miRNA), emerged as interesting molecules with potential therapeutic value for HCC. The practical use of these molecules is however limited by the identification of optimal molecular targets and especially by the lack of effective and targeted HCC delivery systems. Here we focus our discussion on the most recent advances in the identification of siRNAs/miRNAs molecular targets and on the development of suitable siRNA/miRNAs delivery systems.
- Citation: Farra R, Grassi M, Grassi G, Dapas B. Therapeutic potential of small interfering RNAs/micro interfering RNA in hepatocellular carcinoma. World J Gastroenterol 2015; 21(30): 8994-9001
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/8994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.8994
Hepatocellular carcinoma (HCC), the predominant form of primary liver cancer, is the third leading cause of cancer-related death worldwide[1-3]. While HCC is most common in Asia and Africa, its incidence in the western countries is increasing. As usually HCC diagnosis occurs in the advanced stage of the disease, available treatments such as resection, liver transplant, or local ablation are poorly if not at all effective. Also systemic chemotherapy has limited effectiveness due to general HCC resistance to anticancer agents[4]. The only drug that can significantly prolong patient survival is Sorafenib[5]; unfortunately, however, the extent of increased survival is very modest being of few months. Together the above considerations clearly indicate that the development of novel therapeutic approaches for HCC are urgently necessary. In this regard, small interfering RNAs (siRNAs) and micro-interfering RNAs (miRNAs), belonging to the big family of nucleic acid based drugs[6,7], are emerging as attractive therapeutic molecules.
MiRNAs and siRNAs are short RNAs synthesized within the cell or deriving from extracellular sources such as viruses and transposons, respectively. Two double-stranded RNA filaments named sense and antisense strand constitute siRNAs and miRNAs. The antisense strand is incorporated into the protein complex RISC (RNA-induced silencing complex) guiding it to the RNA target. In the case of miRNAs, the antisense strand binds the target RNA via a partial complementarity; a perfect complementarity occurs in contrast for the siRNA antisense strand. miRNA-guided RISC induces the down-regulation of target gene expression either via the impairment of protein translation or via the degradation of the target RNA[8]. siRNA-guided RISC represses target gene expression only via target RNA degradation.
As it is possible to chemically generating siRNAs and miRNAs targeted against any cellular RNA, these molecules can be used to down regulate the expression of virtually any gene causing disease. This explains the great therapeutic potential, well witnessed by the numerous papers so far published[7,9-15].
Despite the great therapeutic value, for the practical use of miRNAs/siRNAs two orders of problems need to be addressed. The first is represented by the off targeting effect and the second deals with the effective delivery.
The off targeting effect[8] can be defined as the possibility of miRNAs/siRNAs to interact, in addition to the target RNA, also with other RNAs. This phenomenon is based on an imperfect hybridization between the miRNA/siRNA antisense strand and RNAs different from the target. This can lead to the unwanted silencing of genes resulting in unpredictable effects in the diseased tissue and, more importantly, in the healthy one. To minimize the side effects, different strategies have been developed. Among these, applicable solutions are the scale down of miRNA/siRNA concentration, the chemical modification and the careful choice of the sequences[8].
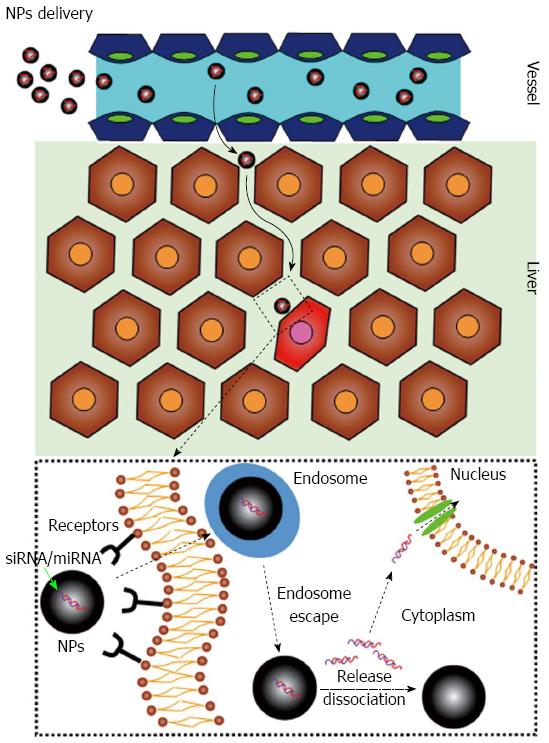
The delivery issue deals with the fact that following systemic administration, siRNAs/miRNAs (Figure 1) can be rapidly degraded by extracellular nucleases, can be eliminated by the reticulo-endothelial system/kidney filtration[16] and may activate the immune system[17,18]. Moreover, being negatively charged hydrophilic molecules, siRNAs/miRNAs are repulsed by the negatively charged surface of the cell membrane and by its hydrophobic layer. Notably, following cellular uptake, siRNAs/miRNAs can be sequestered into endosomes with no possibility to reach the target RNA into the cytoplasm/nucleus. Finally, a targeting strategy to the diseased tissue is utmost desirable to minimize/abolish side effects stemming from the targeting of the healthy tissue (Figure 1). So far, delivery systems based on the use of viral vectors or synthetic vectors have been developed. Here we will briefly discuss the most recent news about the development of synthetic vectors.
Despite the above mentioned problems, active investigations are in progress for: (1) the identification of suitable anti HCC target for siRNA/miRNAs; and (2) the development of effective and targeted delivery systems for siRNA/miRNAs to the liver. Among the several works reported in the past[19], in the following sections, we have reported some of the most notable and recent examples of works aimed at the identification of molecular HCC targets and delivery systems based on the use of miRNAs/siRNAs.
Different strategies have been recently investigated to try to combat HCC (Table 1). Among these, it can be included approaches based on: (1) the targeting of proteins more relevant for HCC cell survival than for normal liver cells; (2) the targeting of genes overexpressed in HCC compared to the healthy liver; and (3) the targeting of genes causing drug resistance.
| No. | Approach | Molecular target | In vivo model | Effects | Ref. |
| 1 | Targeting molecules more relevant for HCC than normal liver | Integrins | Mouse genetically induced HCC | Reduction HCC cell proliferation | 20 |
| 2 | Targeting molecules overexpressed in HCC tissue | ECT2 | Mouse xenograft HCC | Reduction HCC cell proliferation | 21 |
| 3 | Targeting molecules overexpressed in HCC tissue | CDK9 | Mouse orthotopic HCC | Reduction HCC cell proliferation | 22 |
| 4 | Targeting molecules overexpressed in HCC tissue | ISG15 | Mouse xenograft HCC | Reduction HCC cell proliferation | 23 |
| 5 | Targeting of genes causing drug resistance | Mapk14 | Mouse genetically induced HCC | Increased sorafenib sensitivity | 24 |
| 6 | Targeting of genes causing drug resistance | Aurora-A | Mouse xenograft HCC | Increased adriamycin and cisplatin sensitivity | 25 |
Within approach 1, the siRNA-mediated silencing of integrins should be mentioned. Integrins are extracellular matrix receptors playing important and different roles with regard to the regulation of cell motility, survival and proliferation. Bogorad et al[20] have used multiple anti-integrin siRNAs, chemically modified to improve the stability, to reduce the off-target potential and to minimize the possible immune response. As delivery device, nanoparticles made by ionisable lipid or cationic lipid were used to form spontaneous complexes with siRNAs. In vivo (rat model), short term (two weeks) targeting of integrins did not significantly alter liver functions, as evaluated by histological analysis and by checking the biochemical markers of liver function. However, long-term down-regulation (seven weeks) caused alterations in liver morphology. In HCC tissue, integrins targeting invariably down modulate HCC progression, via the reduction of cell proliferation and the increase of tumor cell death. These data indicate that HCC cells are more sensitive to integrins targeting than normal hepatocytes. This supports the concept that siRNA-mediated inhibition of integrins may preferentially affect HCC tissue in so resulting in a specific effect. However, the observed negative effects to the healthy liver in the long-term suggests that a more refined delivery approach has to be developed for futures studies.
More common in the literature are works addressing the silencing of gene over-expressed in HCC compared to normal liver (approach 2). Clinicians know very well that a major problem in improving the survival of HCC patients is represented by the early recurrence of the disease. To study this aspect, Chen et al[21] explored the possibility of down regulating the Epithelial cell transforming sequence 2 (ECT2). Overexpression of ECT2 has been described in several human cancers including lung, oesophageal and glioblastoma. In HCC, the authors found that ECT2 is up-regulated in tumor samples compared to normal tissues. Moreover, ECT2 expression is markedly up-regulated in early recurrent HCC tissues compared to non-recurrent. Notably, patients with high ECT2 expression have a significantly shorter disease free duration compared to patients with low ECT2 expression. In a subcutaneous xenograft model of HCC, it was demonstrated that ECT2 targeting by siRNAs delivered via a viral vector, significantly reduced tumor growth. This was due to a G1 phase arrest secondary to the inhibition of the Rho/ERK pathway. Despite no reported evidence of improved animal survival, this work opens the possibility that ECT2 is an attractive target to reduce HCC recurrence.
Huang et al[22] have used an interesting approach to identify novel anti HCC target. The authors established a murine HCC model driven by Myc overexpression and p53 loss, two genetic signatures commonly found in human HCC. The authors screened in the HCC model developed a custom library of short hairpin RNAs (shRNAs) directed against known drug targets. By this approach, it was possible to identify Cdk9, a key component of the transcription elongation factor b complex. In vitro and in a murine orthotopic model of HCC, it was shown that the siRNA mediated silencing of CdK9 down modulates the aberrant proliferation of Myc-overexpressing HCC tumor. These data indicate CDK9 inhibition as a therapeutic strategy for Myc-overexpressing liver tumors. No evidences of the effects of CdK9 targeting in the normal liver cells were reported, thus a possible toxicity for the normal liver cannot be excluded. Of course, this event may be circumvented by using delivery systems specifically targeting HCC cells but not normal hepatocytes.
To control viral infection, cells produce interferons (IFNs), a series of pro-inflammatory cytokines, which can block viral infection and replication. IFNs exert their action via the activation of a number of effectors among which IFN-stimulated genes 15 (ISG15). This is an ubiquitin-like protein that conjugates to cellular substrates to form ISGylated proteins; the conjugation provides a tag that either marks the labeled protein for degradation or modulates its function. Aberrant functioning of ISG15 correlates with aberrant cell signaling and malignant transformation. Recently it has been shown[23] that ISG15 is highly expressed in HCC tissues and its expression significantly correlates with the HCC differentiation grade and patient survival. Based on these observations, in a subcutaneous xenograft mouse model of HCC, the authors administered by direct tumor injection, an anti ISG15 siRNA. ISG15 silencing significantly inhibited tumor growth and reduced micro-vessel density. Notably, a prolonged animal survival of about 15% was observed. Whereas future investigation on the effects of ISG15 targeting in healthy hepatocytes are required, the data reported constitute a first step in the identification of ISG15 as a valuable siRNA target to counteract HCC.
With regard to approach 3 (targeting of genes causing drug resistance), it should be mentioned the work of Rudalska et al[24]. The authors have proposed a strategy based on the identification of gene products whose inhibition can increase the therapeutic efficacy of the only approved anti HCC drug, i.e., sorafenib. It was thus developed a novel siRNA-based approach to screen in vivo genes whose inhibition increases the therapeutic efficacy of sorafenib. Because of this investigation, the kinase Mapk14 resulted to be a key element of sorafenib resistance in mouse and human liver cancer. The siRNA mediated intra-tumor knockdown of Mapk14, sensitized the mice to sorafenib treatment resulting in a significant longer survival and reduced tumor growth compared to mice treated by sorafenib alone. Notably, Mapk14 inhibition did not result in a significant toxicity for the treated animals, as evaluated by body weight measurement. Moreover, the authors showed that the combinatorial treatment with sorafenib and Mapk14 inhibitor had no negative impact on liver regeneration. This is a pivotal feature for any HCC therapeutic approach; indeed, liver carcinomas mostly arise in cirrhotic livers with impaired function and regenerative capacity, which should not be affected by the therapeutic approach. As the authors demonstrated the significant lack of toxicity following Mapk14 chemical inhibition alone, the same should be done also when using siRNAs.
Aurora kinases are a novel family of serine/threonine kinases which includes Aurora-A, Aurora-B and Aurora-C. Aurora-A is crucial for mitosis and has a relevant role in tumorigenesis. In HCC the expression level of Aurora-A has been proposed as a reliable marker to predict HCC patient prognosis. Based on these observations, Zhang et al[25] studied the effects of Aurora-A silencing by siRNAs. In HCC cell lines, Aurora-A silencing significantly reduced colony formation and promoted apoptosis. Moreover, the down regulation of Aurora improved the chemo-sensitivity to the chemotherapeutic agents adriamycin and cisplatin via the increase of apoptosis. These data were subsequently confirmed in a subcutaneous xenograft mouse model where it was shown that not only Aurora-A silencing reduced tumor growth but also potentiated the effects of adriamycin and cisplatin. Despite no evidences of improved animal survival have been reported and no investigations on the effects of Aurora-A silencing in healthy hepatocytes have been performed, this study may pave the way for a novel approach to HCC treatment.
In addition of being effective, therapeutic approaches should be as specific as possible to avoid significant toxic effects. The works above discussed indicate that a certain level of specificity can be achieved by targeting gene products more relevant for HCC cell survival than normal liver cells. However, this level of specificity has to be improved, especially for drugs administered systemically. To this end, researchers have explored the possibility to develop liver specific delivery following various approaches (Table 2).
| No. | Approach | Molecular target | In vivo model | Effects | Ref. |
| 1 | PEI-GAL nano particles | ApoB | Normal mouse liver | Healthy liver targeting | 26 |
| 2 | PHEA-DEAEMA | E2F1 | - | HCC cell in vitro targeting | 27 |
| 3 | Inu-DETA | E2F1 | - | HCC cell in vitro targeting | 29 |
| 4 | Lipid-PEG | FVII | Normal mouse liver | Healthy liver targeting | 31 |
| 5 | pH-sensitive lipids | miRNA 122 | Normal mouse liver | Healthy liver targeting | 32 |
| 6 | US sensible lipids | BCL-2 | Mouse Xenograft HCC | HCC targeting | 33 |
Sajeesh et al[26] developed nanoparticles (NPs) constituted by siRNAs bound to linear high molecular weight polyethyleneimine (PEI), functionalized with galactose (GAL) units. PEI, a polymer with repeating unit composed of amine, is able to condense nucleic acid molecules into positively charged particles, which in turn can bind to the anionic surface of cells allowing uptake via endocytosis. Galactose is a molecule recognized by the asialoglycoproteins receptors present on liver cells, thus facilitating a receptor-mediated uptake. In vitro, NPs containing GAL modified PEI complexed with Cy3 labeled siRNA, reached significantly better uptake in the liver Hep3B and Hepa 1-6 cells compared to NPs lacking GAL residues. Functional studies, performed using siRNAs targeted against the ApoB gene, showed a marked reduction in the target mRNA which was reduced down to 70% at the siRNA concentration of 100 nmol/L. NPs delivered systemically in mouse, showed the maximum accumulation in the liver region with a remarkable reduction in target mRNA which reached 40%. This interesting approach may represent a starting point to develop HCC specific delivery system; however, it has to be improved the ability to discriminate between healthy and tumor liver cells.
Recently we developed a novel polymeric-based delivery system for siRNAs in HCC cells. We used a copolymer based on α,β-poly(N-2-hydroxyethyl)-D,L-aspartamide (PHEA) bearing positively chargeable side oligochains, with diethylamino ethyl methacrylate as monomer. In vitro, a siRNA against the transcription factor E2F1, was successfully delivered to the HCC cell line HuH7. Consequently, the successful E2F1 targeting resulted in a significant decrease of HuH7 proliferation, as expected due to the relevant role of E2F1 in HCC[27,28]. An advantage of our system is that it can effectively deliver not only siRNA but also plasmid DNA (pDNA). Thus, it allows minimizing possible artifacts introduced by the use of different delivery agents for siRNAs and pDNA. The possibility to employ the same delivery system is very convenient for many experimental settings where long (pDNA) and short (siRNA) needs to be delivered to same target cells. Potentially, our system can allow the co-delivery of a therapeutic siRNA together with a therapeutic long nucleic acid encoded by the plasmid. Future studies in animal models will better define the effectiveness of our copolymer (work in progress).
Another novel siRNA delivery-polymer we recently developed is based on inulin (Inu), an abundant and natural polysaccharide, conjugated with diethylenetriamine (DETA) residues (Inu-DETA)[29]. Our data indicate that Inu-DETA polyplexes can effectively bind siRNAs, are highly cyto-compatible and, in the hepatocellular carcinoma cells JHH6, can effectively deliver functional siRNA targeted against the mRNA of the cell promoter gene E2F1. This, in turn, induces a potent anti-proliferative effect. Notably, we observed that the mechanism of polymer-siRNA uptake and trafficking, i.e., micropinocytosis and clatrin mediated endocytosis, is crucial to allow the proper siRNA cellular distribution and thus effectiveness in JHH6. In contrast, in the bronchial cell line 16HBE where the uptake mechanism (caveolae mediated endocytosis) does not allow the proper distribution of siRNA within the cell, no significant effects are observed. This data stresses the relevance of the proper trafficking for efficient siRNA delivery and suggests that delivery specificity may be also achieved at the trafficking level. Future in vivo investigations will better define this peculiar feature of our delivery polymer.
Lipids are frequently used as siRNA delivery devices in many experimental therapeutic approaches[30] including those targeting the liver. Cationic lipids, bearing different degrees of positive charges, are the most studied system used for nucleic acid delivery. They can protect siRNAs from degradation and, due to the positive charge, can promote the binding to the negatively charged cell surface. Lipid NPs can be combined with polyethylene glycol (PEG), a polymer consisting of ethylene oxide able to stabilize lipid NPs. In addition, PEG can protect lipid NPs shielding them from inactivation by the immune system. Using lipid-PEG carriers, Chen et al[31] studied the possibility to prepare liver directed NPs containing siRNAs via subcutaneous (sc) administration. The advantages of s.c. administration compared to iv include the potential for self-administration and a prolonged therapeutic window due to a depot effect. The authors investigated the effects of NPs size and the influence of hepatocyte-specific targeting ligands. Variation in NP size was obtained by varying the PEG-lipid content. Optimal NPs size for liver accumulation was found to be of approximately 45 nm. These NPs allowed a reduction of the siRNA target (FVII mRNA) down to 80% of control. FVII, one of the proteins that causes blood to clot in the coagulation cascade, is produced and secreted by hepatocytes into the circulation. Notably, following a single sc injection with 1 mg/kg of NPs, the amount of circulating FVII was reduced down to 50% with the effect lasting up to seven days. Smaller particles (approximately 35 nm) were far less effective. However, the incorporation into these smaller NPs of galactose residues, dramatically improved the efficacy as almost 90% of FVII knockdown was obtained with the effect lasting for 7 d. These data confirm the usefulness of using liver targeting molecules and indicate the relevance of NPs physical properties such as the size.
Lipids have been also utilized to deliver nucleic acid molecules without conjugates; in this case, other smart modifications have been introduced. For example, Hatakeyama et al[32] developed lipid NPs (MEND) containing a pH-sensitive cationic lipid YSK05 (YSK05-MEND). These NPs, being pH-sensitive, favor endosome escape and thus nucleic acid diffusion in the cytosol. The authors loaded NPs with a short nucleic acid (anti miRNA 122) targeting miRNA 122. This is a liver specific miRNA playing important roles in liver physiology (lipid metabolism) and HCC. The choice of targeting a liver specific miRNA, confers to the approach an intrinsic specificity. In cultured liver cancer cells (Hepa1c1c7), YSK05-MEND had improved delivery efficacy compared to the commercially available lipid Lipofectamine 2000 (LFN2k), mostly due to the efficient endosomal escape. Notably, significantly reduced toxicity was observed compared to LFN2k. In mouse, following vein tail administration, there was a predominant accumulation in the liver. The authors explained this observation with the fact that neutral liposomes tend to absorb apolipoprotein E (apoE) in the circulation; this in turn enhances the uptake of the liposomes by hepatocytes via specific receptors (low-density lipoprotein receptors). In this regard, it would have been interesting to test whether the delivery system developed has the possibility to discriminate between healthy and cancer hepatocytes in vivo. The accumulation of NPs in the liver was paralleled by the knockdown of miR-122 with the increase of the mRNA levels of the miR-122 target genes. This effect lasted up to two weeks. Notably, no major toxicity was observed as evaluated by mouse body weight measurement over 25 d and by monitoring liver transaminases levels (48 h after NPs injection). This smart work indicates that liver targeting may be achieved also via physiological features such as the lipid absorption of apoE.
Lipid NPs carrying nucleic acids can also be prepared to contain an ultrasound (US) gas. The application of a US pressures above a certain threshold causes a violent NPs collapse which results in NPs disruption and nucleic acid release. In vivo, US also determine the transient disruptions of cell membranes, thus allowing the transport of nucleic acid from NPs to cells. Applying US at a defined site, it is also possible obtaining a specific delivery via a physical approach. Yin et al[33] have used the US sensible NPs (US-NPs) to deliver a siRNA together with the anti-tumor drug paclitaxel (PTX). In the HCC cell line HepG2 in the presence of US, US-NPs loaded by a red fluorescent siRNA and a green vital die, could deliver efficiently the two molecules through the entire cells and to the cytoplasm, respectively. Notably, in the absence of US, the delivery efficiency was dramatically reduced. US-NPs loaded PTX and a siRNA against the mRNA of the anti-apoptotic gene BCL-2, resulted in the remarkable down regulation of BCL-2 in the presence of US. This in turn determined a potent pro-apoptotic effect significantly superior to those obtained by US-NPs loaded with PTX or siRNA alone. For in vivo studies, the authors used a mouse HepG2 xenograft subcutaneous model. Following die labelled US-NPs delivery via the tail vein, it was possible to observe a clear tumor localization of US-NPs only upon US application. Using US-NPs loaded by PTX and siRNA anti BCL-2, the application of US substantially blocked the tumor growth; this resulted in an increase of animal survival compared to all the controls considered (US-NPs with PTX and siRNA without US application, US-NPs with PTX and US application, US-NPs with siRNA and US application). This work is particularly interesting as it shows the possibility to get a target delivery via a physical approach. It remains to be determined how efficient the system could be in an orthotopic model of HCC. Despite this, the work demonstrates the possibility to overcome HCC drug resistance by combining clinically used drug and siRNA.
Effective therapeutic approaches for HCC, especially in its advanced forms, are now not available. In this regard, the use of siRNAs/miRNAs is considered to have a great therapeutic potential. This is substantially because it is possible to generate siRNAs/miRNAs silencing the expression of any genes implicated in HCC. Additionally, by properly addressing the possible off targeting effect, it is feasible to reach a great specificity of biological action. The use of multiple siRNAs/miRNAs also allows to target different members of a pathological pathway, potentially resulting in an improved anti HCC effects. In addition, the combination of siRNAs/miRNAs administered with clinical used drugs may reciprocally potentiate the therapeutic effects of the two types of molecules.
Despite the great therapeutic potential, the use of siRNAs/miRNAs as anti HCC agents has not yet reached the clinical relevance. This is substantially due to the lack of appropriate delivery systems. If administered as naked molecules, siRNAs/miRNAs are rapidly degraded by nucleases, cleared by the kidneys and cannot efficiently cross cellular membranes. Whereas these aspects can be significantly circumvented by the available delivery systems, further optimization is required for the targeting issue. Examples of liver directed delivery systems are described in the literature; however, improvements in the possibility to discriminate between HCC and normal liver cells is still necessary. Additionally, it should be considered that the HCC tissue is almost exclusively arterially vascularized, unlike the normal liver, which has a dual supply with a portal component of 75% to 80% and an arterial component of only 20% to 25%[34]. This implies that it will be important to concentrate the attention on the development of delivery systems suited for the intra-arterial administration rather the iv.
In conclusion, despite further improvements in the use of siRNA/miRNAs as potential novel anti HCC drugs are necessary, the progresses achieved in the last years fully justify further efforts in the field.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 2. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 405] [Article Influence: 25.3] [Reference Citation Analysis (2)] |
| 3. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3126] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 4. | Colombo M. Multidisciplinary approach to hepatocellular carcinoma. Preface. Dig Liver Dis. 2010;42 Suppl 3:S205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10525] [Article Influence: 584.7] [Reference Citation Analysis (9)] |
| 6. | Grassi G, Dawson P, Guarnieri G, Kandolf R, Grassi M. Therapeutic potential of hammerhead ribozymes in the treatment of hyper-proliferative diseases. Curr Pharm Biotechnol. 2004;5:369-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Grassi M, Cavallaro G, Scirè S, Scaggiante B, Dapas B, Farra R, Baiz D, Giansante C, Guarnieri G, Perin D. Current Strategies to Improve the Efficacy and the Delivery of Nucleic Acid Based Drugs. Curr Signal Transduct Ther. 2010;5:92-120. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Scaggiante B, Dapas B, Farra R, Grassi M, Pozzato G, Giansante C, Fiotti N, Grassi G. Improving siRNA bio-distribution and minimizing side effects. Curr Drug Metab. 2011;12:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Dapas B, Farra R, Grassi M, Giansante C, Fiotti N, Uxa L, Rainaldi G, Mercatanti A, Colombatti A, Spessotto P. Role of E2F1-cyclin E1-cyclin E2 circuit in human coronary smooth muscle cell proliferation and therapeutic potential of its downregulation by siRNAs. Mol Med. 2009;15:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Farra R, Dapas B, Pozzato G, Scaggiante B, Agostini F, Zennaro C, Grassi M, Rosso N, Giansante C, Fiotti N. Effects of E2F1-cyclin E1-E2 circuit down regulation in hepatocellular carcinoma cells. Dig Liver Dis. 2011;43:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Werth D, Grassi G, Konjer N, Dapas B, Farra R, Giansante C, Kandolf R, Guarnieri G, Nordheim A, Heidenreich O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur J Cell Biol. 2010;89:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Farra R, Dapas B, Pozzato G, Giansante C, Heidenreich O, Uxa L, Zennaro C, Guarnieri G, Grassi G. Serum response factor depletion affects the proliferation of the hepatocellular carcinoma cells HepG2 and JHH6. Biochimie. 2010;92:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Lang C, Sauter M, Szalay G, Racchi G, Grassi G, Rainaldi G, Mercatanti A, Lang F, Kandolf R, Klingel K. Connective tissue growth factor: a crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med (Berl). 2008;86:49-60. [PubMed] |
| 14. | Agostini F, Dapas B, Farra R, Grassi M, Racchi G, Klingel K, Kandolf R, Heidenreich O, Mercatahnti A, rainaldi G. Potential applications of small interfering RNAs in the cardiovascular field. Drug Future. 2006;31:513-525. [DOI] [Full Text] |
| 15. | Grassi G, Scaggiante B, Dapas B, Farra R, Tonon F, Lamberti G, Barba A, Fiorentino S, Fiotti N, Zanconati F. Therapeutic potential of nucleic acid-based drugs in coronary hyper- proliferative vascular diseases. Curr Med Chem. 2013;20:3515-3538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Huang Y, Hong J, Zheng S, Ding Y, Guo S, Zhang H, Zhang X, Du Q, Liang Z. Elimination pathways of systemically delivered siRNA. Mol Ther. 2011;19:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 427] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 819] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 19. | Scaggiante B, Kazemi M, Pozzato G, Dapas B, Farra R, Grassi M, Zanconati F, Grassi G. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20:1268-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Bogorad RL, Yin H, Zeigerer A, Nonaka H, Ruda VM, Zerial M, Anderson DG, Koteliansky V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat Commun. 2014;5:3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Chen J, Xia H, Zhang X, Karthik S, Pratap SV, Ooi LL, Hong W, Hui KM. ECT2 regulates the Rho/ERK signalling axis to promote early recurrence in human hepatocellular carcinoma. J Hepatol. 2015;62:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Huang CH, Lujambio A, Zuber J, Tschaharganeh DF, Doran MG, Evans MJ, Kitzing T, Zhu N, de Stanchina E, Sawyers CL. CDK9-mediated transcription elongation is required for MYC addiction in hepatocellular carcinoma. Genes Dev. 2014;28:1800-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, Liu H, Zhu H. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014;5:8429-8441. [PubMed] |
| 24. | Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang TW, Hohmeyer A, Pesic M, Leibold J, von Thun A. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med. 2014;20:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 25. | Zhang K, Chen J, Chen D, Huang J, Feng B, Han S, Chen Y, Song H, De W, Zhu Z. Aurora-A promotes chemoresistance in hepatocelluar carcinoma by targeting NF-kappaB/microRNA-21/PTEN signaling pathway. Oncotarget. 2014;5:12916-12935. [PubMed] |
| 26. | Sajeesh S, Lee TY, Kim JK, Son da S, Hong SW, Kim S, Yun WS, Kim S, Chang C, Li C. Efficient intracellular delivery and multiple-target gene silencing triggered by tripodal RNA based nanoparticles: a promising approach in liver-specific RNAi delivery. J Control Release. 2014;196:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Farra R, Dapas B, Baiz D, Tonon F, Chiaretti S, Del Sal G, Rustighi A, Elvassore N, Pozzato G, Grassi M. Impairment of the Pin1/E2F1 axis in the anti-proliferative effect of bortezomib in hepatocellular carcinoma cells. Biochimie. 2015;112:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Zhan L, Huang C, Meng XM, Song Y, Wu XQ, Miu CG, Zhan XS, Li J. Promising roles of mammalian E2Fs in hepatocellular carcinoma. Cell Signal. 2014;26:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Sardo C, Farra R, Licciardi M, Dapas B, Scialabba C, Giammona G, Grassi M, Grassi G, Cavallaro G. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. Eur J Pharm Sci. 2015;75:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Bochicchio S, Dalmoro A, Barba AA, Grassi G, Lamberti G. Liposomes as siRNA delivery vectors. Curr Drug Metab. 2014;15:882-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Chen S, Tam YY, Lin PJ, Leung AK, Tam YK, Cullis PR. Development of lipid nanoparticle formulations of siRNA for hepatocyte gene silencing following subcutaneous administration. J Control Release. 2014;196:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, Harashima H. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J Control Release. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Yin T, Wang P, Li J, Wang Y, Zheng B, Zheng R, Cheng D, Shuai X. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials. 2014;35:5932-5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Cazejust J, Bessoud B, Colignon N, Garcia-Alba C, Planché O, Menu Y. Hepatocellular carcinoma vascularization: from the most common to the lesser known arteries. Diagn Interv Imaging. 2014;95:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Balaban YH, Dang SS, Xu Y, Zhang XC S- Editor: Yu J L- Editor: A E- Editor: Ma S