Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.977
Peer-review started: July 9, 2014
First decision: August 15, 2014
Revised: September 2, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: January 21, 2015
Processing time: 196 Days and 1.8 Hours
AIM: To investigate the safety and effectiveness of endoscopic therapy with a paclitaxel-eluting balloon (PEB) for biliary anastomotic stricture (AS) after liver transplantation (LT).
METHODS: This prospective pilot study enrolled 13 consecutive eligible patients treated for symptomatic AS after LT at the University Hospital of Münster between January 2011 and March 2014. The patients were treated by endoscopic therapy with a PEB and followed up every 8 wk by endoscopic retrograde cholangiopancreatography (ERCP). In cases of re-stenosis, further balloon dilation with a PEB was performed. Follow-up was continued until 24 mo after the last intervention.
RESULTS: Initial technical feasibility, defined as successful balloon dilation with a PEB during the initial ERCP procedure, was achieved in 100% of cases. Long-term clinical success (LTCS), defined as no need for further endoscopic intervention for at least 24 mo, was achieved in 12 of the 13 patients (92.3%). The mean number of endoscopic interventions required to achieve LTCS was only 1.7 ± 1.1. Treatment failure, defined as the need for definitive alternative treatment, occurred in only one patient, who developed recurrent stenosis with increasing bile duct dilatation that required stent placement.
CONCLUSION: Endoscopic therapy with a PEB is very effective for the treatment of AS after LT, and seems to significantly shorten the overall duration of endoscopic treatment by reducing the number of interventions needed to achieve LTCS.
Core tip: Biliary anastomotic stricture is common after liver transplantation and can significantly impair both organ and patient survival. Endoscopic treatment of an anastomotic stricture usually requires many interventions before long-term resolution is achieved. This study investigated the safety and efficacy of an innovative approach using endoscopic therapy with a paclitaxel-eluting balloon for the treatment of biliary anastomotic stricture after liver transplantation. Our data are very promising and show excellent long-term results. Furthermore, use of a paclitaxel-eluting balloon seems to reduce the number of endoscopic interventions needed to achieve sustained resolution of the stricture.
- Citation: Hüsing A, Reinecke H, Cicinnati VR, Beckebaum S, Wilms C, Schmidt HH, Kabar I. Paclitaxel-eluting balloon dilation of biliary anastomotic stricture after liver transplantation. World J Gastroenterol 2015; 21(3): 977-981
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/977.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.977
Biliary complications are common after liver transplantation (LT) because the vascular supply to the biliary tract is easily injured, and such complications are a major cause of morbidity and graft failure. Biliary complications are reported to occur in 10%-25% of LT recipients, and may include stricture, leakage, and formation of biliary stones, casts, and sludge[1-3]. Biliary stricture is one of the most commonly reported complications after LT and can be divided into two types: anastomotic stricture (AS) and non-anastomotic stricture. This categorization helps to predict the response to treatment, with AS responding more favorably to endoscopic intervention than non-anastomotic stricture[3,4].
AS is the most frequent biliary complication after LT, accounting for 8%-20% of all complications. Endoscopic retrograde cholangiopancreatography (ERCP) is considered to be the gold standard for diagnosis and treatment of biliary complications after LT. Current standard endoscopic interventions for AS include balloon dilation and stent placement. Most patients require repeated endoscopic procedures for 12 to 24 mo, during which they undergo an average of 5-6 endoscopic interventions. The reported success rate of balloon dilation alone is about 40%, and the reported overall long-term success rate after endoscopic interventions is 75%-80%. However, AS recurrence after successful endoscopic treatment has been reported in up to 30% of cases[5-7].
In recent years, fully covered self-expandable metal stents have been used to treat biliary strictures after LT when standard endoscopic treatment failed. Treatment with such stents has a success rate of nearly 80%, but also has a high complication rate of up to 47%, mainly due to stent migration, stent occlusion, and de novo stricture formation[1,8,9].
New treatment modalities are needed to reduce procedure-related morbidity and the overall duration of endoscopic treatment, as well as to improve long-term results. The short-term outcome data of this study published in 2012 were very promising[10]. The aim of this pilot study was to evaluate the long-term safety and effectiveness of endoscopic therapy with a paclitaxel-eluting balloon (PEB) for AS after LT.
This prospective study was conducted between January 2011 and March 2014 in the Department of Transplant Medicine, University Hospital of Münster. Thirteen consecutive eligible patients were enrolled between January 2011 and September 2011, and follow-up was performed until March 2014.
All patients gave written informed consent, and the study was conducted in accordance with the guidelines of the Declaration of Helsinki (2004 revision). The study protocol was finalized after consultation with the local institutional review board.
All patients with newly diagnosed AS after LT who had undergone end-to-end choledochocholedochostomy were eligible for inclusion in the study. Patients who had undergone choledochojejunostomy were excluded.
AS was defined as a narrowing of the bile duct, predominantly at the anastomotic site, with markedly reduced passage of contrast material observed on fluoroscopy during ERCP. ERCP was performed if there were clinical, biochemical, or histological signs of cholestasis, or dilated bile ducts on imaging examinations (ultrasonography, computed tomography, or magnetic resonance tomography). Four of the 13 patients (30.8%) had dilated bile ducts on non-invasive imaging examinations.
ERCP was performed using a therapeutic duodenoscope (TJF-180V, Olympus Corp., Tokyo, Japan) under conscious sedation (midazolam and propofol, with or without opiates) and antibiotic prophylaxis (ciprofloxacin 750 mg orally 2 h before and 6 h after the procedure). The bile duct was accessed using a sphincterotome (Tri-Tome, TRI 20, Cook Medical, Winston-Salem, NC, United States) and a wire (0.035 inches, THSF-35-480, Cook Medical) to guide cannulation. Cholangiography was performed to assess the biliary anastomosis. The guidewire was then advanced through the stricture.
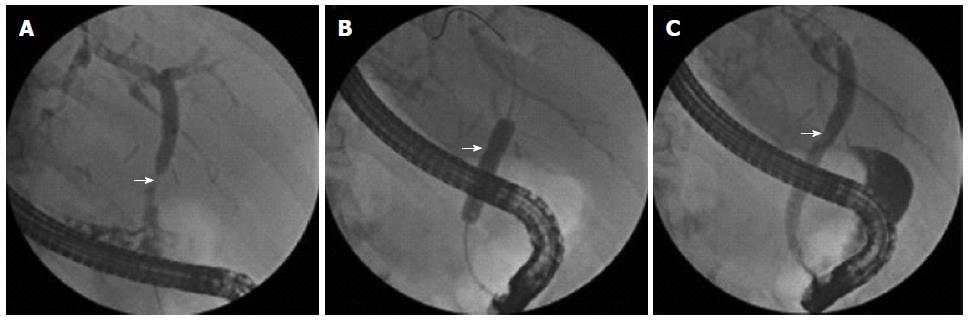
If AS was diagnosed, endoscopic treatment was performed, comprising sphincterotomy followed by dilation with a PEB (DIOR paclitaxel coated coronary balloon dilatation catheter, paclitaxel 3 μg/mm2; Eurocor, Bonn, Germany). The PEB was coated with a 1:1 mixture of paclitaxel and shellac that prevented release of paclitaxel in the working channel of the endoscope. Contact of the hydrophilic shellac mixture with a body fluid such as bile opens the structure to allow pressure-induced release of paclitaxel on the inflated balloon. The PEB was inflated at the anastomotic site to a pressure of up to 12 bar, and left in place for 30 s (Figure 1). After the initial balloon dilation, ERCP was performed every 8 wk to re-evaluate the stricture. If cholangiography showed a patent anastomosis, no further endoscopic treatment was performed. If the stricture persisted, further dilation with a PEB was performed. Follow-up was continued until 24 mo after the last intervention.
The main outcome parameter of this prospective study was long-term clinical success (LTCS), defined as a period of at least 24 mo without the need for further endoscopic intervention, confirmed by the absence of stricture recurrence on follow-up ERCP examinations, laboratory test results indicating cholestasis, or clinical signs of jaundice or cholangitis. The secondary outcome parameters were initial technical feasibility, sustained clinical success (SCS), and treatment failure.
Initial technical feasibility was defined as successful advancement of a guidewire through the AS followed by balloon dilation during the initial ERCP procedure. SCS was defined as a period of at least 6 mo with no need for further endoscopic intervention, confirmed by the absence of stricture recurrence on follow-up ERCP examinations. Treatment failure was defined as the need for definitive alternative treatment at any time during the follow-up period, such as stent placement, percutaneous transhepatic drainage, or surgical intervention.
Descriptive statistical analyses were performed. Data are presented as the mean ± SD. All statistical analyses were performed using SPSS version 20 for Windows.
A total of 13 patients (2 women, 11 men, mean age 50.4 ± 13.8 years) who were referred for endoscopic treatment of newly diagnosed AS after LT were included in the study. The mean time from LT to the initial ERCP procedure was 9.4 ± 3.2 mo. The indications for LT were alcoholic cirrhosis (n = 5), hepatitis B virus infection (n = 2), hepatitis C virus infection (n = 2), hepatocellular carcinoma (n = 3), and primary biliary cirrhosis (n = 1).
The initial technical feasibility rate was 100%, and no intra-procedural complications were recorded. Liver enzyme levels and cholestasis parameters decreased after the initial procedure in all patients (Table 1). Two patients developed acute cholangitis within 3 d after the last intervention, which resolved after antibiotic therapy with imipenem. There were no cases of post-ERCP pancreatitis. The mean follow-up period was 30.3 ± 3.3 mo. SCS was achieved in 12 of 13 patients (92.3%): after one dilation in nine patients (69%), after two dilations in one patient, and after three dilations in two patients. Despite initial technical feasibility, the remaining patient developed AS recurrence with increasing bile duct dilatation proximal to the stenosis after three dilations with a PEB, and eventually underwent conventional stent placement. This case was considered to be a treatment failure. AS recurrence after SCS was observed in 2/12 patients (16.6%). The time interval between the initial balloon dilation and AS recurrence was 8.89 ± 0.63 mo. In each of these two patients, one additional dilation was needed to achieve LTCS. LTCS was eventually achieved in all 12 patients (92.3%) who achieved SCS, after a mean of 1.7 ± 1.1 balloon dilations.
| Before intervention | 7 d after intervention | 24 mo after last intervention | |
| Bilirubin | 6.8 ± 4.1 | 2.2 ± 1.3 | 1.4 ± 0.6 |
| (reference value < 1.2 mg/dL) | |||
| GPT | 149.1 ± 120.1 | 51.5 ± 21.3 | 35.1 ± 3.9 |
| (reference value 10-35 U/L) | |||
| GGT | 614.5 ± 330.0 | 107.1 ± 62.8 | 37.7 ± 10.1 |
| (reference value < 39 U/L) |
After successful endoscopic treatment of the stenosis, the bilirubin and liver enzyme levels decreased over time in all patients (Table 1).
Biliary tract complications are common after LT, and present a therapeutic challenge for endoscopists. Endoscopic intervention is currently the gold standard treatment for AS after LT[1-3], but the optimal treatment strategy for this complication is still unclear. Repeated endoscopic interventions over a prolonged treatment period of up to 24 mo are usually needed to achieve SCS[4-7]. Moreover, there are few data available regarding the rate of AS recurrence after SCS[4]. When endoscopic therapy is unsuccessful, surgical treatment is indicated[2,11].
The limitations of the currently available treatment options clearly demonstrate the need for development of new interventions. Considering the fibroproliferative aspect of AS, a therapeutic approach that combines balloon dilation with application of an antiproliferative agent directly to the site of the stenosis seems logical. The mitotic inhibitor paclitaxel has antiproliferative effects as well as antifibrotic effects via inhibition of transforming growth factor-beta/Smad activity[12,13]. Use of PEBs has previously been shown to be clinically safe and effective for the treatment of coronary and femoro-popliteal arterial stenoses[14,15].
This is the first study to investigate endoscopic treatment with a PEB for AS after LT. SCS was achieved in 92.3% of cases, and was achieved after only one balloon dilation in 69% of cases. AS recurrence after SCS occurred in 16.6% of patients, which is much less frequent than the previously reported recurrence rate of about 30%[5-7]. All cases of AS recurrence were successfully treated by repeat balloon dilation. LTCS was achieved in 92.3% of cases, after 1.7 ± 1.1 balloon dilations. This is a notable achievement in comparison with previous reports that five or six interventions were generally required to achieve sustained resolution of AS[1-3,5].
Successful endoscopic treatment was associated with significantly decreased levels of bilirubin and liver enzymes (Table 1).
In this pilot study, endoscopic therapy with a PEB achieved a high rate of LTCS and a low rate of AS recurrence after SCS, indicating very promising outcomes for our treatment approach for AS after LT. Use of a PEB seems to reduce the number of interventional procedures required to achieve LTCS, thereby shortening the overall duration of endoscopic treatment. A reduced number of endoscopic interventions may also result in a decreased overall complication rate. Larger randomized clinical trials are needed to confirm our findings.
Biliary anastomotic stricture (AS) is an important cause of morbidity and graft failure after liver transplantation (LT). The reported success rate of endoscopic therapy for AS after LT is variable, and the optimal treatment for this complication is still unclear.
Patients with AS after LT usually undergo repeated endoscopic interventions, with balloon dilation with or without stent implantation, over a long time period. However, the reported success rate of balloon dilation alone is relatively low, and stent placement has a high complication rate.
Paclitaxel is an antifibrotic and antiproliferative agent. As AS after LT results from inflammatory fibrosis, this study investigated the effectiveness of endoscopic therapy with a paclitaxel-eluting balloon (PEB) for the treatment of such strictures. This is the first study to investigate the use of a PEB for the treatment of AS after LT.
The results of this study show that endoscopic therapy with a PEB may significantly reduce the number of interventions required to achieve complete resolution of AS after LT, thereby shortening the overall duration of endoscopic therapy.
After LT, AS may develop at the site of bile duct anastomosis. AS is caused by fibrosis during healing, and most cases develop within the first year after LT. Paclitaxel is a mitotic inhibitor that is used as a chemotherapeutic agent for the treatment of malignant tumors. Because of its antiproliferative effects, paclitaxel is also used to treat and prevent coronary restenosis.
In this paper, the authors reported the results of an extended follow up of their series of patients, included in a previous publication from the same group. Although new information is limited, the paper is interesting and well written.
| 1. | Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Pascher A, Neuhaus P. Bile duct complications after liver transplantation. Transpl Int. 2005;18:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Williams ED, Draganov PV. Endoscopic management of biliary strictures after liver transplantation. World J Gastroenterol. 2009;15:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, Treichel U, Gerken G. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kulaksiz H, Weiss KH, Gotthardt D, Adler G, Stremmel W, Schaible A, Dogan A, Stiehl A, Sauer P. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy. 2008;40:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Sauer P, Chahoud F, Gotthardt D, Stremmel W, Weiss KH, Büchler M, Schemmer P, Weitz J, Schaible A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Traina M, Tarantino I, Barresi L, Volpes R, Gruttadauria S, Petridis I, Gridelli B. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kabar I, Cicinnati VR, Beckebaum S, Cordesmeyer S, Avsar Y, Reinecke H, Schmidt HH. Use of paclitaxel-eluting balloons for endotherapy of anastomotic strictures following liver transplantation. Endoscopy. 2012;44:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Buxbaum JL, Biggins SW, Bagatelos KC, Ostroff JW. Predictors of endoscopic treatment outcomes in the management of biliary problems after liver transplantation at a high-volume academic center. Gastrointest Endosc. 2011;73:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhou J, Zhong DW, Wang QW, Miao XY, Xu XD. Paclitaxel ameliorates fibrosis in hepatic stellate cells via inhibition of TGF-beta/Smad activity. World J Gastroenterol. 2010;16:3330-3334. [PubMed] [DOI] [Full Text] |
| 13. | Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, Liu Y, Peng Y, Haruna Y, Kanwar YS. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest. 2010;90:436-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113-2124. [PubMed] [DOI] [Full Text] |
| 15. | Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831-840. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Camellini L, Guo XZ S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM