Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8943
Peer-review started: December 23, 2014
First decision: February 2, 2015
Revised: March 25, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: August 7, 2015
Processing time: 229 Days and 2.8 Hours
AIM: To investigate the feasibility, advantages and disadvantages of two types of anvil insertion techniques for esophagojejunostomy after laparoscopic total gastrectomy.
METHODS: This was an open-label prospective cohort study. Laparoscopy-assisted radical total gastrectomy with D2 lymph node dissection was performed in 84 patients with primary non-metastatic gastric cancer confirmed by pre-operative histological examination. Overweight patients were excluded, as well as patients with peritoneal dissemination and invasion of adjacent organs. After total gastrectomy, all patients were randomized into two groups. Patients in Group I underwent esophagojejunostomy using a transorally-inserted anvil (OrVilTM), while patients in Group II underwent esophagojejunostomy using the hemi-double stapling technique (HDST). Both types of esophagojejunostomy were performed under laparoscopy. Patients’ baseline characteristics, preoperative characteristics, perioperative characteristics, short-term postoperative outcomes and operation cost were compared between the two groups. The primary endpoint was evaluation of the surgical outcome (operating time, time of digestive tract reconstruction and time of anvil insertion) and the medical cost of each operation (operation cost and total cost of hospitalization). The secondary endpoints were time to solid diet, post-surgical hospitalization time, time to defecation, time to ambulation and intra-operative blood loss. In addition, complications were assessed and compared.
RESULTS: Laparoscopic total gastrectomy and esophagojejunostomy were successfully performed in all 84 patients, without conversion to laparotomy. There were no significant differences in the operative time and time for total gastrectomy between the two groups (287.8 ± 38.4 min vs 271.8 ± 46.1 min, P = 0.09, and 147.7 ± 31.6 min vs 159.8 ± 33.8 min, P = 0.09, respectively). The time for digestive tract reconstruction and for anvil insertion were significantly decreased in Group II compared with Group I (47.8 ± 12.1 min vs 55.4 ± 15.7 min, P = 0.01, and 12.6 ± 4.7 min vs 18.7 ± 7.5 min, P = 0.001, respectively). Intra-operative blood loss (96.4 ± 32.7 mL vs 88.2 ± 36.9 mL, P = 0.28), time to defecation (3.5 ± 0.9 d vs 3.2 ± 1.1 d, P = 0.12), time to ambulation (3.9 ± 0.7 d vs 3.6 ± 1.1 d, P = 0.12), time to solid diet (7.6 ± 1.4 d vs 8.0 ± 2.7 d, P = 0.31) and total hospitalization (10.6 ± 2.6 d vs 10.8 ± 3.5 d, P = 0.80) were similar between the two groups. In addition, the total costs of hospitalization were similar between the two groups (73848.7 ± 11781.0 RMB vs 70870.3 ± 14003.5 RMB, P = 0.296), but operation cost was significantly higher in Group I compared with Group II (32401.9 ± 1981.6 RMB vs 26961.9 ± 2293.8 RMB, P < 0.001).
CONCLUSION: Anvil insertion was faster and easier using the HDST technique compared with OrVilTM, and was more cost-effective. There was no significant difference in safety.
Core tip: Reconstruction of the digestive tract after total gastrectomy is technically difficult using laparoscopy. This study investigated two different methods to simplify this technique: a transorally inserted anvil (OrVilTM) and the hemi-double stapling technique (HDST). The patients were randomized for comparison of these methods after laparoscopy-assisted radical total gastrectomy with D2 lymph node dissection. Both methods had similar safety and operation success. However, anvil insertion was faster and easier with HDST than with OrVilTM, and was more cost-effective.
- Citation: Wang H, Hao Q, Wang M, Feng M, Wang F, Kang X, Guan WX. Esophagojejunostomy after laparoscopic total gastrectomy by OrVilTM or hemi-double stapling technique. World J Gastroenterol 2015; 21(29): 8943-8951
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8943.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8943
Surgery is the main treatment for gastric cancer[1]. Laparoscopy-assisted radical gastrectomy for gastric cancer has been used for more than 20 years[2]. The improvement of these techniques and apparatus have led to a gradual expansion of the available laparoscopy-assisted surgical methods for gastric cancer, allowing a more complete dissection of lymph nodes[3,4]. However, there are still some technical issues, and reconstruction of the digestive tract after total gastrectomy is one of these.
Delayed development of laparoscopic total gastrectomy is mainly attributed to the high technical requirements for laparoscopic esophagojejunostomy. Currently, many methods are available for reconstruction of the digestive tract after total gastrectomy[5-7]. An upper vertical midline incision with a length of about 5-7 cm in the abdominal wall is usually used to perform esophagojejunostomy after laparoscopic total gastrectomy. The length of the incision might even reach 8-11 cm in some obese patients, resulting in a more invasive treatment[8]. Side-to-side esophagojejunal anastomosis has been used for many years. This method has a large anastomotic diameter and anastomotic stricture is not easy to perform after surgery, but could solve some problems of esophagojejunostomy[7,9,10]. End-to-side esophagojejunal anastomosis is still a widely used anastomotic method[11]. However, some procedures are very difficult to perform, such as placement of the stapler anvil on the esophageal stump. If an open operation is performed, the first step of this process is to perform a purse-string suture of the lower edge of the esophagus. However, this procedure is difficult under laparoscopy, and could easily lead to potential problems in the operation, failure in anastomosis and prolonged surgery.
Many methods have been suggested for improving the placement of anvils under laparoscopy[12-15]; however, there is no consensus about their use. Currently, the major methods of anvil insertion involve transoral and intra-abdominal placements. The transorally-inserted anvil (OrVilTM)[16] and the hemi-double stapling technique (HDST)[17] were recently developed. Although these two techniques have only been used for a short period, they have attracted much attention because they are simple and omit the need for a purse-string suture of the esophagus[18-21]. The OrVilTM technique inserts the stapler anvil through a transoral esophageal approach. A tube is connected with the central rod of the stapler. The tube is inserted in the esophagus and pulled out from the esophageal stump, and the anvil is placed under the guide of the tube. In the HDST method, the anvil is inserted through the lower esophagus, and the needles and threads are pulled out from the anterior wall of the esophagus. Subsequently, the lower esophagus is closed, and finally, the anvil is pulled out from the anterior wall close to the esophageal stump guided by the needles and threads, thereby completing the anvil insertion. In this technique, the anvil is inserted in a lower-upper pattern, and purse-string suturing is not required. These two methods are skillfully designed, simple and practical, and they can be completely mastered after simple training. However, it is not clear which of these methods is the best one.
Therefore, this study used either OrVilTM or HDST anvil insertion methods to perform esophagojejunostomy after laparoscopic total gastrectomy and compared the effectiveness of these two methods in an open-label prospective cohort study. The results should provide important information on selection of the best method for anvil insertion for esopagojejunostomy in the clinical practice.
Patients with gastric cancer and who were admitted to the Department of General Surgery, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China from May 2011 to October 2013 were approached for participation in this study. Inclusion criteria were: (1) willing to participate in the study; (2) male or female subjects aged ≤ 75 years; (3) newly diagnosed gastric adenocarcinoma confirmed by gastroscopy and histopathology; and (4) body mass index (BMI) ≤ 26.0 kg/m2. Exclusion criteria were: (1) severe cardiac, hepatic or renal insufficiency, or hematopoietic dysfunction; (2) paraaortic lymph node metastasis or lymph node invasion to major blood vessels revealed by pre-operative or intra-operative exploration; (3) metastatic gastric cancer; (4) metastases to the liver, lung and other organ according to enhanced abdominal CT scan and chest X-ray; (5) tumor invasion into adjacent organs revealed by pre-operative examination or intra-operative exploration; (6) peritoneal dissemination; or (7) carcinoma of the gastric cardia involving the esophagus.
The study was approved by the Ethics Committee of the Drum Tower Hospital, Medical School of Nanjing University, Nanjing. Written informed consent was obtained from all participants.
This was an open-label prospective cohort study. After resection of the stomach and lymph node dissection, all subjects were randomized into two groups using a computer-generated random number table in a 1:1 ratio. Patients in Group I underwent esophagojejunostomy using OrVilTM, while patients in Group II underwent HDST. Randomization was implemented using individual sealed envelopes (n = 84) prepared in advance by a statistician, and envelopes were opened by the surgeon according to the operation order. Researchers were blinded to the study grouping.
The primary endpoint was evaluation of the surgical outcome (operating time, time of digestive tract reconstruction and time of anvil insertion) and the medical cost of each operation (operation cost and total cost of hospitalization). The secondary endpoints were time to solid diet, post-surgical hospitalization time, time to defecation, time to ambulation and intra-operative blood loss. In addition, complications were assessed and compared.
Background demographic and clinical data were collected from the patients’ medical records. The surgical evaluations (time for the procedures and blood loss) were collected during the procedure. The post-surgical information was collected by the clinical nursing staff. The patients were followed-up once every two months for the first postoperative year, and once every three months for the second postoperative year.
In accordance with other Asian countries, but in contrast to many Western countries, D2 lymphadenectomy was performed for all patients[1]. All surgeries were performed by the same surgeon and the same surgical team. Prior to the completion of this study, the surgeon and all members of the group had performed laparoscopic gastrectomy for more than 50 cases.
Patients were placed in the supine position, with legs wide apart. The surgeon stood on the left side of the patient, with the assistant on the right side and the laparoscope holder between the patient’s two legs. A CO2 pneumoperitoneum was established by CO2 injection through an umbilical port, and the 10-mm port served as the observation port for the laparoscope. A 12-mm port was used on the left anterior axillary line below the costal margin, as the main operating port. A 5-mm port 5 cm to the left side of the umbilicus served as the auxiliary operating port, while a 12-mm port on the right anterior axillary line below the costal margin and a 5-mm port superior to the umbilicus on the right midclavicular line were used as the assistant operating ports.
Total gastrectomy and lymph node dissection were performed. The stomach was dissected along the left gastrocolic ligament, the roots of the left gastroepiploic vessels and short gastric arteries were ligated, and lymph nodes 4sa and 4sb were dissected. The right gastroepiploic artery and vein were ligated along the right pancreatic surface and the sixth group of lymph nodes was dissected. The stomach was dissected along the gastroduodenal artery and the common hepatic artery, and the root of the right gastric artery was ligated. Lymph nodes 5, 8a and 12a were dissected. The stomach was turned over to the head side, and the left gastric artery and vein along with the splenic artery were exposed and ligated. Lymph nodes 7, 9 and 11p were dissected. The duodenum was cut with a linear stapler (Ethicon Endosurgery; Cincinnati, OH, United States).
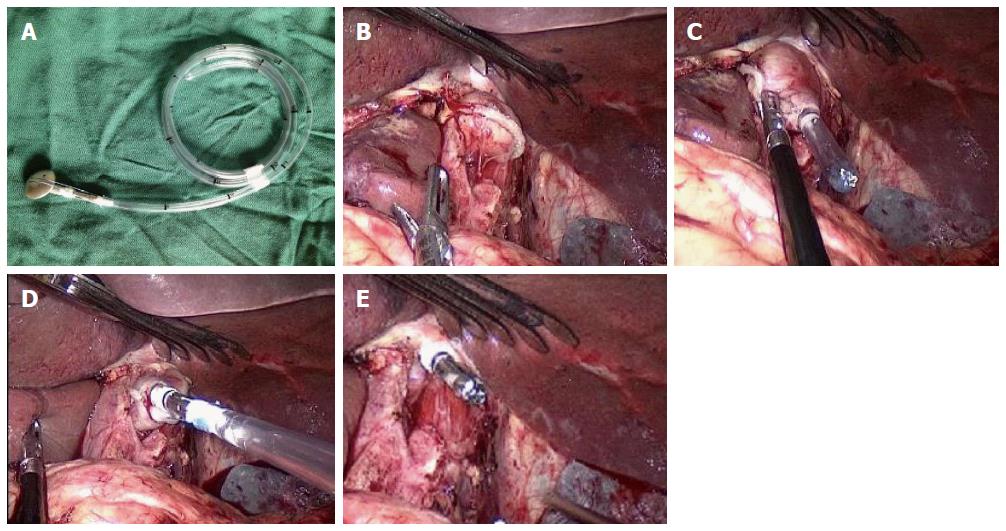
Anvil insertion into the end of the esophagus was performed using the OrVilTM system or HDST. Briefly, the OrVilTM technique was performed as follows. The lower edge of the esophagus was fully dissociated, and the esophagus 3 cm superior to the cardia was cut down using a linear stapler (Figure 1A-E). The tube of the OrVilTM system (OrVilTM; Covidien, Mansfield, MA, United States) was transorally inserted into the esophagus with the anesthetist’s assistance. Once the head of the tube reached the esophageal stump, a small port was pushed into the esophageal stump using an ultrasound scalpel. The tube was pulled out from the port until the anvil connecting with the end of the tube reached the esophageal stump. The connection line between the tube and the anvil was cut, and the tube was pulled out.
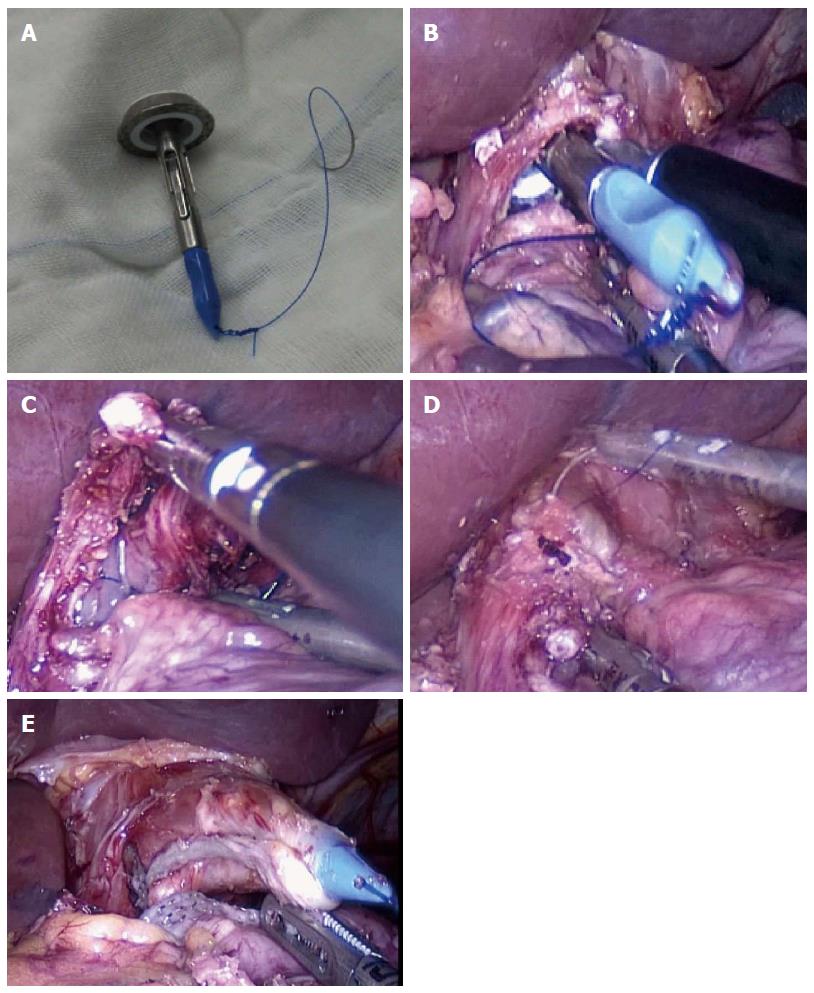
The HDST method was performed as follows (Figure 2A-E). The tip of the rod on the anvil was sutured with a needle containing sutures 4-5 cm in length. The prepared anvil was inserted into the abdomen. An incision of 2 cm in diameter was cut on the anterior wall of the cardia. The anvil was longitudinally inserted into the esophagus until the rod of the anvil was totally inserted into the esophagus and exceeded the tangent level of the esophagus. Then, the needle and thread were pulled out from the anterior wall of the esophagus. The esophagus was cut down using a linear stapler, and the needle and thread were further pulled outside until the rod of the anvil was completely pulled out from the anterior wall of the esophagus.
A Roux-en-Y esophagojejunostomy was performed. A 3.5-cm incision was made 2 cm to the left side of the umbilicus, and specimens were sampled via the incision on the abdominal wall. End-to-side jejunojejunal anastomosis was performed via the incision, and a jejunal portion of about 50 cm in length was retained. The circular stapler was inserted into the jejunum and temporarily fixed with rubber bands. The anastomotic device and small intestine were placed into the abdomen, and a pneumoperitoneum was re-established by clipping the abdominal wall with towel forceps. End-to-side esophagojejunal anastomosis was performed under a laparoscope, and the jejunal stump was closed using a linear stapler. Digestive tract reconstruction was performed, and a drainage tube was routinely placed beside the anastomotic stoma.
All data are expressed as mean ± standard deviation (SD). All statistical analyses were performed using SPSS 17.0 (IBM, Armonk, NY, United States). Continuous variables were compared using the Student’s t test if the variances in both groups were equal; otherwise, the Welch’s t test was used (time of post-surgical hospitalization). Categorical data (sex, TNM stage, tumor site) were compared with the Fisher’s exact test. A P value < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by Dr Guan from Drum Tower Hospital, Medical School of Nanjing University, Nanjing.
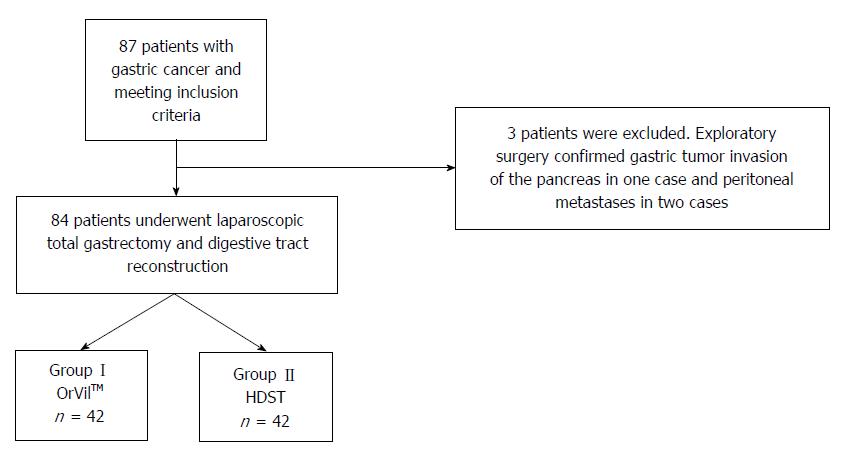
Figure 3 presents the patients’ flowchart. Eighty-seven patients were initially included in the study, but three were excluded because of tumor invasion or metastasis. Therefore, 84 patients were finally included and randomized.
There was no significant difference in age, BMI, tumor location and TNM stage between the two groups (Table 1). There were 24.4 and 26.7 lymph nodes dissected in groups I and II, respectively (P > 0.05).
| OrVilTM | HDST | P value | |
| Sex (M/F) | 31/11 | 27/15 | 0.345 |
| Age | 58.4 ± 8.0 | 56.5 ± 7.9 | 0.28 |
| BMI (kg/m2) | 23.1 ± 2.5 | 22.5 ± 2.7 | 0.28 |
| Resection margin (cm) | 2.9 ± 0.7 | 2.8 ± 0.5 | 0.40 |
| Length of surgical incision | 3.9 ± 0.6 | 3.7 ± 0.7 | 0.38 |
| Number of retrieved lymph nodes | 24.4 ± 6.8 | 26.7 ± 5.5 | 0.09 |
| TNM Stage | 0.802 | ||
| IA | 4 | 7 | |
| IB | 13 | 12 | |
| IIA | 10 | 8 | |
| IIB | 7 | 10 | |
| IIIA | 5 | 3 | |
| IIIB | 3 | 2 | |
| Tumor Site | 0.165 | ||
| Cardia and gastric fundus | 31 | 25 | |
| Body of stomach | 11 | 17 |
The primary endpoint of the study was the surgical difficulty. All 84 patients underwent successful esophagojejunostomy, without conversion to laparotomy. The mean operative time was 287.8 ± 38.4 min in Group I, including 147.7 ± 31.6 min for total gastrectomy and lymph node dissection, and 55.4 ± 15.7 min for digestive tract reconstruction. The mean operative time was 271.8 ± 46.1 min in Group II, including 159.8 ± 33.8 min for total gastrectomy and lymph node dissection, and 47.8 ± 12.1 min for digestive tract reconstruction.
There was no significant difference in the mean operative time, and in the mean time for total gastrectomy and lymph node dissection between the two groups (P > 0.05). The mean time for stapler anvil insertion using the OrVilTM system was 18.7 ± 7.5 min, while it was 12.6 ± 4.7 min with HDST (P < 0.05), indicating that anvil insertion took less time when the HDST technique was used, and the mean time of the digestive tract reconstruction was, accordingly, shorter (Table 2).
| Variable | Group I(n = 42) | Group II(n = 42) | P value |
| Time of surgery (min) | 287.8 ± 38.4 | 271.8 ± 46.1 | 0.09 |
| Time of lymph node dissection and total gastrectomy (min) | 147.7 ± 31.6 | 159.8 ± 33.8 | 0.09 |
| Time of digestive tract reconstruction (min) | 55.4 ± 15.7 | 47.8 ± 12.1 | 0.011a |
| Time of anvil insertion (min) | 18.7 ± 7.5 | 12.6 ± 4.7 | 0.001a |
| Intra-operative bleeding volume (mL) | 96.4 ± 32.7 | 88.2 ± 36.9 | 0.28 |
| Time of defecation (d) | 3.5 ± 0.9 | 3.2 ± 1.1 | 0.12 |
| Time to get out of bed (d) | 3.9 ± 0.7 | 3.6 ± 1.1 | 0.12 |
| Time to post-surgical eating (d) | 7.6 ± 1.4 | 8.0 ± 2.7 | 0.31 |
| Time of post-surgical hospitalization (d) | 10.6 ± 2.6 | 10.8 ± 3.5 | 0.80 |
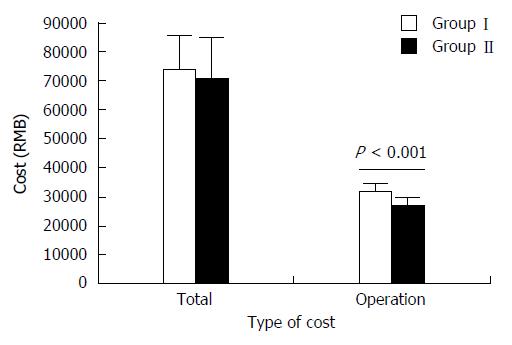
The total costs of hospitalization were 73848.7 ± 11781.0 RMB in Group I, including operation cost of 32401.9 ± 1981.6 RMB, and 70870.3 ± 14003.5 RMB in Group II, including operation cost of 26961.9 ± 2293.8 RMB. The operation cost in Group II was significantly lower than in Group I (P < 0.001) but the total cost of hospitalization was not different between the two groups (P = 0.296) (Figure 4).
In Group I, mean intra-operative blood loss was 96.4 ± 32.7 mL, the mean time to defecation was 3.5 ± 0.9 d, the mean time to ambulation was 3.9 ± 0.7 d, the mean time to post-surgical eating was 7.6 ± 1.4 d and the mean time of hospitalization was 10.4 ± 2.6 d. In Group II, the mean intra-operative bleeding volume was 88.2 ± 36.9 mL, the mean time to defecation was 3.2 ± 1.1 d, the mean time to ambulation was 3.6 ± 1.1 d, the mean time to post-surgical eating was 8.0 ± 2.7 d and the mean time of hospitalization was 10.8 ± 3.5 d. There was no significant difference in all these parameters between the two groups (all P > 0.05) (Table 2). The mean distance from the surgical margin was 2.9 ± 0.7 cm and 2.8 ± 0.5 cm on proximal esophagus to the cancer in the patients with carcinoma of gastric cardia from Groups I and II, respectively.
No residual cancer tissue was found in all cases. A high post-surgical short-term therapeutic efficacy was achieved in both groups with no bile reflux.
Intra-operative complications occurred in some patients. After surgery, two cases with atelectasis, two cases with incision dehiscence and three cases with throat pain were observed in Group I. One case had pleural effusion and one case had esophageal-jejunal anastomotic fistula (diagnosed by radiographic examination) in Group II, which was cured with drainage and enteral nutrition for 18 d.
All patients were followed up for 10-28 mo after surgery, with a median follow-up period of 16 mo. During follow-up, other adverse events occurred. Four patients, two in each group, suffered from an esophageal-jejunal anastomotic stenosis (difficulty in swallowing, which was confirmed by esophagography) that was relieved by endoscopic dilatation. In Group I, one case had peritoneal implantation metastases 7 mo postoperatively and died 14 mo postoperatively. Another case had a liver metastasis and entered remission with chemotherapy. In Group II, one case had peritoneal implantation metastases 9 mo postoperatively and entered remission with chemotherapy.
The aim of the present trial was to compare two different methods of anvil insertion for esophagojejunostomy after laparoscopic total gastrectomy, primarily in terms of surgical difficulty. All procedures were successfully completed during the 84 total gastrectomy surgeries in patients with gastric cancer. In addition, no technical problems occurred in any case during the reconstruction of the digestive tract and none of the cases needed conversion to laparotomy or expansion of the surgical incision, indicating that these two methods had a high reliability and stability.
Novel methods have been introduced for esophagojejunostomy because of the technical difficulties of this procedure in a laparoscopic setting. Purse-string suturing is a difficult technique to perform in a narrow space with a restricted view, which requires experience and skill from the surgeon. When purse-string suturing is required, a lot of surgical time is consumed. Therefore, most surgeons are reluctant to perform such procedures. Both the OrVilTM system[22-24] and HDST[25,26] have been shown to simplify esophagojejunostomy, with high success rates and few requirements for transfer to laparotomy. To our knowledge this is the first randomized study to compare these two methods after total gastrectomy, although one previous study compared these methods on a smaller cohort alongside a conventional anvil head method and side-to-side esophagojejunostomy with a linear stapler[21]. That study showed that surgery with OrVilTM was similar in time course to a more traditional method and concluded that none of the methods tested were entirely satisfactory. In the present study, the mean time of digestive tract anastomosis was 55.4 min using the OrVilTM system and 47.8 min using the HDST technique. In addition, the mean time for stapler anvil insertion was 18.7 min using the OrVilTM technique and 12.6 min using the HDST technique. Both of these techniques completed anvil insertion in a short time period, indicating that they were simple and easy to perform. Anvil insertion using the OrVilTM technique took a longer time than the HDST technique, which might be attributed to transoral placement procedures. In addition, the flexible tube operated by the anesthesiologist was difficult to control. In order to enable the head of the tube to be fixed in a good position, repeated adjustment was required that led to a longer operation time. However, due to omission of purse-string suturing, these two methods exhibited a significant superiority over the traditional purse-string suture methods in terms of the time of operation, which in our experience take approximately 20-25 min longer.
An important factor in survival after total gastrectomy is achieving a negative surgical margin. This is influenced by a number of factors, but most importantly the extent of the tumor and the extent of surgery[27]. The mean distance between the surgical margin on the proximal esophagus and the tumor is an important marker for a negative surgical margin[28]. If an open operation is performed, the surgical margin of the esophagus can reach 3-5 cm superior to the cardia. In the present study, among the 56 cases with carcinoma involving the gastric cardia, the mean distance between the surgical margin on the proximal esophagus and the cancer was about 3 cm, and no residual cancer tissues were observed, which ensured the completion of tumor resection.
Comparison of open and laparoscopic techniques for total gastrectomy suggests that these methods have similar outcome and success rates, but laparoscopic total gastrectomy can be associated with an increased complication rates in comparison with open surgery[29]. The most common of these complications is an anastomotic fistula, although recent laparoscopic methods have shown decreased rates as the techniques have improved[30]. Of the 84 patients who underwent esophagojejunostomy, anastomotic fistula occurred in only one case from Group II. This case was diagnosed as esophageal-jejunal anastomotic micro-fistula using radiographic examination and was cured with post-surgical drainage and enteral nutrition. If anastomosis is unsatisfactory, it is suggested that the suture be strengthened and the drainage tube be routinely indwelled beside the anastomotic stoma. Radiographic examinations should be performed in some suspected patients prior to removal of the drainage tube in order to exclude anastomotic fistula.
Anastomotic stenosis is another complication commonly observed in patients after undergoing laparoscopic total gastrectomy. In the present study, 4 out of 84 patients developed anastomotic stenosis. Shim et al[21] reported that anastomic stenosis occurred in 5 out of 26 patients after esophagojejunostomy using the OrVilTM and HDST techniques. Umemura et al[31] reported that the incidence of stenosis after esophagojejunostomy using a linear stapler was only 1.8%. Therefore, we suggest that the incidence of anastomotic stenosis after esophagojejunostomy using circular stapler methods was higher than that using a linear stapler. However, further study is necessary to address this issue.
Based on previous reports, some surgeons have made several modifications to these two surgical methods with the aim of overcoming the corresponding shortcomings. Indeed, the OrVilTM technique often results in a dog ear, and Hirahara et al[26] tried to solve this issue with a loop-shaped thread wrapped around the esophageal stump opening. With this modification, esophagojejunostomy was completed without a dog-ear. This approach may decrease the incidence of anastomotic fistula, but studies with larger sample size are required to support this assertion due to the small number of cases in this study. Muguruma et al[19] described a similar technique based on the procedure described by Omori et al[17], except that they used the OrVilTM anvil instead of the ECS25 (Ethicon Endo-Surgery stapler). It was found that the OrVilTM anvil was much easier to use and that surgeons with relatively little experience in gastric laparoscopy were able to conduct the operation and avoid complications. The median operative time was 318 min including 5 min for the placement of the anvil on the esophageal stump. Compared with these two retrospective studies, the present study focused on the prospective comparison between the two methods. We believe that these two methods for esophagojejunostomy will gain an increasing recognition, and will be widely used by more and more surgeons.
Compared with the HDST technique, the OrVilTM system requires a specific stapler and has a higher cost, while the HDST technique does not need a specific stapler and has a relatively low cost. Therefore, the use of the HDST technique might be more practical in undeveloped areas of the world.
The present study has some limitations. As the main purpose of the study was to evaluate two different methods of anvil insertion for esophagojejunostomy, we did not collect detailed data on tumor differentiation status, since these would not generally impact upon the surgical difficulty. If these data had been collected, a more detailed comparison between the groups and long-term outcomes could be performed. A larger multicenter study would also add more data and provide more convincing evidence for the most suitable anvil insertion method. A longer follow-up period would provide more information on the long-term effects of surgery and should be considered in future studies.
In summary, we conclude that the OrVilTM and HDST techniques were simple and reliable without any significant differences in safety and difficulty of operation. Both were reliable techniques for laparoscopic esophagojejunostomy.
Laparoscopic total gastrectomy is still not widespread because of the technical difficulty of the reconstruction. Although various types of esophagojejunal anastomosis are described in the literature, the optimal procedure has yet to be established. The placement of the stapler anvil is one of the limiting steps of laparoscopic total gastrectomy.
There are many suggested methods for improving the placement of anvils under a laparoscope. The transorally-inserted anvil (OrVilTM) and the hemi-double stapling technique (HDST) are recently developed methods of anvil insertion. However, there is no consensus on the best technique.
Previous reports have been issued on the feasibility of OrVilTM and HDST. To the best of our knowledge, this is the first report that compares the two types of anastomosis after laparoscopic total gastrectomy.
Both methods had similar safety and operation success. However, anvil insertion was faster and easier with HDST than with OrVilTM, and was more cost-effective.
This study is excellent with adequate sample size records. Case sheets should be used to check the genuineness of findings as this study could change treatment as well as the technique used. This article can be accepted with these conditions and with the corrections mentioned to the author.
| 1. | Tegels JJ, De Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH. Improving the outcomes in gastric cancer surgery. World J Gastroenterol. 2014;20:13692-13704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 3. | Kojima K, Yamada H, Inokuchi M, Hayashi M, Sekita Y, Kawano T, Sugihara K. [Current status and evaluation of laparoscopic surgery for gastric cancer]. Nihon Geka Gakkai Zasshi. 2006;107:77-80. [PubMed] |
| 4. | Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM, Memon B, Memon MA. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, Osugi H. Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg. 2007;94:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y. Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc. 2009;23:2167-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Matsui H, Uyama I, Sugioka A, Fujita J, Komori Y, Ochiai M, Hasumi A. Linear stapling forms improved anastomoses during esophagojejunostomy after a total gastrectomy. Am J Surg. 2002;184:58-60. [PubMed] |
| 8. | Kim SG, Lee YJ, Ha WS, Jung EJ, Ju YT, Jeong CY, Hong SC, Choi SK, Park ST, Bae K. LATG with extracorporeal esophagojejunostomy: is this minimal invasive surgery for gastric cancer? J Laparoendosc Adv Surg Tech A. 2008;18:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc. 2010;24:2475-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Scurtu R, Groza N, Otel O, Goia A, Funariu G. Quality of life in patients with esophagojejunal anastomosis after total gastrectomy for cancer. Rom J Gastroenterol. 2005;14:367-372. [PubMed] |
| 12. | Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008;11:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Takiguchi S, Sekimoto M, Fujiwara Y, Miyata H, Yasuda T, Doki Y, Yano M, Monden M. A simple technique for performing laparoscopic purse-string suturing during circular stapling anastomosis. Surg Today. 2005;35:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc. 2010;24:2908-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. 2009;197:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Cianchi F, Macrì G, Indennitate G, Mallardi B, Trallori G, Biagini MR, Badii B, Staderini F, Perigli G. Laparoscopic total gastrectomy using the transorally inserted anvil (OrVil™): a preliminary, single institution experience. Springerplus. 2014;3:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Muguruma K, Tanaka H, Sakurai K, Toyokawa T, Kubo N, Yamashita Y, Sawada T, Ohira M, Hirakawa K. Laparoscopy-assisted total gastrectomy: a simplified approach. Int Surg. 2014;99:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Xie JW, Huang CM, Zheng CH, Li P, Wang JB, Lin JX, Jun L. A safe anastomotic technique of using the transorally inserted anvil (OrVil) in Roux-en-Y reconstruction after laparoscopy-assisted total gastrectomy for proximal malignant tumors of the stomach. World J Surg Oncol. 2013;11:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Shim JH, Yoo HM, Oh SI, Nam MJ, Jeon HM, Park CH, Song KY. Various types of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. 2013;16:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Chong-Wei K, Dan-Lei C, Dan D. A modified technique for esophagojejunostomy or esophagogastrostomy after laparoscopic gastrectomy. Surg Laparosc Endosc Percutan Tech. 2013;23:e109-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | LaFemina J, Viñuela EF, Schattner MA, Gerdes H, Strong VE. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. 2013;20:2975-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 24. | Liao GQ, Ou XW, Liu SQ, Zhang SR, Huang W. Laparoscopy-assisted total gastrectomy with trans-orally inserted anvil (OrVil™): a single institution experience. World J Gastroenterol. 2013;19:755-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kim DH, Jeon TY, Kim DH, Kim DI, Sim MS, Kim S. Roux-en-Y gastrojejunostomy using modified hemi-double stapling. Dig Surg. 2009;26:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Hirahara N, Tanaka T, Yano S, Yamanoi A, Minari Y, Kawabata Y, Ueda S, Hira E, Yamamoto T, Nishi T. Reconstruction of the gastrointestinal tract by hemi-double stapling method for the esophagus and jejunum using EEA OrVil in laparoscopic total gastrectomy and proximal gastrectomy. Surg Laparosc Endosc Percutan Tech. 2011;21:e11-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Raziee HR, Cardoso R, Seevaratnam R, Mahar A, Helyer L, Law C, Coburn N. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer. 2012;15 Suppl 1:S116-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Kim MG, Lee JH, Ha TK, Kwon SJ. The distance of proximal resection margin dose not significantly influence on the prognosis of gastric cancer patients after curative resection. Ann Surg Treat Res. 2014;87:223-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer. 2015;18:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, Akiyama Y, Wakabayashi G. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. 2015;38:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Desiderio J, Rao KS S- Editor: Yu J L- Editor: O’Neill M E- Editor: Wang CH