Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8776
Peer-review started: April 4, 2015
First decision: April 23, 2015
Revised: May 8, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: August 7, 2015
Processing time: 127 Days and 19.1 Hours
Medical treatment has progressed significantly over the past decade towards achieving and maintaining clinical remission in patients with refractory ulcerative colitis (UC). Proposed mediators of inflammation in UC include pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-2, and the cell-surface adhesive molecule integrin α4β7. Conventional therapeutics for active UC include 5-aminosalicylic acid, corticosteroids and purine analogues (azathioprine and 6-mercaptopurine). Patients who fail to respond to conventional therapy are treated with agents such as the calicineurin inhibitors cyclosporine and tacrolimus, the TNF-α inhibitors infliximab or adalimumab, or a neutralizing antibody (vedolizumab) directed against integrin α4β7. These therapeutic agents are of benefit for patients with refractory UC, but are not universally effective. Our recent research on TNF-α shedding demonstrated that inhibition of annexin (ANX) A2 may be a new therapeutic strategy for the prevention of TNF-α shedding during inflammatory bowel disease (IBD) inflammation. In this review, we provide an overview of therapeutic treatments that are effective and currently available for UC patients, as well as some that are likely to be available in the near future. We also propose the potential of ANX A2 as a new molecular target for IBD treatment.
Core tip: The main goal of ulcerative colitis (UC) therapy is to induce and maintain long-term corticosteroid-free remission. Therapies such as anti-tumor necrosis factor (TNF)-α and integrin α4β7 neutralizing antibodies have emerged in recent times, but are not universally efficacious; additional treatments are needed. We have recently demonstrated that annexin (ANX) A2 inhibition may be a new therapeutic strategy to prevent TNF-α shedding during inflammatory bowel disease (IBD) inflammation. Here we focus on effective therapies for UC patients that are currently available, or will be in the near future, and the potential of ANX A2 as a new molecular target for IBD treatment.
- Citation: Tanida S, Mizoshita T, Ozeki K, Katano T, Kataoka H, Kamiya T, Joh T. Advances in refractory ulcerative colitis treatment: A new therapeutic target, Annexin A2. World J Gastroenterol 2015; 21(29): 8776-8786
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8776
Ulcerative colitis (UC) is a chronic inflammatory disease that affects the colonic mucosa and that is characterized by repeated periods of remission and deterioration[1]. Pharmacologic management of UC to achieve clinical remission, and improve the quality of life currently consists of 5-aminosalicylic acids (5-ASA)[2], corticosteroids[3], purine analogues [azathioprine (AZA) and 6-mercaptopurine (6-MP)][4], cytapheresis[5] [granulocyte and monocyte adsorptive apheresis (GMA) and leukocytapheresis (LCAP)], calcineurin inhibitors[6,7] [cyclosporine and tacrolimus (TAC)], and biologics including tumor necrosis factor (TNF)-α inhibitors[8,9]. In particular, anti-TNF-α antibodies such as infliximab (IFX)[8] and adalimumab (ADA)[9] can induce clinical remission in patients with refractory UC by inhibiting the activity of TNF-α, a member of the TNF superfamily that mediates a series of immune responses. However, responses to anti-TNF-α antibodies are often diminished during scheduled maintenance therapy; consequently, patients develop flare-ups[10]. Therefore, new therapeutic targets are needed for UC patients who no longer respond to these therapeutic agents.
A disintegrin and metalloproteinase (ADAM)17, also known as TNF-α converting enzyme (TACE)[11,12], is a key enzyme for the shedding of the membrane-anchored TNF-α (proTNF-α). We have recently demonstrated that annexin (ANX) A2 is involved in the shedding of proTNF-α through ADAM17[13]. Inhibition of ANX A2 may be a new therapeutic strategy for the prevention of TNF-α shedding during inflammatory bowel disease (IBD) inflammation.
The present review focuses on therapeutic treatments that are effective and currently available for UC patients, or will be in the near future, and the potential of ANX A2 as a new molecular target for IBD treatment.
A systematic review and meta-analysis of the effect of 5-ASA on UC demonstrated that 5-ASA is highly effective for inducing remission in UC with a relative risk (RR) of failure to achieve remission of 0.79 (95%CI: 0.73-0.85; P = 0.009). This finding was based on analysis of data showing that remission of UC was not achieved in 887 (60.3%) of 1470 patients randomized to receive 5-ASA, compared with 494 (80.2%) of 616 patients allocated to placebo[14]. In addition, when remission was defined as endoscopic healing[15-19], 5-ASA was of benefit in inducing remission in active UC (RR = 0.76; 95%CI: 0.69-0.84). Moreover, a systematic review and meta-analysis that investigated the effect of high- or standard-dose 5-ASA (≥ 2 g) vs low-dose 5-ASA (< 2 g) on induction of remission demonstrated that doses of ≥ 2 g/d were more effective than doses of < 2 g/d for inducing remission with a RR of failure to achieve remission of 0.91 (95%CI: 0.85-0.98)[14]. This finding was based on data showing that 380 (58.7%) of 647 patients receiving high- or standard-dose 5-ASA failed to achieve remission, compared with 257 (69.8%) of 368 patients assigned to low-dose 5-ASA[18,20-26].
A systematic review and meta-analysis of the efficacy of 5-ASA vs placebo in preventing relapse in quiescent UC demonstrated that 5-ASA is highly effective for preventing relapse in UC with a RR of relapse of 0.65 (95%CI: 0.55-0.76)[14]. This finding was based on data showing that 342 (40.3%) of 849 patients randomized to 5-ASA relapsed, compared with 409 (62.6%) of 653 patients allocated to placebo[27-37].
It was also suggested that doses of ≥ 2 g/d may be more effective than doses of < 2 g/d for preventing relapse with a RR of relapse of 0.79 (95%CI: 0.64-0.97). This finding was based on data showing that 225 (34.7%) of 649 patients receiving high- or standard-dose 5-ASA relapsed, compared with 379 (42.8%) of 885 patients assigned to low-dose 5-ASA[14].
A systematic review and meta-analysis of the efficacy of corticosteroids in UC demonstrated that standard corticosteroids were superior to placebo for UC remission with a RR of failure to achieve remission of 0.65 (95%CI: 0.45-0.93)[38]. This finding was based on analysis of data showing that 122 (54.0%) of 226 patients assigned to standard oral glucocorticoids failed to achieve remission, compared with 173 (79.0%) of 219 patients allocated to placebo[3,39-42]. Based on the above, standard corticosteroids are probably effective in inducing remission in UC.
This systematic review also showed that there was no evidence of increased adverse events in patients taking standard corticosteroids, compared with placebo, even though the absolute rate was higher (14.3% compared with 7.0%, RR = 1.69; 95%CI: 0.30-9.62)[38].
Cytapheresis including GMA (Adacolumn®) and LCAP (Cellsoba®) is an extracorporeal therapy that selectively depletes activated granulocytes and monocytes, or leukocytes, resulting in amelioration of the gut inflammation of UC.
A systematic review and meta-analysis of the effect of GMA in both active and corticosteroid-dependent or resistant UC demonstrated that GMA appeared superior to conventional medical therapy. This conclusion was based on data showing that 26 (74%) of 35 patients assigned to GMA achieved remission, compared with 16 (49%) of 35 patients receiving prednisolone (PSL) (P = 0.02)[43,44]. In addition, there was also evidence for corticosteroid-sparing effects with GMA, with significantly lower cumulative doses of corticosteroids, and significantly higher rates of corticosteroid-free remission in patients receiving GMA. These findings were based on data that showed that (1) during the 12 wk of treatment, the cumulative amount of PSL received per patient was 1157 mg in 46 patients assigned to GMA, compared with 1938 mg in 23 patients assigned to receiving the mean dose of PSL up to 30 mg daily (P = 0.001)[45]; and that (2) 27 (77%) of the GMA-treated patients achieved corticosteroid-free at 12 wk, compared with 5 (14%) of the patients allocated to PSL (P = 0.008)[43]. However, GMA did not achieve significantly higher remission rates compared with a sham procedure in achieving remission in UC[46]. Interestingly further subgroup analysis demonstrated that GMA is of benefit in patients with confirmed endoscopically active disease. This conclusion was based on the data of a total of 63 patients with histological evidence of mucosal erosions or ulcerations at baseline, which showed that clinical remission was achieved in 11 (24%) of 46 patients randomized to GMA, compared with 0 (0%) of 17 patients allocated to sham apheresis (P = 0.03). On the other hand, a randomized trial comparing LCAP with a sham column also suggested benefit[47]. Interestingly, a systematic review and meta-analysis of the effect of intensive GMA regimens (two sessions per week) over conventional GMA regimens (one session per week) in achieving remission in UC demonstrated that intensive GMA regimens had higher remission rates[48-50] and shorter time-to-remission than conventional regimens[48,49]. Serious adverse side effects have been rare in patients receiving GMA. Based on the above, cytapheresis appears of some benefit in UC.
The traditional pyramid of therapy for the management of UC suggests that patients are prescribed immunosuppressive agents when 5-ASA and corticosteroids fail[51]. A systematic review and meta-analysis of the effect of the immunosuppressant AZA on active UC demonstrated a trend to benefit of AZA over placebo in a total of 130 UC patients allocated to AZA or placebo, with no statistical significance (RR = 0.85; 95%CI: 0.71-1.01; P = 0.07)[4,52,53]. However, AZA is of benefit in preventing relapse in quiescent UC (RR = 0.60; 95%CI: 0.37-0.95; P = 0.03)[52]. This finding was based on data that 26 (39.3%) of 66 patients receiving AZA experienced a relapse of UC, compared with 40 (65.6%) of 61 patients allocated to placebo[4,53,54], with a statistically significant benefit of AZA.
Based on the above, AZA/6-MP appears to be of little benefit for inducing remission in active UC, but may prevent relapse in quiescent UC.
This systematic review also showed that there was no evidence of increased adverse events in patients taking purine analogues, compared with placebo[52]. However, there has been one trial that reported that one patient was dying of an infection associated with an immunocompromised state that occurred when taking AZA[55]. AZA/6-MP are also associated with a 4-6 fold increased risk of lymphoma[56,57] and a 2-6 fold increase in non-melanoma skin cancer[58,59]. Thus, immunosuppressive therapy with AZA/6-MP is never without risk.
Calcineurin inhibitors including cyclosporine and TAC are useful for the treatment of refractory UC due to their potent immunosuppressive properties that inhibit the transcription of the early activation genes encoding interleukin (IL)-2, TNF-α, and interferon-γ, which contribute to the development of inflammation[60].
A clinical trial that investigated the effect of cyclosporine on severely active UC, in which a response was defined as symptomatic improvement demonstrated that cyclosporine was of benefit over placebo in improving symptoms (RR no improvement with cyclosporine, 0.22; 95%CI: 0.07-0.67). This finding was based on data showing that 2 (18%) of 11 patients receiving cyclosporine had no response, as compared with 9 of 9 patients allocated to placebo[7,52].
A recent systematic review of pertinent literature in the Cochrane Database that investigated the efficacy of TAC in inducing remission or clinical improvement of symptoms of UC in a total of 63 moderate-to-severe UC patients randomized to TAC or placebo demonstrated that TAC was of benefit in inducing short-term clinical improvement in patients with refractory UC. This conclusion was based on data showing that 21 (48.8%) of 43 patients randomized to TAC achieved clinical improvement at 2 wk, compared with 2 (10.0%) of 20 patients allocated to placebo (odds ratio (OR), 8.66; 95%CI: 1.79-42.00), with a statistically significant benefit of TAC[60,61]. However, TAC is of little benefit in inducing remission. This conclusion is based on data showing that 6 (13.9%) of 43 patients randomized to TAC achieved remission at 2 wk, compared with 1 (5.0%) of 20 patients allocated to placebo (OR = 2.27; 95%CI: 0.35-14.75), with no statistically significant benefit of TAC over placebo.
Regarding safety concerns, patients in the high serum target concentration group were significantly more likely than placebo patients to experience adverse events related to treatment (P = 0.043). Finger tremor (n = 6) was the most common adverse event in 43 patients receiving TAC. Other adverse events included: gastroenteritis, sepsis, sleepiness, hot flush, headache, queasiness and stomach discomfort[60,61].
Based on the above, TAC may be effective for short-term clinical improvement in patients with refractory UC.
IBDs are characterized by chronic inflammation involving the surplus or excessive activity of the immune system in the gut. In order to block this excessive immune reaction, many approaches to the treatment of IBD with biological agents against inflammatory cytokines and adhesive molecules have been developed. The most popular approach to IBD treatment is to block TNF-α, a pro-inflammatory cytokine, which activates inflammatory cells, up-regulates adhesion molecules, and ultimately induces gut inflammation. Treatments with monoclonal antibodies against TNF-α are currently successful in many patients. However, only a third or less will achieve remission and many of those who do will eventually lose their response[62,63]. Monoclonal antibodies (vedolizumab) that block integrin α4β7, which mediates the infiltration of leukocytes into the gut mucosa, have also been developed, and will hopefully be used in clinical practice in the near future.
A very recent systematic review and network meta-analysis of the efficacy of biological agents on UC in a total of 2282 mild-to-moderate UC patients randomized to biological agents (n = 1167) or placebo (n = 1115) also demonstrated that all biological agents (ADA, golimumab (anti-TNF-α), IFX, and vedolizumab) were superior to placebo for induction of clinical response, clinical remission, and mucosal healing, except for ADA for clinical remission. Furthermore, IFX was shown to be more likely to induce a favorable clinical outcome than ADA for induction of clinical response (OR = 2.36, 95%CI: 1.22-4.63), clinical remission (OR = 2.79, 95%CI: 0.95-8.83), and mucosal healing (OR = 2.02, 95%CI: 1.13-3.59)[8,9,64-68]. In addition, all biological agents also suggested superiority over placebo for maintenance[64].
Another systematic review and meta-analysis of the efficacy of all anti-TNF-α antibodies on moderately to severely active UC demonstrated that IFX antibodies are superior to placebo in inducing remission (RR of failure to achieve remission, 0.72; 95%CI: 0.57-0.91). This conclusion is based on data showing that remission of UC was not achieved in 231 (42.9%) of 539 patients that were randomized to receive IFX for 6 to 12 wk, compared with 201 (69.8%) of 288 patients allocated to placebo[8,69-72].
Regarding safety concerns, it was also suggested that the number of patients experiencing any adverse event was not greater with IFX in moderate-to-severe UC[69]. Based on the above, IFX is of benefit over placebo in inducing remission in active UC.
IFX and calcineurin inhibitors such as cyclosporin and TAC are effective for the treatment of patients with moderate or severe corticosteroid-dependent/refractory UC. Whether cyclosporin or TAC therapy should precede IFX as a second-line therapy currently remains controversial. A parallel, open-label randomized controlled trial compared the efficacy of cyclosporin and IFX on acute severe UC that was refractory to intravenous corticosteroids. In this trial, a total of 115 severe UC patients were randomized to cyclosporine (n = 58) or IFX (n = 57), and this trial demonstrated that cyclosporine was not more effective than IFX. This conclusion was based on data showing that 35 (60%) of 58 patients receiving cyclosporine failed to respond to the treatment by day 98, compared with 31 (54%) of 57 patients receiving IFX, with no statistically significant benefit of cyclosporine over IFX (OR = 1.3; 95%CI: 0.6-2.7; P = 0.52). Furthermore, 50 (86%) of 58 patients receiving cyclosporine achieved a clinical response by day 7, compared with 48 (84%) of 57 patients receiving IFX, with no statistically significant benefit of cyclosporine over IFX (OR = 1.2; 95%CI: 0.4-3.3; P = 0.76)[73].
A retrospective study that investigated the efficacy of IFX salvage therapy for patients with severe or moderate UC who failed to respond to TAC demonstrated that IFX salvage therapy following TAC tended to be more efficacious in TAC responders (loss of response or no tolerance) than in non-responders (refractoriness), and that sequential therapy may prove useful and well tolerated. These conclusions were based on data showing the following: (1) in 13 patients receiving IFX for severe or moderate UC who showed refractoriness or loss of response to TAC, or no tolerance, the mean partial Mayo score of UC activity was significantly decreased (P < 0.05) to 5.69, 3.07, and 2.77 at baseline, 8, and 30 wk, respectively; (2) six (46.2%) of the 13 patients showed clinical remission at 8 wk and four (30.8%) showed clinical remission at 30 wk; and (3) rates of clinical remission at 8 and 30 wk of IFX therapy were 60.0% and 40.0%, respectively in TAC responders, and good remission rates of 37.5% and 25.0%, respectively, were also obtained in TAC non-responders[74]. More interestingly, some recent investigations demonstrated that biological therapy could be terminated after achieving complete remission in response to scheduled maintenance therapy with TNF-α biologics in patients with refractory UC[75,76].
A randomized, double-blind, double-dummy trial evaluated the efficacy of IFX alone, AZA alone, and combination therapy with IFX and AZA, at week 16 for the treatment of moderate-to-severe UC that was naïve to anti-TNF-α antibodies in a total of 239 patients. This trial demonstrated that the patients receiving combination therapy with IFX and AZA were more likely to achieve corticosteroid-free remission at week 16 than those receiving either monotherapy. This conclusion was based on data showing that 31 (39.7%) of 78 patients receiving a combination of IFX and AZA achieved corticosteroid-free remission, compared with 17 (22.1%) of 77 patients receiving IFX alone (P = 0.017) and 18 (23.7%) of 76 patients receiving AZA alone (P = 0.032)[77]. In addition, combination therapy led to significantly better mucosal healing than AZA alone, based on data showing that 49 (62.8%) of 78 patients receiving IFX and AZA combination therapy achieved mucosal healing at week 16, compared with 42 (54.6%) of 77 patients receiving IFX alone (P = 0.295) and 28 (36.8%) of 76 patients receiving AZA alone (P = 0.001)[77].
A recent systematic review of pertinent literature in the Cochrane Database that investigated the efficacy of vedolizumab for induction and maintenance of remission in a total of 606 moderate-to-severe UC patients randomized to vedolizumab demonstrated that vedolizumab is significantly more effective than placebo in inducing clinical remission and response as well as endoscopic remission. This conclusion was based on the following data: (1) 293 (77%) of 382 patients that were randomized to vedolizumab failed to achieve clinical remission by week 4 to 6, compared with 205 (92%) of 224 patients allocated to placebo, with a statistically significant effect in favor of vedolizumab (RR = 0.86; 95%CI: 0.80-0.91); (2) 48% of patients randomized to vedolizumab failed to have a clinical response at week 6, compared with 72% of patients allocated to placebo (RR = 0.68; 95%CI: 0.59-0.78); and (3) 68% of patients failed to achieve endoscopic remission at week 4 to 6, compared with 81% of patients allocated to placebo (RR = 0.82; 95%CI: 0.75-0.91)[78]. In addition, vedolizumab was of benefit over placebo in preventing relapse in patients who were in remission, based on data that showed that 140 (54%) of 247 patients randomized to vedolizumab experienced a clinical relapse at week 52, compared with 106 (84%) of 126 patients allocated to placebo (RR = 0.67; 95%CI: 0.59-0.77)[78]. Regarding safety concerns, patients receiving vedolizumab were no more likely than those receiving placebo to experience adverse events or serious adverse events[78].
Based on the above, vedolizumab is superior to placebo for induction of clinical remission, response, and endoscopic remission in patients with moderate-to-severe UC, and for prevention of relapse in patients with quiescent UC.
Tofacitinib is an inhibitor of Janus kinases 1, 2 and 3 that are believed to block lymphocyte activation, function, and proliferation through inhibition of signaling involving gamma chain-containing cytokines including IL-2, -4, -7, -9, and -15[79,80]. Consequently tofacitinib is expected to be a therapeutic agent for the treatment of active UC.
A double-blind, placebo-controlled, phase II trial that evaluated the efficacy of tofacitinib in 194 patients with moderate-to-severe UC demonstrated that clinical response at week 8 occurred in 20 (42%) of 48 patients allocated to placebo (95%CI: 28-56) compared with 10 (32%) of 31 patients randomized to 0.5 mg of tofacitinib (95%CI: 16-49; P = 0.39), 16 (48%) of 33 randomized to 3 mg of tofacitinib (95%CI: 31-66; P = 0.55), 20 (61%) of 33 randomized to 10 mg of tofacitinib (95%CI: 44-77; P = 0.10), 38 (78%) of 49 randomized to 15 mg of tofacitinib (95%CI: 66-89; P < 0.001)[81]. In addition, clinical remission at week 8 occurred in 5 (10%) of 48 patients receiving placebo (95%CI: 2-19), compared with 4 (13%) of 31patients receiving 0.5 mg of tofacitinib (95%CI: 1-25; P = 0.76), 11 (33%) of 33 receiving 3 mg of tofacitinib (95%CI: 17-49; P = 0.01), 16 (48%) of 33 receiving 10 mg of tofacitinib (95%CI: 31-66; P < 0.001), and 20 (41%) of 49 receiving 15 mg of tofacitinib (95%CI: 27-55; P < 0.001)[81]. Regarding safety concerns, there was a dose-dependent increase in both low-density lipoprotein and high-density lipoprotein cholesterol concentrations at week 8 with tofacitinib, which reversed after discontinuation of the study drug.
Based on the above, patients with moderate-to-severe UC treated with tofacitinib were more likely to achieve clinical response and remission than those receiving placebo.
As described above, TNF-α blockade using anti-TNF-α antibodies is not always successful. A better understanding of the TNF-α shedding process may lead to new methods of blocking TNF-α shedding and thereby attenuating the inflammation of UC. A recent investigation demonstrated a mechanism for the regulation of TNF-α shedding in which ANX A2 regulates ADAM17-mediated cleavage and subsequent shedding of proTNF-α from the cell membranes of monocytes and colon epithelial cells[13].
ANX A2 was initially isolated as a substrate for the tyrosine kinase of the oncogene protein pp60 (v-src)[82]. ANX A2 is a pleiotropic calcium- and anionic phospholipid-binding protein that exists as a monomer and as a heterotetrameric complex with the plasminogen receptor protein, S100A10[83]. A recent extensive study of the detailed biological functions of ANX A2 showed that ANX A2 in complex with S100A10 participates in Ca2+-evoked exocytosis, and, in the endocytic pathway, ANX A2 in combination with acylated caveolin is considered to be involved in the internalization/transport of lipids[84]. Therefore, ANX A2 has been proposed to play a key role in many processes including exocytosis, endocytosis, membrane organization, ion channel conductance, and in linking the F-actin cytoskeleton to the plasma membrane[83,85].
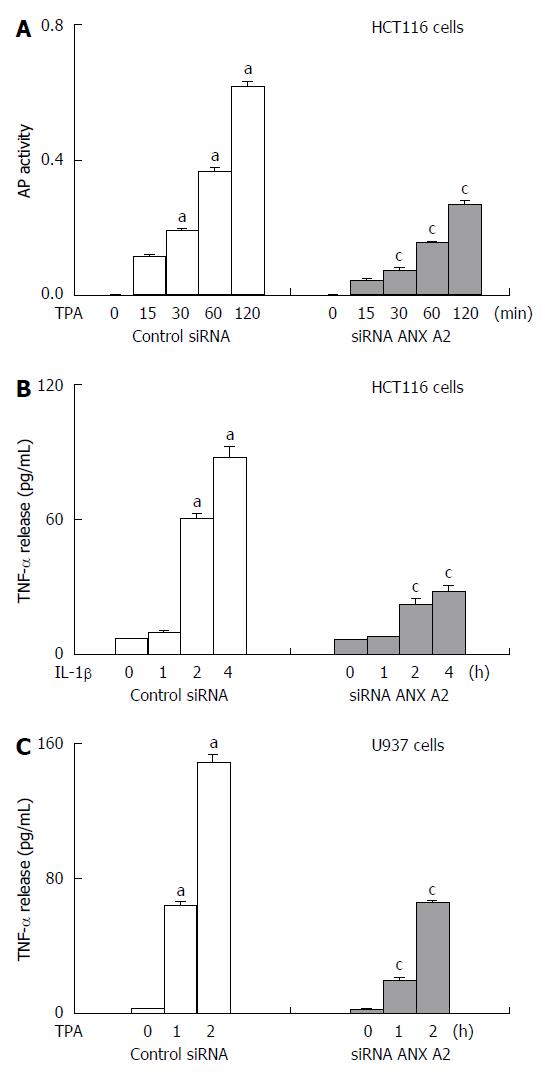
The molecular mechanism by which TNF-α shedding is induced by interaction of ANX A2 with ADAM 17 has now become clear. TNF-α is known to be expressed on the cell membranes of monocytes. Western blotting that examined the endogenous protein expression levels of TNF-α, ADAM17 and ANX A2 in cell lines such as the colon epithelial cell lines, HCT116 and HT29, and the monocyte cell line, U937, showed that a high level of TNF-α protein was constitutively expressed in U937 cells, whereas HCT116 and HT29 cells expressed TNF-α at very low levels. High expression levels of ADAM17 and ANX A2 were observed in all three cell lines. Immunoprecipitaion with an anti-ANX A2 antibody followed by Western blotting with an anti-ADAM17 antibody demonstrated that ADAM17 directly interacts with ANX A2. It is known that the ectodomain of proTNF-α is mainly shed through the activity of ADAM17, although ADAM10 can also mediate a small amount of proTNF-α shedding[86]. The role of ADAM17 in TNF-α shedding was confirmed by analysis of the inhibitory effects of KB-R7785, an ADAM inhibitor, and of ADAM17 short interfering RNAs (siRNAs) on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced shedding of TNF-α from HCT116 cells overexpressing alkaline-phosphatase (AP)-tagged proTNF-α, which allowed quantitative analyses of shed AP-tagged TNF-α in the culture medium during TPA stimulation using an AP assay. Forced depletion of ANX A2 using siRNAs targeted towards ANX A2 resulted in a significant suppression of TPA-induced TNF-α shedding (Figure 1A). In accordance with these data, the expression level of the AP-tagged proTNF-α protein of these HCT116 cells was decreased after TPA stimulation, which was partially inhibited by siRNA-mediated depletion of ANX A2. Furthermore, forced depletion of ANX A2 using siRNAs targeted toward ANX A2 resulted in a significant suppression of stimulation-induced endogenous TNF-α release from HCT116 and U937 cells, which was assessed using an ELISA of TNF-α shed into the conditioned medium (Figure 1B and C). These data suggested that ANX A2 is involved in TNF-α shedding and release in colon epithelial cells and monocytes.
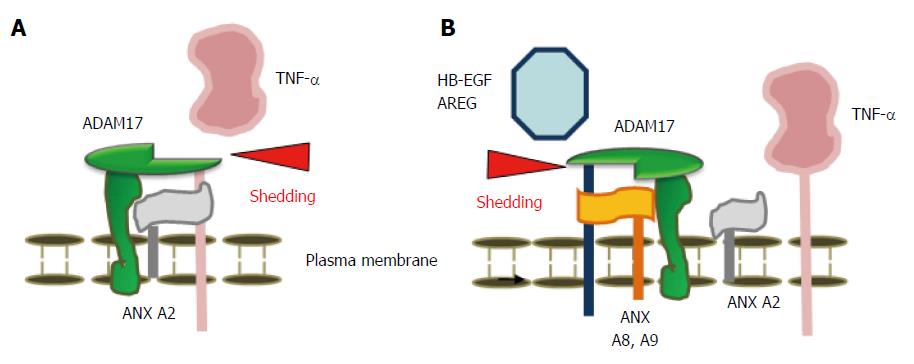
ADAM17 is also a key enzyme for the shedding of various other membrane proteins in addition to TNF-α, including epidermal growth factor receptor (EGFR) ligands[87]. The C-terminus of type 1 membrane proteins such as EGFR ligands [e.g., amphiregulin (AREG) and heparin-binding epidermal growth factor-like growth factor (HB-EGF)] is in the cytoplasm, whereas the N-terminus of type 2 membrane proteins (e.g., TNF-α) is in the cytoplasm. The detailed mechanism by which ADAM17 cleaves type 1 and 2 membrane proteins is unclear. An AP assay of HCT116 cells overexpressing AP-tagged proAREG and proHB-EGF demonstrated that, in contrast to its effect on TNF-α shedding, depletion of ANX A2 with siRNAs significantly increased AREG and HB-EGF shedding[13]. These experiments confirmed that ANX A2 is involved in the ectodomain shedding of AREG and HB-EGF.
The combined data indicated that depletion of ANX A2 inhibited ADAM17-mediated ectodomain shedding of proTNF-α; conversely, depletion of ANX A2 upregulated ADAM17-mediated ectodomain shedding of proAREG and proHB-EGF. These results suggest that depletion of ANX A2 ameliorates gut inflammation by suppressing TNF-α cleavage and induces cell proliferation and mucosal repair by promoting AREG and HB-EGF cleavage.
More interestingly, depletion of other members of the ANX family, ANX A8 and A9 abrogated the shedding of EGFR ligands, suggesting that ANX A8 and A9 are required for their shedding[88]. In contrast, decreased levels of TNF-α shedding were observed during stimulation from HCT116 cells overexpressing ANX A8 and A9, compared with mock cells, suggesting that ANX A8 and A9 inhibit TNF-α shedding.
Based on the above studies, ANX A2, A8 and A9 are responsible for regulation of the cleavage of the type 2 membrane-anchored protein TNF-α, and the cleavage of the type 1 membrane-anchored proteins AREG and HB-EGF (Figure 2). Clearly, ANX A2 is a new candidate molecular target for overcoming the failure of TNF-α blockade, and inhibition of ANX A2 may be a new therapeutic strategy for the prevention of TNF-α shedding during IBD inflammation.
As detailed in this review, many agents with different mechanisms of action are available, or are likely to be available in the near future, for the treatment of UC. However, these therapeutic strategies are not always satisfactory and there also remains the problem of refractory UC. Of the current treatments, calcineurin inhibitors, TNF-α blockade, and vedolizumab, which block or neutralize the production and functions of proinflammatory cytokines including IL-2 and TNF-α, and adhesive molecules, can be effective for the treatment of patients with refractory UC, but they have limitations. To address these limitations, the development and clinical trials of new therapeutic agents that target the surplus or excessive activity of the immune system are needed. ANX A2, which mediates TNF-α shedding, is also one such new candidate molecular target. Progress in understanding the pathogenesis of UC is expected to result in the emergence of many potentially useful treatments, such as the targeting of ANX A2, for UC treatment in the future.
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1656] [Article Influence: 118.3] [Reference Citation Analysis (6)] |
| 2. | Sutherland LR, May GR, Shaffer EA. Sulfasalazine revisited: a meta-analysis of 5-aminosalicylic acid in the treatment of ulcerative colitis. Ann Intern Med. 1993;118:540-549. [PubMed] |
| 3. | Lennard-Jones JE, Longmore AJ, Newell AC, Wilson CW, Jones FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut. 1960;1:217-222. [PubMed] |
| 4. | Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627-630. [PubMed] |
| 5. | Rembacken BJ, Newbould HE, Richards SJ, Misbah SA, Dixon ME, Chalmers DM, Axon AT. Granulocyte apheresis in inflammatory bowel disease: possible mechanisms of effect. Ther Apher. 1998;2:93-96. [PubMed] |
| 6. | Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, Koyanagi K, Hibi T. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1191] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 8. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2951] [Article Influence: 140.5] [Reference Citation Analysis (2)] |
| 9. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-265.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 970] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 10. | Zampeli E, Gizis M, Siakavellas SI, Bamias G. Predictors of response to anti-tumor necrosis factor therapy in ulcerative colitis. World J Gastrointest Pathophysiol. 2014;5:293-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2422] [Cited by in RCA: 2439] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 12. | Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1280] [Article Influence: 44.1] [Reference Citation Analysis (9)] |
| 13. | Tsukamoto H, Tanida S, Ozeki K, Ebi M, Mizoshita T, Shimura T, Mori Y, Kataoka H, Kamiya T, Fukuda S. Annexin A2 regulates a disintegrin and metalloproteinase 17-mediated ectodomain shedding of pro-tumor necrosis factor-α in monocytes and colon epithelial cells. Inflamm Bowel Dis. 2013;19:1365-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, Moayyedi P. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:601-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Baron JH, Connell AM, Lennard-Jones JE, Jones FA. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;1:1094-1096. [PubMed] |
| 16. | Dick AP, Grayson MJ, Carpenter RG, Petrie A. Controlled trial of sulphasalazine in the treatment of ulcerative colitis. Gut. 1964;5:437-442. [PubMed] |
| 17. | Hetzel DJ, Shearman DJ, Labrooy J, Bochner F, Imhoff DM, Gibson GE, Fitch RJ, Hecker R, Rowland R. Olsalazine in the treatment of active ulcerative colitis: a placebo controlled clinical trial and assessment of drug disposition. Scand J Gastroenterol Suppl. 1988;148:61-69. [PubMed] |
| 18. | Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, Safdi M. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:1188-1197. [PubMed] |
| 19. | Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, Lyne A, Stephenson D, Palmen M, Joseph RE. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132:66-75; quiz 432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 20. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2326] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 21. | Sninsky CA, Cort DH, Shanahan F, Powers BJ, Sessions JT, Pruitt RE, Jacobs WH, Lo SK, Targan SR, Cerda JJ. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350-355. [PubMed] |
| 22. | Miglioli M, Bianchi Porro G, Brunetti G. Oral delayed- release mesalazine in the treatment of mild ulcerative colitis: a dose-ranging study. Eur J Gastroenterol Hepatol. 1990;2:229-234. |
| 23. | Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, Bell JK, Johnson LK. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | D’Haens G, Hommes D, Engels L, Baert F, van der Waaij L, Connor P, Ramage J, Dewit O, Palmen M, Stephenson D. Once daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: a phase II, dose-ranging study. Aliment Pharmacol Ther. 2006;24:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut. 1988;29:669-674. [PubMed] |
| 26. | Kruis W, Bar-Meir S, Feher J, Mickisch O, Mlitz H, Faszczyk M, Chowers Y, Lengyele G, Kovacs A, Lakatos L. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003;1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Misiewicz JJ, Lennard-Jones JE, Connell AM, Baron JH, Jones Avery F. Controlled trial of sulphasalazine in maintenance therapy for ulcerative colitis. Lancet. 1965;285:185-188. [DOI] [Full Text] |
| 28. | Dissanayake AS, Truelove SC. A controlled therapeutic trial of long-term maintenance treatment of ulcerative colitis with sulphazalazine (Salazopyrin). Gut. 1973;14:923-926. [PubMed] |
| 29. | Riis P, Anthonisen P, Wulff HR, Folkenborg O, Bonnevie O, Binder V. The prophylactic effect of salazosulphapyridine in ulcerative colitis during long-term treatment. A double-blind trial on patients asymptomatic for one year. Scand J Gastroenterol. 1973;8:71-74. [PubMed] |
| 30. | Sandberg-Gertzén H, Järnerot G, Kraaz W. Azodisal sodium in the treatment of ulcerative colitis. A study of tolerance and relapse-prevention properties. Gastroenterology. 1986;90:1024-1030. [PubMed] |
| 31. | Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. Use of colonic eicosanoid concentrations as predictors of relapse in ulcerative colitis: double blind placebo controlled study on sulphasalazine maintenance treatment. Gut. 1988;29:1316-1321. [PubMed] |
| 32. | Wright JP, O’Keefe EA, Cuming L, Jaskiewicz K. Olsalazine in maintenance of clinical remission in patients with ulcerative colitis. Dig Dis Sci. 1993;38:1837-1842. [PubMed] |
| 33. | Miner P, Hanauer S, Robinson M, Schwartz J, Arora S. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Pentasa UC Maintenance Study Group. Dig Dis Sci. 1995;40:296-304. [PubMed] |
| 34. | Group TMS. An oral preparation of mesalamine as long-term maintenance therapy for ulcerative colitis. A randomized, placebo-controlled trial. The Mesalamine Study Group. Ann Intern Med. 1996;124:204-211. [PubMed] |
| 35. | Hawkey CJ, Dube LM, Rountree LV, Linnen PJ, Lancaster JF. A trial of zileuton versus mesalazine or placebo in the maintenance of remission of ulcerative colitis. The European Zileuton Study Group For Ulcerative Colitis. Gastroenterology. 1997;112:718-724. [PubMed] |
| 36. | Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Bianchi Porro G. Is maintenance therapy always necessary for patients with ulcerative colitis in remission? Aliment Pharmacol Ther. 1999;13:373-379. [PubMed] |
| 37. | Lichtenstein GR, Gordon GL, Zakko S, Murthy U, Sedghi S, Pruitt R, Merchant K, Shaw A, Bortey E, Forbes WP. Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis - a 6-month placebo-controlled trial. Aliment Pharmacol Ther. 2010;32:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, Moayyedi P. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590-599; quiz 600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 39. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [PubMed] |
| 40. | Angus P, Snook JA, Reid M, Jewell DP. Oral fluticasone propionate in active distal ulcerative colitis. Gut. 1992;33:711-714. [PubMed] |
| 41. | Rizzello F, Gionchetti P, D’Arienzo A, Manguso F, Di Matteo G, Annese V, Valpiani D, Casetti T, Adamo S, Prada A. Oral beclometasone dipropionate in the treatment of active ulcerative colitis: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2002;16:1109-1116. [PubMed] |
| 42. | Bossa F, Latiano A, Rossi L, Magnani M, Palmieri O, Dallapiccola B, Serafini S, Damonte G, De Santo E, Andriulli A. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103:2509-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Kageoka M, Ikeya K, Yamada M, Kikuyama M, Iwaoka Y. Intensive granulocyte and monocyte adsorption versus intravenous prednisolone in patients with severe ulcerative colitis: an unblinded randomised multi-centre controlled study. Dig Liver Dis. 2008;40:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka T, Maruyama Y, Matsushita I. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S; Adacolumn Study Group. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Sawada K, Kusugami K, Suzuki Y, Bamba T, Munakata A, Hibi T, Shimoyama T. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am J Gastroenterol. 2005;100:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, Oshima T, Kunisaki R, Matsumoto T. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104:2990-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Sakuraba A, Sato T, Naganuma M, Morohoshi Y, Matsuoka K, Inoue N, Takaishi H, Ogata H, Iwao Y, Hibi T. A pilot open-labeled prospective randomized study between weekly and intensive treatment of granulocyte and monocyte adsorption apheresis for active ulcerative colitis. J Gastroenterol. 2008;43:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Ricart E, Esteve M, Andreu M, Casellas F, Monfort D, Sans M, Oudovenko N, Lafuente R, Panes J. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: a pilot, prospective, multicenter, randomized study. World J Gastroenterol. 2007;13:2193-2197. [PubMed] |
| 51. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 956] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 52. | Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one-year, placebo-controlled, randomized trial. Indian J Gastroenterol. 2000;19:14-16. [PubMed] |
| 54. | Sood A, Kaushal V, Midha V, Bhatia KL, Sood N, Malhotra V. The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. J Gastroenterol. 2002;37:270-274. [PubMed] |
| 55. | O’Donoghue DP, Dawson AM, Powell-Tuck J, Bown RL, Lennard-Jones JE. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet. 1978;2:955-957. [PubMed] |
| 56. | Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol. 2010;105:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 58. | Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390-399.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 59. | Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, Carbonnel F, Colombel JF, Dupas JL, Godeberge P. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621-1628.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 362] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 60. | Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, Hibi T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 61. | Baumgart DC, Macdonald JK, Feagan B. Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane Database Syst Rev. 2008;CD007216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 63. | Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, Bonovas S. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 65. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 687] [Article Influence: 45.8] [Reference Citation Analysis (1)] |
| 66. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 711] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 67. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96-109.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 530] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 68. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1965] [Article Influence: 151.2] [Reference Citation Analysis (1)] |
| 69. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 70. | Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, Targan SR, Podolsky DK. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83-88. [PubMed] |
| 71. | Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, Forbes A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52:998-1002. [PubMed] |
| 72. | Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson A, Verbaan H. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805-1811. [PubMed] |
| 73. | Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 462] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 74. | Tsukamoto H, Tanida S, Mizoshita T, Ozeki K, Ebi M, Shimura T, Mori Y, Kataoka H, Kamiya T, Joh T. Infliximab salvage therapy for patients with ulcerative colitis who failed to respond to tacrolimus. Eur J Gastroenterol Hepatol. 2013;25:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Steenholdt C, Molazahi A, Ainsworth MA, Brynskov J, Østergaard Thomsen O, Seidelin JB. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. 2012;47:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Tanida S, Mizoshita T, Ozeki K, Tsukamoto H, Mori Y, Kubota E, Kataoka H, Kamiya T, Joh T. The first case of biological therapy discontinuation after a complete remission induced by maintenance therapy with adalimumab for refractory ulcerative colitis. J Clin Med Res. 2015;7:118-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 716] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 78. | Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, MacDonald JK, Khanna R, Feagan BG. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;8:CD007571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, Doty JL, Elliott EA, Fisher MB, Hines M. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468-8484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 80. | Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR. The specificity of JAK3 kinase inhibitors. Blood. 2008;111:2155-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 82. | Glenney JR. Phosphorylation of p36 in vitro with pp60src. Regulation by Ca2+ and phospholipid. FEBS Lett. 1985;192:79-82. [PubMed] |
| 83. | Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259-6305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 84. | Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1144] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 85. | Sarkar S, Kantara C, Singh P. Clathrin mediates endocytosis of progastrin and activates MAPKs: role of cell surface annexin A2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G712-G722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Rosendahl MS, Ko SC, Long DL, Brewer MT, Rosenzweig B, Hedl E, Anderson L, Pyle SM, Moreland J, Meyers MA. Identification and characterization of a pro-tumor necrosis factor-alpha-processing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem. 1997;272:24588-24593. [PubMed] |
| 87. | Tanida S, Joh T, Itoh K, Kataoka H, Sasaki M, Ohara H, Nakazawa T, Nomura T, Kinugasa Y, Ohmoto H. The mechanism of cleavage of EGFR ligands induced by inflammatory cytokines in gastric cancer cells. Gastroenterology. 2004;127:559-569. [PubMed] |
| 88. | Nakayama H, Fukuda S, Inoue H, Nishida-Fukuda H, Shirakata Y, Hashimoto K, Higashiyama S. Cell surface annexins regulate ADAM-mediated ectodomain shedding of proamphiregulin. Mol Biol Cell. 2012;23:1964-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ahluwalia NK, Arasaradnam RP, Perakath B, Romano C S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH