Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8629
Peer-review started: February 6, 2015
First decision: March 10, 2015
Revised: March 26, 2015
Accepted: May 2, 2015
Article in press: May 4, 2015
Published online: July 28, 2015
Processing time: 174 Days and 20.2 Hours
AIM: To investigate a newly designed stent and its dilatation effect in a rabbit model of benign esophageal stricture.
METHODS: Thirty-four New Zealand white rabbits underwent a corrosive injury in the middle esophagus for esophageal stricture formation. Thirty rabbits with a successful formation of esophageal strictures were randomly allocated into two groups. The control group (n = 15) was implanted with a conventional stent, and the study group (n = 15) was implanted with a detachable “pieced” stent. The study stent (30 mm in length, 10 mm in diameter) was composed of three covered metallic pieces connected by surgical suture lines. The stent was collapsed by pulling the suture lines out of the mesh. Two weeks after stricture formation, endoscopic placement of a conventional stent or the new stent was performed. Endoscopic extraction was carried out four weeks later. The extraction rate, ease of extraction, migration, complications, and survival were evaluated.
RESULTS: Stent migration occurred in 3/15 (20%) animals in the control group and 2/15 (13%) animals in the study group; the difference between the two groups was not statistically significant. At the end of four weeks, the remaining stents were successfully extracted with the endoscope in 100% (11/11) of the animals in the study group, and 60% (6/10) of the animals in the control group; this difference was statistically significant (P < 0.05). There was no difference in the mean number of follow-up days between the control and study groups (25.33 vs 25.85). Minor bleeding was reported in five cases in the study group and four in the control group. There were no severe complications directly associated with stent implantation or extraction in either of the two groups.
CONCLUSION: In this experimental protocol of benign esophageal strictures, the novel “pieced” stent demonstrated a superior removal rate with a similar migration rate compared to a conventional stent.
Core tip: At present, esophageal stent retrieval may be a difficult and traumatic procedure because of tissue adhesion and the stent’s radial force. Currently, all types of stents used in clinical practice retain some of the radial force during the removal procedure. We designed a novel type of stent that has a detachable property and no radial force during removal procedure. We investigated the efficacy and removal feasibility of the stent in an animal model, and we anticipate that it could be used in humans in the future.
- Citation: Liu J, Shang L, Liu JY, Qin CY. Newly designed “pieced” stent in a rabbit model of benign esophageal stricture. World J Gastroenterol 2015; 21(28): 8629-8635
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8629.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8629
Benign esophageal strictures, due to esophageal acid exposure, Schatzki’s rings, corrosive injury, esophageal surgery, radiation injury, ablative therapies, and achalasia, are a common clinical problem[1,2]. Refractory benign esophageal strictures (RBES) are a subgroup of strictures (30%-40%) with more complex characteristics (i.e., > 2 cm, tortuous, and associated with a severely narrow diameter or presence of leaks or fistulas). This type of stricture cannot maintain an adequate diameter (≥ 14 mm) despite several repeated sessions of dilatation. Esophageal dilation with bougies or balloons has been the primary therapy for the treatment of RBES; however, in RBES patients, dilation may not be adequate[3,4]. Although surgical management including resection or gastric pull-up and enteric replacement may potentially be curative, these measures have higher morbidity rates and are more invasive than endoscopic procedures[5,6]. Other alternative treatment modalities utilized for the treatment of esophageal strictures include temporary stent placement, glucocorticoid injection with dilation, and self-dilation[7,8].
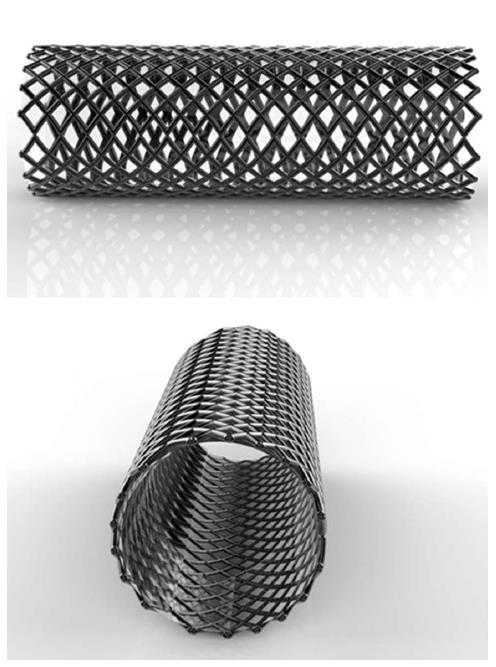
Ideally, the temporary placement of an esophageal stent (Figure 1) should be easy to remove without damaging tissue after it remolds the esophagus in the strictured area through a long-term dilation effect and maintain luminal patency while simultaneously stretching the stenosis. Previous studies utilized self-expandable metal stents (SEMS) with titanium-nickel or stainless steel structure and self-expandable plastic stents (SEPS). Although the SEMS was initially found to be successful in the treatment of malignant strictures, problems occurred in the treatment of RBES. These included tissue ingrowth, new stricture formation due to granulation, necrosis, and ulceration along the rigid mesh. Hence, the removal of the stent often proved difficult and traumatic[9-11]. Although the SEPS has a lower rate of tissue imbedding than SEMS, it is associated with a higher migration rate compared with metal stents[12], often requiring removal. Recent studies have introduced a biodegradable (BD) stent composed of polydioxanone that can be degraded by hydrolysis of ester bonds[13,14]. BD stents are suitable for those patients who do not want or cannot tolerate the subsequent stent removal procedures. Currently, there is no ideal stent for RBES; therefore, exploration for the development of a better stent is worthwhile.
Thus far, none of the available stents can completely change their radial force during removal procedure. We recently designed a detachable “pieced” SEMS with no radial force during retraction. The present study was designed to evaluate the dilation effect and ease of stent removal between our newly designed stent and a conventional SEMS, as well as to investigate the safety of the stent in a rabbit model of esophageal stricture.
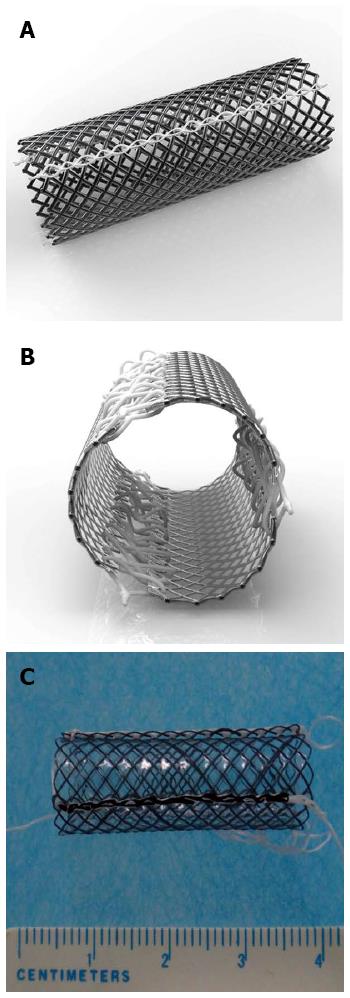
The detachable “pieced” esophageal stent (invention patent No. ZL201110323099.5; Shandong Provincial Institute of Medical Instruments, Jinan, Shandong, China) has the following characteristics: (1) the stent is a fully covered SEMS; (2) three pieces of curved nickel-titanium mesh are connected by silk braided non-absorbable surgical suture lines; (3) there are three slipknots of surgical suture lines on the proximal end of the stent (similar to the string slipknots commonly present on bags of flour); (4) all margins of the pieces are round, dull-braided and with dull ends; and (5) the stent is 10 mm in diameter and 30 mm in length to fit the rabbit’s esophagus (Figure 2).
Thirty-four New Zealand white male rabbits (5-8-mo-old, 2.5 ± 0.5 kg) were obtained from the Shandong Provincial Laboratory Animal Center, Jinan, Shandong, China. The study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. The animals were kept in separate cages in a temperature-controlled (23 °C) room with 50% humidity, under a 12/12 h light/dark cycle, and provided ad libitum access to food and water.
After 24 h of fasting, each rabbit was weighed and anesthetized with an intravenous injection of 3% sodium pentobarbital (30 mg/kg). Rabbits were then placed in the left lateral decubitus position with the head elevated up to approximately 30°[15,16]. Esophageal corrosive injury was induced at approximately 12.5 cm inferior to the maxillary incisors by 1 mL of 4% sodium hydroxide solution injected into a #10 Foley catheter with obliteration of the front opening and creation of an aperture above the balloon.
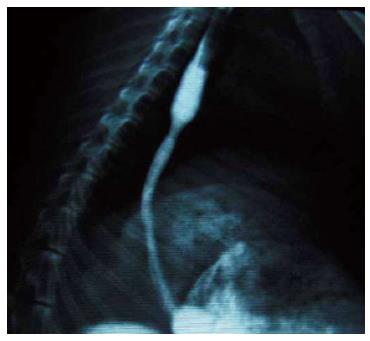
Two weeks after inducing the corrosive injury, endoscopic and fluoroscopic observations were performed to confirm the condition of the site of injury. The degree of the stricture was measured during endoscopy and esophagography. For the procedure to be considered successful, the diameter of stricture had to be < 0.5 maximal diameter of the more proximal normal esophagus.
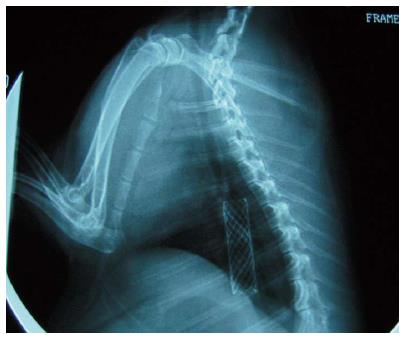
The stents were implanted into the animal’s esophagus two weeks after corrosive injury. The animals with successful stricture were randomly assigned to one of two groups: a control stent group (n = 15) and a study group (n = 15). The control group received the implantation of conventional stents. The conventional stents were of the same size as the detachable “pieced” stent, 10 mm in diameter and 30 mm in length. These stents were fully covered nickel-titanium SEMS without purse string and no flange. The conventional stent was designed for the rabbit’s esophagus by the Shandong Provincial Institute of Medical Instruments, Jinan, Shandong, China. The study group received the detachable “pieced” stent. A guide wire and an ultra-slim upper endoscope (GIF-XP260N; Olympus Optical Co. Ltd., Tokyo, Japan) with a 2.0 mm working channel and a 5.0 mm tip diameter were used for the implantation of the stents. After insertion of the stents, the rabbits were weighed and received a fluoroscopic and endoscopic performance at one-week intervals for four weeks.
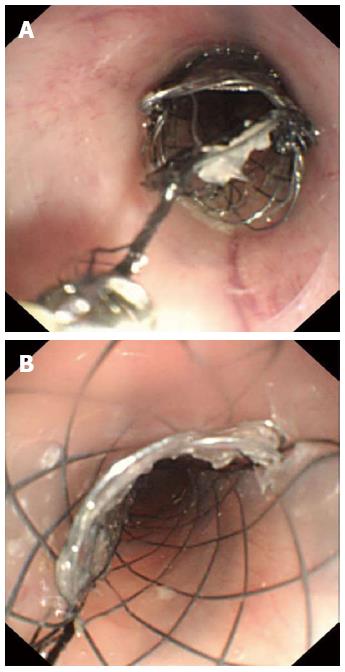
Stent removal was carried out using an ultra-slim upper endoscope and a biopsy forceps (FB-19K-1; Olympus Optical Co. Ltd., Tokyo, Japan). Feasibility of stent removal and the number of failed retrieval cases were studied. The stent retrieval procedures were as follows. For the control stents, the stents were retrieved using a biopsy forceps with an ultra-slim upper endoscope. For the study stents: (1) the residue of the surgical suture line was grasped and pulled upward with the biopsy forceps; (2) the slipknots of the surgical suture lines were unfastened; (3) three pieces of nickel-titanium mesh connected by the surgical suture lines were subsequently detached; and (4) each stent piece was pulled out of the esophagus with biopsy forceps. All endoscopic procedures were performed by a single skilled physician.
The extraction rate, ease of extraction, migration, stent-related complications, and survival were evaluated. An outcome was defined as safe if the stent was either implanted or extracted safely with no or minor bleeding. Stent-related complications that were considered severe were hemorrhage, tissue tearing, perforation, aspiration, fistula, and death.
Data were analyzed using the SPSS version 17.0 (SPSS, Inc., Chicago, IL, United States). The Fisher’s exact test was used to compare the stent migration rate and extraction rate. P≤ 0.05 was considered statistically significant. The statistical methods in this study were reviewed by Qing-Qing Zhang from the Shandong Provincial Hospital Affiliated to Shandong University, Shandong, China.
Esophagography showed the enlarged upper part of the esophagus, while the lower part narrowed preventing the passage of the ultra-slim upper endoscope through the stricture (Figure 3). The stricture model failed in 4/34 rabbits (success rate of 88%). In both groups, all stents were successfully placed with stents expanding in the esophagus and covering the stricture area (Figure 4). Migration occurred in five animals: three in the control group and two in the study group (20% vs 13%). There were no severe complications directly associated with the study stent implantation, such as hemorrhage, perforation, aspiration, or fistula. Three rabbits in the study group and four in the control group had minor bleeding after stent placement. Two rabbits died of malnutrition in the study group before the end of the study (4 wk). In the control group, one animal died of malnutrition, and one died of wound infection (Table 1). All stents were patent at the end of the follow-up period.
| Condition | Control (n = 15) | Test (n = 15) | P-value |
| Implantation outcomes | |||
| Minor bleeding | 4 | 3 | - |
| Migration | 3 (20) | 2 (13) | 1.0 |
| Death | 2 | 2 | - |
| Number of rabbits available for retrieval | 10 | 11 | - |
| Number of removable stents | 6 | 11 | 0.035 |
| Stent-related complication during removal | |||
| Minor bleeding | 4 | 5 | |
| Hemorrhage | 1 | 0 | |
| Tissue tearing | 2 | 0 | |
| Death | 1 | 0 | |
| Mean number of follow-up days | 25.33 | 25.85 | 0.831 |
In the control group, three stents migrated and two animals died; therefore, 10 control rabbits were available for analysis of the stent removal. In the study group, two stents migrated and two animals died with a total of 11 animals available for analysis. Those 11 remaining stents in the study group were all extracted successfully through the ultra-slim upper endoscope (Figure 5). Although the suture lines broke in two rabbits during the removal procedure, the slipknots were unfastened when the operator clamped the residual string and pulled upward. None of the study group rabbits experienced severe bleeding or perforation. Minor bleeding was reported in five cases in the study group and four in the control group. In the control group, stent retrieval failed in four cases. The failure was due to esophageal tissue tearing (n = 2), bleeding (n = 1), and death (n = 1). The rate of stent removal was significantly different between the control and study groups (60% vs 100%; P = 0.035).
RBES can have a harmful impact on the patient’s quality of life. The condition can cause severe complications including weight loss, aspiration, anemia, and malnutrition, and can be life-threatening. In 1983, Frimberger[17] proposed SEMS for the treatment of esophageal strictures resulting in significant therapeutic benefit. However, the long-term complications associated with stent implantation and removal have hindered the application of conventional stents in the treatment of esophageal strictures, especially RBES.
The removal of partially covered SEMS can be difficult because of the high rate of hyperplasia, foreign body reactions, and bleeding[7,9,10,18]. The use of SEPS has shown a less frequent rate of hyperplastic tissue ingrowth and overgrowth in comparison with that of partially covered metal stents. However, plastic stents have several limitations, including the need to be “prepared for delivery,” weaker radial force, and other complications. Moreover, decreased friction of the covered surface of the stent with the esophageal wall often leads to stent migration (45%-62%)[7,19]. Therefore, many stent designs have focused on anti-migration, such as an SX-ELLA stent (Hradec Kralove, Czech Republic). This stent has an anchoring flap or flared end[20] with an anti-migration ring at the proximal end. Other examples include a stent with dog-bone ends (90 stent; Taewoong Medical, Seoul, South Korea) and the stent with multiple protuberances[21]. The Niti-S double-layered covered nitinol stent was developed to overcome the major drawback of stent implantation. The Niti-S stent has migration rates lower than other covered stents due to the design with the enlarged flares at both ends and double-layer structure with an outer uncovered nitinol wire tube[22]. Although the Niti-S stent demonstrated excellent results in inoperable carcinoma of the esophagus[23,24], the rigid shape of the stent prevents removal from benign esophageal strictures. To overcome problems associated with retrieval of the implanted stent, the BD stent was introduced, rendering later stent removal procedures unnecessary[13,14]. However, in cases where patients do not tolerate a BD stent or stent migration occurs after BD stent implantation, stent removal is unavoidable.
Difficult stent retrievals often lead to a high incidence of esophageal injury. Therefore, we sought to find a better solution to this problem. The primary aim of designing the detachable “pieced” stent was to enhance the removal of the implanted stent after the dilation effect is attained or if stent migration has occurred. Our animal model experiments demonstrate the following properties of our newly designed stent: (1) the stent preserves the strong supporting property of the conventional SEMS to prevent migration; (2) the stent is much easier to extract because it can be detached into three pieces with no tension; and (3) the duration of stent implantation can be controlled. These features may prevent serious complications associated with long-term use of stents.
The detachable “pieced” stent has an equivalent dilation effect and safety compared with that of a conventional SEMS. The rate of stent removal was significantly higher in the study group compared with the control group. The control group had four failed removal procedures due to tissue overgrowth at the end of the stent; furthermore, bleeding occurred in four rabbits. There was no obvious bleeding or resistance in any of the removals in the study group. Two cases in the study group had rough surgical suture lines, which snapped during clamping. However, the broken end of the suture line was grasped with biopsy forceps, and the slipknot was then unfastened sequentially by pulling the residual suture lines forward. Our work indicates that the study stent can be extracted even if the surgical suture line is broken. Different suture materials should be addressed in the future studies.
The limitation of this study is that it is an experimental animal study with only one style of conventional stent used for comparison. Further studies are necessary to investigate the duration of the detachable “pieced” stent placement, the feasibility of the retrieval, the optimal length and diameter, the tissue reaction, and the long-term complications in animals and humans. Clinical trials involving patients with esophageal stricture are needed in future investigations.
In conclusion, the detachable “pieced” stent may offer a new option for the treatment of benign esophageal strictures in humans. The stent may sustain a long-lasting dilation effect, potentially reducing complications associated with stent extraction. The time of stent removal can be controlled, and the procedure of stent retrieval is simple. The stent may potentially become an option for those patients who need to have a stent extracted or who have had a stent migration. Its use in other areas of the gastrointestinal tract should also be explored.
We would like to express our gratitude to Dr. David E Fleischer, who revised the paper and gave us writing assistance.
Benign esophageal stricture is one of the most common digestive diseases and can reduce the patient’s quality life. Short-term stent implantation treatment is considered to palliate dysphagia and discomfort by sustained dilatation.
The main problem in stent removal is its radial force. So far, no stent can completely change its radial force during the removal procedure.
The newly designed detachable “pieced” stent possesses the properties of not only strong radial force for sustained dilatation, but also easy removal without radial force. It presents a better alternative management for treatment of refractory benign esophageal stricture.
The study results suggest that the newly designed detachable “pieced” stent can provide sufficient dilatation effect and good patency to remold the esophageal tract and can be easily removed during stent extraction.
Refractory benign esophageal strictures usually occur in the patients who have had failed 1-3 consecutive sessions of bougies or balloons dilatation. These are more complex strictures (30%-40%) of > 2 cm in size, tortuous, and associated with a severely narrow diameter or presence of leaks or fistulas. Therefore, the strictures cannot maintain an adequate diameter (≥ 14 mm) through several repeated sessions of dilatation or can be relieved for a very short interval.
This experimental protocol investigated a newly designed stent for benign esophageal stricture in a rabbit esophageal stricture model. The authors designed the detachable “pieced” esophageal stent, which had no radial force during retraction. The new stent could achieve the goal of temporary stent placement for dilation effect and easy removal.
| 1. | de Wijkerslooth LR, Vleggaar FP, Siersema PD. Endoscopic management of difficult or recurrent esophageal strictures. Am J Gastroenterol. 2011;106:2080-2091; quiz 2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Ferguson DD. Evaluation and management of benign esophageal strictures. Dis Esophagus. 2005;18:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Hourneaux de Moura EG, Toma K, Goh KL, Romero R, Dua KS, Felix VN, Levine MS, Kochhar R, Appasani S, Gusmon CC. Stents for benign and malignant esophageal strictures. Ann N Y Acad Sci. 2013;1300:119-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Siersema PD. Treatment options for esophageal strictures. Nat Clin Pract Gastroenterol Hepatol. 2008;5:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Song HY, Choi EK, Kim KR, Shin JH, Lim JO. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients. Eur Radiol. 2009;19:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Little AG, Naunheim KS, Ferguson MK, Skinner DB. Surgical management of esophageal strictures. Ann Thorac Surg. 1988;45:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Siersema PD, de Wijkerslooth LR. Dilation of refractory benign esophageal strictures. Gastrointest Endosc. 2009;70:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Dzeletovic I, Fleischer DE, Crowell MD, Pannala R, Harris LA, Ramirez FC, Burdick GE, Rentz LA, Spratley RV, Helling SD. Self-dilation as a treatment for resistant, benign esophageal strictures. Dig Dis Sci. 2013;58:3218-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Baron TH. Esophageal avulsion following removal of a partially covered esophageal stent: lessons learned 10 years later. Clin Gastroenterol Hepatol. 2012;10:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | van Heel NC, Haringsma J, Wijnhoven BP, Kuipers EJ. Endoscopic removal of self-expandable metal stents from the esophagus (with video). Gastrointest Endosc. 2011;74:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Siersema PD. Stenting for benign esophageal strictures. Endoscopy. 2009;41:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Verschuur EM, Repici A, Kuipers EJ, Steyerberg EW, Siersema PD. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol. 2008;103:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenterol. 2007;13:3977-3980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Stivaros SM, Williams LR, Senger C, Wilbraham L, Laasch HU. Woven polydioxanone biodegradable stents: a new treatment option for benign and malignant oesophageal strictures. Eur Radiol. 2010;20:1069-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Thompson JN. Corrosive esophageal injuries. II. An investigation of treatment methods and histochemical analysis of esophageal strictures in a new animal model. Laryngoscope. 1987;97:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 16. | Zhou JH, Jiang YG, Wang RW, Fan SZ, Gong TQ, Tan QY, Ma Z, Zhao YP, Deng B. Prevention of stricture development after corrosive esophageal burn with a modified esophageal stent in dogs. J Thorac Cardiovasc Surg. 2008;136:1336-142, 1336-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Frimberger E. Expanding spiral--a new type of prosthesis for the palliative treatment of malignant esophageal stenoses. Endoscopy. 1983;15 Suppl 1:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Baron TH, Burgart LJ, Pochron NL. An internally covered (lined) self-expanding metal esophageal stent: tissue response in a porcine model. Gastrointest Endosc. 2006;64:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Repici A, Hassan C, Sharma P, Conio M, Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Holm AN, de la Mora Levy JG, Gostout CJ, Topazian MD, Baron TH. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest Endosc. 2008;67:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Ji JS, Lee BI, Kim HK, Cho YS, Choi H, Kim BW, Kim SW, Kim SS, Chae HS, Choi KY. Antimigration property of a newly designed covered metal stent for esophageal stricture: an in vivo animal study. Gastrointest Endosc. 2011;74:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Verschuur EM, Homs MY, Steyerberg EW, Haringsma J, Wahab PJ, Kuipers EJ, Siersema PD. A new esophageal stent design (Niti-S stent) for the prevention of migration: a prospective study in 42 patients. Gastrointest Endosc. 2006;63:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Fujita T, Tanabe M, Shimizu K, Iida E, Matsunaga N. Radiological image-guided placement of covered Niti-S stent for palliation of dysphagia in patients with cervical esophageal cancer. Dysphagia. 2013;28:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Choi SJ, Kim JH, Choi JW, Lim SG, Shin SJ, Lee KM, Lee KJ. Fully covered, retrievable self-expanding metal stents (Niti-S) in palliation of malignant dysphagia: long-term results of a prospective study. Scand J Gastroenterol. 2011;46:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Spiliopoulos S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN