Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8580
Peer-review started: January 25, 2015
First decision: February 10, 2015
Revised: March 30, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: July 28, 2015
Processing time: 186 Days and 20.5 Hours
AIM: To investigate the effects of restraint stress on chronic colitis in interleukin (IL)-10 deficient (IL-10-/-) mice.
METHODS: The first experiment compared the effect of restraint stress on the development of intestinal inflammation in wild-type and IL-10-/- mice. Both wild-type and IL-10-/- mice were physically restrained in a well-ventilated, 50 cm3 conical polypropylene tube for 2 h per day for three consecutive days. The second experiment was performed to assess the effect of restraint stress on exacerbation of colitis induced by piroxicam in IL-10-/- mice. The IL-10-/- mice were exposed to restraint stress for 2 h per day for 3 consecutive days, and then treated with piroxicam for 4 d at a dose of 200 ppm administered in the rodent chow.
RESULTS: In the first experiment, none of the wild-type mice with or without restraint stress showed clinical and histopathological abnormality in the gut. However, IL-10-/- mice exposed to restraint stress exhibited histologically significant intestinal inflammation as compared to those without restraint stress. In the second experiment, restraint stress significantly reduced body weight and increased the severity of intestinal inflammation assessed by histopathologic grading in IL-10-/- mice. Colonic IL12p40 mRNA expression was strongly increased in mice exposed to restraint stress.
CONCLUSION: This novel animal model could be useful in future study of psychological stress in the pathogenesis of inflammatory bowel disease.
Core tip: We investigated the effect of restraint stress on the development and worsening of bowel inflammation in interleukin (IL)-10-/- mice and developed a novel animal model. This is the first study to demonstrate the effect of restrain stress in inducing and exacerbating chronic colitis in IL-10-/- mice. We believe that this novel animal model could be useful in future study of psychological stress in the pathogenesis of inflammatory bowel disease because this model develops chronic colitis due to interaction of genetic, immune dysregulation, microbial environment, and stress factor.
- Citation: Koh SJ, Kim JW, Kim BG, Lee KL, Kim JS. Restraint stress induces and exacerbates intestinal inflammation in interleukin-10 deficient mice. World J Gastroenterol 2015; 21(28): 8580-8587
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8580
Stress is associated with numerous gastrointestinal disorders[1,2]. Peptic ulcer disease is associated with stress-induced acid secretion[3], and inpatients with psychological stress have been noted to have increased rates of irritable bowel syndrome (IBS) and functional dyspepsia[4]. Stress is an important factor for both the development and occurrence of relapse in inflammatory bowel disease (IBD)[5]. Patients with IBD are reported to have an increased response rate to placebo treatment, supporting the role of stress in the pathogenesis of IBD[6,7].
It has been proposed that stress modulates intestinal inflammation in experimental models. Restraint stress was shown to induce mucosal erosion and subepithelial hemorrhage in rats[8]. Restraint stress exacerbates dextran sulfate sodium (DSS)-induced colitis as well as the oxidative stress damage caused by 2,4,6-trinitrobenzene sulfonic acid (TNBS)[9,10]. Stress increases colonic permeability, facilitating bacterial translocation and activation of CD4+ T cell response[11]. These data support the importance of stress in both the development and recurrence of bowel inflammation.
The precise pathogenesis of IBD remains obscure, but it is known to result from a complex interaction of genetic, microbial, immune dysregulation, and environmental factors[12]. Interleukin (IL)-10 knockout (-/-) mice, in which IL-10 deficiency allows for the unrestrained augmentation of a type 1 T helper (Th-1)-related immune response, represent an animal model of Crohn’s disease[13]. IL-10-/- mice develops chronic colitis due to a combination of the same factors in the pathogenesis of IBD, including genetic predisposition, immune dysregulation, and microbial environment. Therefore, IL-10-/--associated colitis is more suitable than chemically-induced colitis models to evaluate the effect of stress on intestinal inflammation.
In the present study, we investigated the effect of restraint stress on the development and worsening of bowel inflammation in IL-10-/- mice and developed a novel animal model.
Seven to eight-week-old specific pathogen-free male (SPF) C57BL/6 IL-10-/- mice were obtained from the Center for Animal Resource and Development (Seoul, South Korea). Age- and sex-mached wild-type (C57BL/6NCrljBgi male mice) littermates were purchased from Orient (Seongnam, South Korea). All mice were maintained on a 12-h/12-h light/dark cycle under SPF conditions. Mice had ad libitum access to water and standard rodent food. All animal procedures and stress protocols were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital.
The first experiment compared the effect of restraint stress on the development of intestinal inflammation in wild-type (stress positive, n = 4; stress negative, n = 4) and IL-10-/- mice (stress positive, n = 4; stress negative, n = 10). Both wild-type and IL-10-/- mice were physically restrained in a well-ventilated, 50 cm3 conical polypropylene tube for 2 h per day for three consecutive days. The mice were euthanized 5 d after the final exposure to restraint stress.
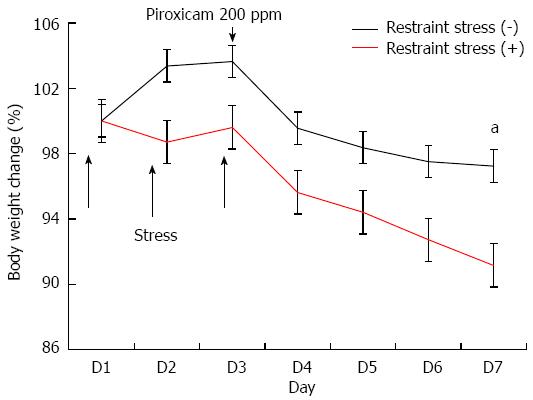
Because the onset and severity of colitis are variable in IL-10-/- mice, the second experiment was performed using piroxicam, which is known to induce rapid and uniform colitis in IL-10-/- mice[14]. The IL-10-/- mice (stress positive, n = 4; stress negative, n = 6) were exposed to restraint stress for 2 h per day for 3 consecutive days, and then treated with piroxicam for 4 days at a dose of 200 ppm administered in the rodent chow. We checked the amount of piroxicam-containing chow daily. IL-10-/- mice consumed approximately 1.5-2 g of piroxicam-containing chow, which was similar in both two groups regardless of stress exposure. Mice were sacrificed on the 7th day after the first exposure to restraint stress. Mice were monitored daily for behavior, water intake, food consumption, and body weight by a researcher blinded to the restraint stress protocol.
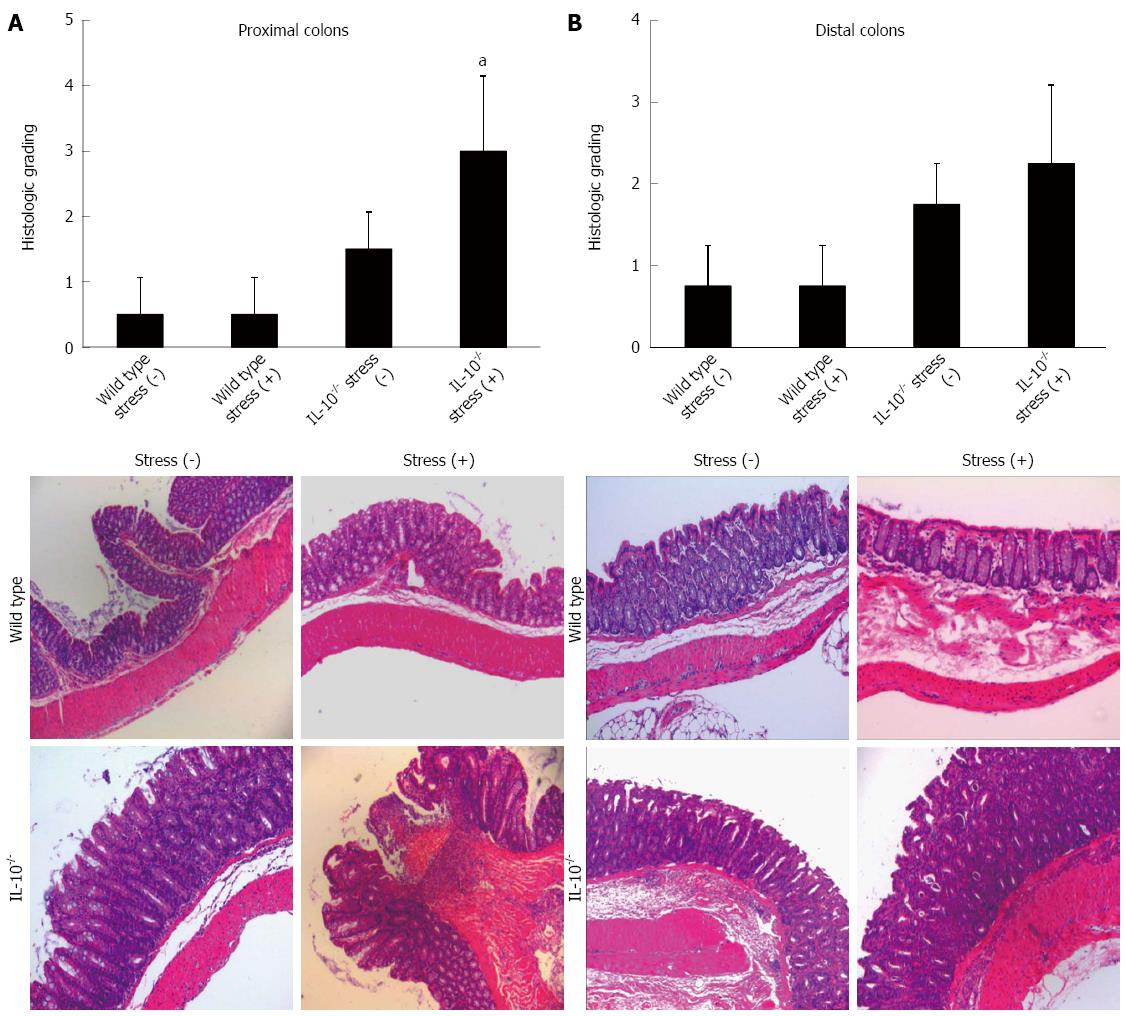
Histopathological evaluation was performed as described previously[15]. Mouse colons were immediately extracted and examined for macroscopic damage. Both the proximal and distal 2-cm portions of the colon were removed and opened longitudinally for histopathological evaluation. The resected colons from mice were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hematoxylin-eosin for light microscopic evaluation or frozen in liquid nitrogen for biochemical analysis. The severity of colitis was evaluated by a pathologist blinded to the restraint stress protocol according to a previously published grading system based on mononuclear cell infiltration, epithelial cell hyperplasia, goblet cell depletion, ulceration, crypt damages, and transmural inflammation[16]: 0 - no change from normal tissue; 1 - a few mononuclear cell infiltrates in the lamina propria with minimal epithelial hyperplasia and slight depletion of mucus from goblet cells; 2 - several multifocal, mild inflammatory cell infiltrates in the lamina propria with mild epithelial hyperplasia and mucin depletion; 3 - moderate inflammation that often involves the submucosa but is rarely transmural; 4 - intense inflammation that is sometimes transmural with marked epithelial hyperplasia, ulceration, and crypt abscesses.
Quantitative real-time reverse-transcriptase polymerase chain reaction (RT-RCR) was performed as described previously[17]. Mouse colon tissues were homogenized and total RNA was extracted by using Trizol (GIBCO). One microgram of extracted RNA was reverse-transcribed and amplified using the SYBR green PCR master mix and an ABI prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) with specific primers for IL-12p40 and IFN-γ. The obtained data were normalized to the level of β-actin. The following PCR primers were used: IL-12p40 forward, 5’-gga agc acg gca gaa ta-3’, IL-12p40 reverse, 3’-aac ttg agg gag aag tag gaa tgg-5’; TNF-α forward, 5’-agc cca cgt cgt agc aac cac caa-3’, TNF-αreverse, 3’-aca ccc att ccc ttc aca gag-5’; β-actin forward, 5’-gtg ggc cgc tct agg cac caa-3’, β-actin reverse, 3’-ctc ttt gat gat gac acg cac gat ttc-5’.
The difference between the two groups was analyzed using the Mann-Whitney U test. P-values less than 0.05 were considered statistically significant.
Before evaluating the effect of restraint stress, we assessed the basal level of intestinal inflammation in IL-10-/- mice for our facility. IL-10-/- mice at 6 to 7 wk of age were grossly healthy and had no intestinal inflammation. In addition, intestinal inflammation was not observed until IL-10-/- mice were 11 wk old.
To assess the role of restraint stress in the inflammatory responses in the colon, 7-wk-old IL-10-/- and wild-type mice were physically restrained in a well-ventilated, 50 cm3 conical polypropylene tube for 2 h per day for three consecutive days. There was no significant difference in body weight change among the four groups. None of the wild-type mice had intestinal inflammation (average colitis score 0.50 ± 0.57 and 0.75 ± 0.50 in proximal and distal colon, respectively). Mild colitis was observed in 20% of IL-10-/- mice without restraint stress (average colitis score 1.20 ± 0.57 and 1.30 ± 0.50 in proximal and distal colon, respectively); the majority (80%) of IL-10-/- mice without stress was completely normal. In contrast, epithelial hyperplasia, crypt abscess, ulceration, and transmural inflammation were observed in IL-10-/- mice exposed to restraint stress. Histological grading showed that restraint stress significantly aggravated the overall score of colitis (average colitis score 3.00 ± 1.15 and 2.25 ± 0.96 in proximal and distal colon, respectively) (Figure 1).
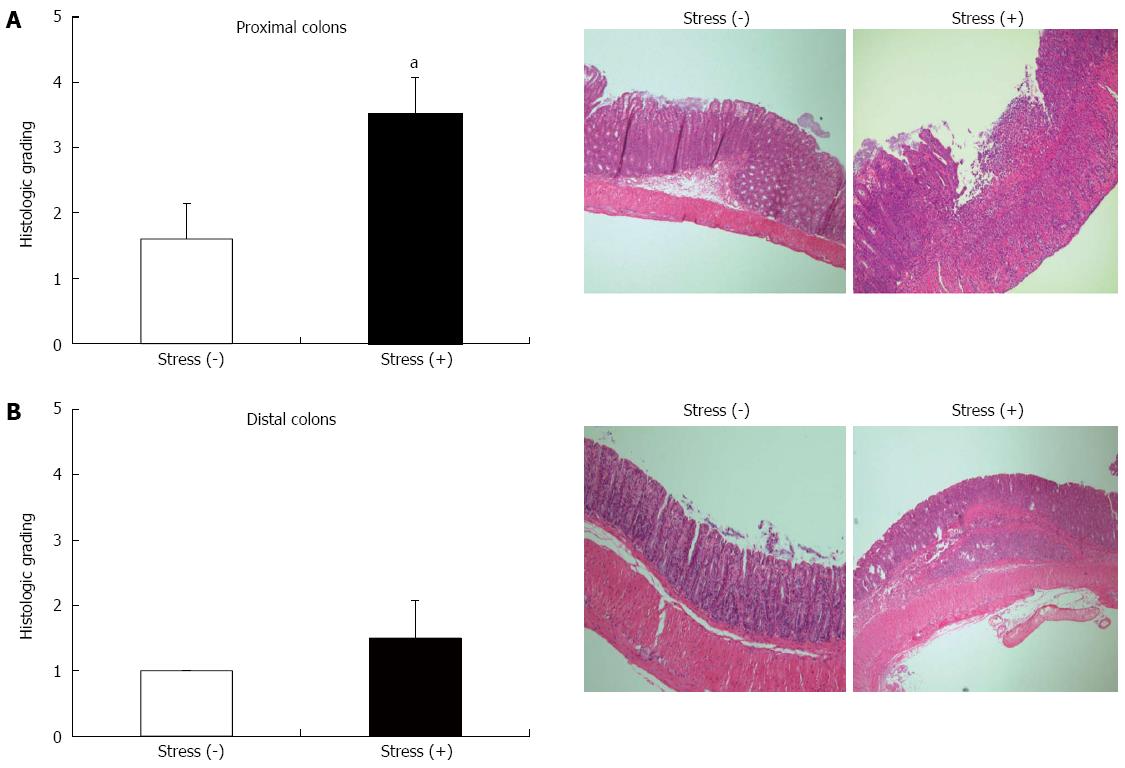
Because the onset and severity of colitis are variable in IL-10-/- mice, we performed a study using a rapid colitis model induced by piroxicam in IL-10-/- mice. Body weight was significantly reduced in IL-10-/- mice exposed to restraint stress 5 d after administration of piroxicam (Figure 2). The histologic examination of the proximal colons from IL-10-/- mice exposed to restraint stress showed severe colitis including ulceration, transmural inflammation, and severe infiltration of inflammatory cells. In contrast, epithelial hyperplasia and mild infiltration were observed in IL-10-/- mice without restraint stress. Histologic grading revealed that restraint stress exposure significantly amplified the severity score of colitis in IL-10-/- mice (average colitis score 3.50 ± 0.58 and 1.60 ± 0.55) (Figure 3A). Histologic evaluation revealed less severe intestinal inflammation in the distal colon than in the proximal colon of both groups. There was no significant difference in the histologic grade between the two groups (Figure 3B).
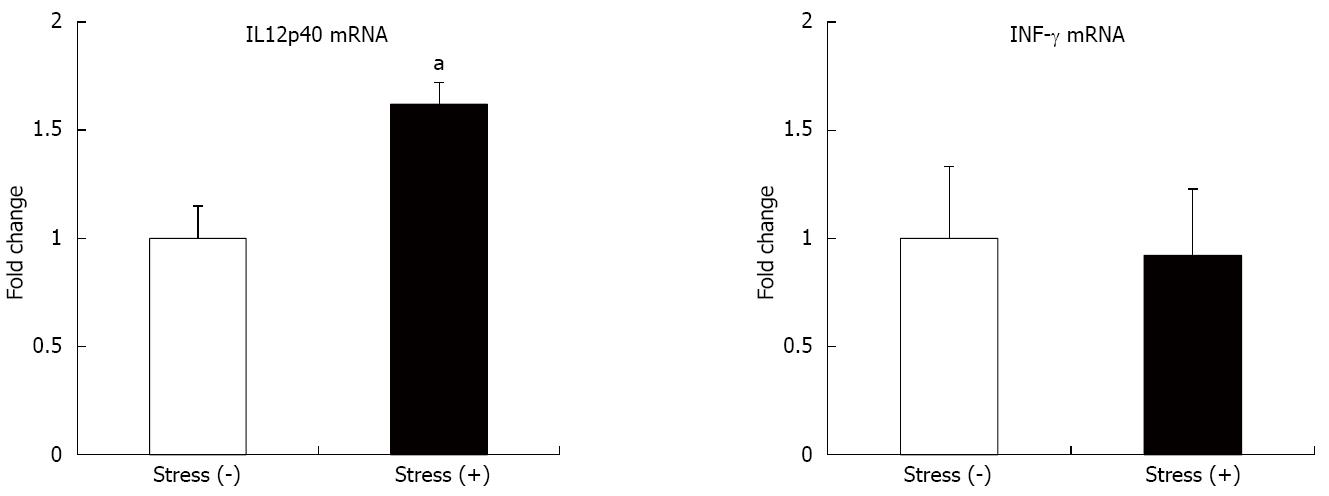
To determine whether restraint stress affects pro-inflammatory cytokine production, we performed real-time RT-PCR using extracted colons from IL-10-/- mice. As shown in Figure 4, the level of IL12p40 mRNA expression was increased in the colon of the mice exposed to restraint stress. However, there was no significant difference in IFN-γ level between the two groups.
It has been proposed that IBD is caused by the interplay of host genetic factors (including barrier function and immune dysregulation), intestinal microbiota, and various environmental factors such as psychological stress[18]. However, the precise role of psychological stress in the pathogenesis of IBD remains unclear. In an animal study, antidepressants reduce the susceptibility to the development of colitis in a model of depression induced by maternal separation[19]. In addition, recent data demonstrated that early life stress exacerbates colitis in IL-10-/- mice by inducing colonic barrier dysfunction[20], suggesting that psychological factors such as chronic stress, adverse life events, and depression are some of the critical factors affecting the disease activity of IBD. Our data prove that restraint stress triggers the development of severe colitis in IL-10-/- mice. In addition, colitis in mice exposed to restraint stress was rapidly induced by the administration of piroxicam, compared to mice without stress. Colitis induced by restraint stress in IL-10-/- mice was associated with elevated pro-inflammatory cytokine. Our data therefore provide additional information regarding the role of stress in the pathogenesis of chronic colitis, suggesting that the stress-induced IL-10-/- colitis model may be useful for elucidating the role of stress in IBD.
A previous study showed that restraint stress is associated with worsening of TNBS-induced colitis in rats[9]. In addition, restraint stress increases disease activity of colitis in mice with DSS-induced colitis[21]. These self-limiting and chemically induced colitis models are inadequate for use in studying chronic colitis, and the extent of mucosal damage may be influenced by duration or concentration of exposure of the chemical toxin. To overcome this weakness, we used IL-10-/- mice, which exhibit spontaneous chronic colitis. Moreover, the histopathology of colitis in these mice is similar to that seen Crohn’s disease. Our data demonstrate that restraint stress exacerbates colitis by both a clinical and histologic index in a Crohn’s disease-like murine model, strengthening the evidence for the role of psychological stress in the pathogenesis of IBD.
The effect of psychological stress in intestinal inflammation is mediated by the alteration of epithelial barrier function, activation of mucosal mast cells, and hormonal changes in the hypothalamus-pituitary-adrenal gland axis[22,23]. However, the molecular mechanism by which restraint stress results in worsening colitis remains obscure. Our data showed no difference in the expression of IFN-γ in the colonic mucosa regardless of restraint stress. A previous study suggested that IFN-γ may not be a critical factor for stress mediated colitis, which is consistent with our data[21]. However, severe colitis caused by restraint stress was associated with increased IL12p40 expression in the present study. IL-12 production is critical for inducing Th-1 differentiation[24]. In addition, anti-IL-12 monoclonal antibody attenuates intestinal inflammation in IL-10-/- mice[25]. Furthermore, a human study demonstrated that psychological stress induces pro-inflammatory cytokine production and Th1-like response in stress-induced anxiety[26]. Therefore, we believe that restraint stress may contribute to activation of Th1-related immune mechanism, resulting in exacerbations of chronic colitis. Further studies are needed to explain the precise mechanism of psychological stress in the development of colitis.
Although we have demonstrated that restraint stress aggravates intestinal inflammation, the molecular mechanism remains obscure. It is suggested that oxidative stress is one of the etiological factors of IBD[27]. In several studies, patients with IBD demonstrated excessive reactive oxygen molecules in various specimens including colon tissues[28-30]. More importantly, an experimental colitis study in rats showed that immobilization stress increased susceptibility to oxidative damage[31]. Based on these results, oxidative stress seems to be an important factor in the molecular pathogenesis of stress-induced colitis. Further studies are needed to elucidate the molecular pathogenesis between stress and intestinal inflammation.
IBS and IBD are distinct diseases; however, they have some important overlapping features such as genetic factor, impaired gut barrier function, and immune activation[32]. Interestingly, stress can activate both IBS and IBD symptoms. However, it remains unclear whether stress overlaps in the pathogenesis of both diseases. Previously, Mozaffari et al[33] used restraint stress to create an animal model of IBS. In this study, five-day restraint stress induced rapid small bowel and colonic transit. Our data showed that restraint stress aggravates the severity of histopathology in IL-10-/- mice. Therefore, we believe that restraint stress animal models may be useful in investigating the common pathogenesis of IBS and IBD.
This study has several limitations. First, there are ethical considerations limiting the extent to which mice can be exposed to restraint stress, however, the Institutional Animal Care and Use Committee in our facility approved our study protocol methods after careful review. In addition, we tried to minimize the number of animals exposed to restraint stress in our study. Second, restraint stress aggravated the severity of colitis in the proximal colon. As known, IL-10-/- mice exhibit minor histological change in the distal colon, compared to that in the proximal colon. Although we could not confirm statistical significance, the trend toward aggravating the severity of inflammation was shown for the distal colon. Finally, we could not provide a precise molecular mechanism by which restraint stress results in chronic intestinal inflammation.
In conclusion, our study showed that restraint stress induces and exacerbates intestinal inflammation and pro-inflammatory cytokine production in IL-10-/- mice. This novel animal model may prove useful in future study of psychological stress in the pathogenesis of IBD.
The precise pathogenesis of inflammatory bowel disease (IBD) remains obscure, but it is known to result from a complex interaction of genetic, microbial, immune dysregulation, and environmental factors. It has been proposed that stress modulates intestinal inflammation. The authors investigated the effect of restraint stress on the development and worsening of bowel inflammation in interleukin (IL)-10-/- mice and developed a novel animal model.
The authors investigated the effect of restraint stress on the development and worsening of bowel inflammation in IL-10-/- mice and developed a novel animal model.
This study demonstrates for the first time that restraint stress induces and exacerbates intestinal inflammation and pro-inflammatory cytokine production in IL-10-/- mice.
This novel animal model may prove useful in future study of psychological stress in the pathogenesis of IBD.
IL-10 is an anti-inflammatory cytokine. IL-10-/- mice exhibit spontaneous Crohn’s disease-like colitis. IL12p40 is a cytokine for the differentiation of T-cells.
The authors examined the effect of restraint stress in a chronic colitis model. IL-10-/- mice exposed to restraint stress exhibited histologically significant intestinal inflammation. Colonic IL12p40 mRNA expression was strongly increased in mice exposed to restraint stress. These results establish a novel model of stress-aggravated IBD-like colitis.
| 1. | Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154 Suppl 1:S63-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Koh SJ, Kim M, Oh da Y, Kim BG, Lee KL, Kim JW. Psychosocial stress in nurses with shift work schedule is associated with functional gastrointestinal disorders. J Neurogastroenterol Motil. 2014;20:516-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Richardson CT. Pathogenetic factors in peptic ulcer disease. Am J Med. 1985;79:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 5. | Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Andreoli A, Luzi C. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am J Gastroenterol. 1994;89:1219-1225. [PubMed] |
| 6. | Ilnyckyj A, Shanahan F, Anton PA, Cheang M, Bernstein CN. Quantification of the placebo response in ulcerative colitis. Gastroenterology. 1997;112:1854-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology. 2004;126:1257-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Stein TA, Keegan L, Auguste LJ, Bailey B, Wise L. Stress induced experimental colitis. Mediators Inflamm. 1993;2:253-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Israeli E, Hershcovici T, Berenshtein E, Zannineli G, Wengrower D, Weiss O, Chevion M, Goldin E. The effect of restraint stress on the normal colon and on intestinal inflammation in a model of experimental colitis. Dig Dis Sci. 2008;53:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Milde AM, Murison R. A study of the effects of restraint stress on colitis induced by dextran sulphate sodium in singly housed rats. Integr Physiol Behav Sci. 2002;37:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1570] [Article Influence: 82.6] [Reference Citation Analysis (2)] |
| 13. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3189] [Cited by in RCA: 3254] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 14. | Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol. 2014;29:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Koh SJ, Kim JW, Kim BG, Lee KL, Chun J, Kim JS. Fexofenadine regulates nuclear factor-κB signaling and endoplasmic reticulum stress in intestinal epithelial cells and ameliorates acute and chronic colitis in mice. J Pharmacol Exp Ther. 2015;352:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Koh SJ, Kim JM, Kim IK, Kim N, Jung HC, Song IS, Kim JS. Fluoxetine inhibits NF-κB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3415] [Article Influence: 179.7] [Reference Citation Analysis (12)] |
| 19. | Varghese AK, Verdú EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10-/- mice. Inflamm Bowel Dis. 2013;19:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Xu Y, Hunt NH, Bao S. The effect of restraint stress on experimental colitis is IFN-gamma independent. J Neuroimmunol. 2008;200:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 23. | Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008-4027. [PubMed] |
| 25. | Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143-3149. [PubMed] |
| 26. | Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 27. | Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 445] [Article Influence: 23.4] [Reference Citation Analysis (3)] |
| 28. | Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 288] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Sampietro GM, Cristaldi M, Cervato G, Maconi G, Danelli P, Cervellione R, Rovati M, Bianchi Porro G, Cestaro B, Taschieri AM. Oxidative stress, vitamin A and vitamin E behaviour in patients submitted to conservative surgery for complicated Crohn’s disease. Dig Liver Dis. 2002;34:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Colón AL, Madrigal JL, Menchén LA, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress increases susceptibility to oxidative/nitrosative mucosal damage in an experimental model of colitis in rats. Dig Dis Sci. 2004;49:1713-1721. [PubMed] |
| 32. | Spiller R, Lam C. The shifting interface between IBS and IBD. Curr Opin Pharmacol. 2011;11:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Mozaffari S, Esmaily H, Rahimi R, Baeeri M, Sanei Y, Asadi-Shahmirzadi A, Salehi-Surmaghi MH, Abdollahi M. Effects of Hypericum perforatum extract on rat irritable bowel syndrome. Pharmacogn Mag. 2011;7:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Marie JC, Trifan A, Zouiten-Mekki L S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN