Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8389
Peer-review started: February 11, 2015
First decision: March 10, 2015
Revised: March 31, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: July 21, 2015
Processing time: 161 Days and 13.4 Hours
AIM: To investigate the splenic hilar vascular anatomy and the influence of splenic artery (SpA) type in laparoscopic total gastrectomy with spleen-preserving splenic lymphadenectomy (LTGSPL).
METHODS: The clinical anatomy data of 317 patients with upper- or middle-third gastric cancer who underwent LTGSPL in our hospital from January 2011 to December 2013 were collected. The patients were divided into two groups (concentrated group vs distributed group) according to the distance between the splenic artery’s furcation and the splenic hilar region. Then, the anatomical layout, clinicopathologic characteristics, intraoperative variables, and postoperative variables were compared between the two groups.
RESULTS: There were 205 patients with a concentrated type (64.7%) and 112 patients with a distributed type (35.3%) SpA. There were 22 patients (6.9%) with a single branch of the splenic lobar vessels, 250 (78.9%) with 2 branches, 43 (13.6%) with 3 branches, and 2 patients (0.6%) with multiple branches. Eighty seven patients (27.4%) had type I splenic artery trunk, 211 (66.6%) had type II, 13 (4.1%) had type III, and 6 (1.9%) had type IV. The mean splenic hilar lymphadenectomy time (23.15 ± 8.02 vs 26.21 ± 8.84 min; P = 0.002), mean blood loss resulting from splenic hilar lymphadenectomy (14.78 ± 11.09 vs 17.37 ± 10.62 mL; P = 0.044), and number of vascular clamps used at the splenic hilum (9.64 ± 2.88 vs 10.40 ± 3.57; P = 0.040) were significantly lower in the concentrated group than in the distributed group. However, the mean total surgical time, mean total blood loss, and the mean number of harvested splenic hilar lymph nodes were similar in both groups (P > 0.05 for each comparison). There were also no significant differences in clinicopathological and postoperative characteristics between the groups (P > 0.05).
CONCLUSION: It is of value for surgeons to know the splenic hilar vascular anatomy when performing LTGSPL. Patients with concentrated type SpA may be optimal patients for training new surgeons.
Core tip: Japanese Gastric Cancer Association guidelines recommend splenic hilar lymphadenectomy in patients with upper- and middle-third gastric cancer. However, the vessels in the splenic hilum are intricate and variable. The areas adjacent to the splenic hilum are located in a deep, narrow operating space, which makes it difficult to identify the proper vessels and successfully complete splenic regional lymphadenectomy. Therefore, familiarity with the vascular anatomy is useful for surgeons performing laparoscopic total gastrectomy with spleen-preserving splenic hilar lymphadenectomy (LTGSPL) and may reduce the complications. This is the first study to investigate the splenic vascular anatomy in vivo and its influence on LTGSPL.
- Citation: Zheng CH, Xu M, Huang CM, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M. Anatomy and influence of the splenic artery in laparoscopic spleen-preserving splenic lymphadenectomy. World J Gastroenterol 2015; 21(27): 8389-8397
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8389
The splenic hilar lymph nodes (No. 10 LNs) are easily invaded in advanced upper- or middle-third gastric cancer. The metastatic frequency in No. 10 LNs is 9.8%-20.9%[1]. According to the Japanese Gastric Cancer Association (JGCA), No. 10 LNs must be dissected in upper- or middle-third gastric cancer patients treated with radical total gastrectomy[2]. The feasibility and safety of laparoscopic-assisted total gastrectomy with D2 LN dissection in upper- or middle-third gastric cancer patients have been accepted[3,4]. Laparoscopic total gastrectomy with spleen-preserving splenic hilar lymphadenectomy (LTGSPL) has been gradually offered to these patients as a result of new interest in surgical treatments and progress in gastric cancer surgical and anatomical techniques[5-7]. The feasibility of LTGSPL in these patients has also been confirmed[5-9].
The vessels in the splenic hilum are intricate and variable. The areas adjacent to the splenic hilum are complex and located in a deep, narrow operating space, which makes it difficult to identify the correct vessels accurately, and successfully complete splenic regional lymphadenectomy. Furthermore, it is difficult to control bleeding once vessels are injured. Therefore, highly skilled surgeons are required for this procedure[7,9]. Both experience and knowledge of minimally invasive surgical anatomy are helpful for laparoscopic surgery[10]. Additionally, familiarity with the splenic hilar vascular anatomy is useful for surgeons performing LTGSPL, and may reduce the complications. However, studies on the splenic hilar vascular anatomy in vivo and the impact of splenic artery (SpA) type on LTGSPL have not been reported. Therefore, we conducted this study to investigate SpA vascular anatomy and evaluate the influence of different vessel types on the surgical outcomes in patients undergoing LTGSPL.
Between January 2011 and December 2013, 317 patients with upper- or middle-third gastric cancer underwent LTGSPL in the Department of Gastric Surgery, Fujian Medical University Union Hospital. All subjects were preoperatively confirmed as having upper- or middle-third gastric cancer by analyses of endoscopic biopsy specimens. Preoperative imaging was routinely performed following endoscopic examination, including computer tomography (CT) scanning, ultrasonography (US) of the abdomen, and endoscopic US. Patients with T4b gastric cancer were preoperatively excluded from this study. Patients with enlargement and integration of No.10 LNs were not considered candidates for surgery. The surgical procedure, including its advantages and risks, was explained to all candidates for surgery. The ethics committee of Fujian Union Hospital approved this retrospective study. Written consent was given by the patients for their information to be stored in the hospital database and used for research.
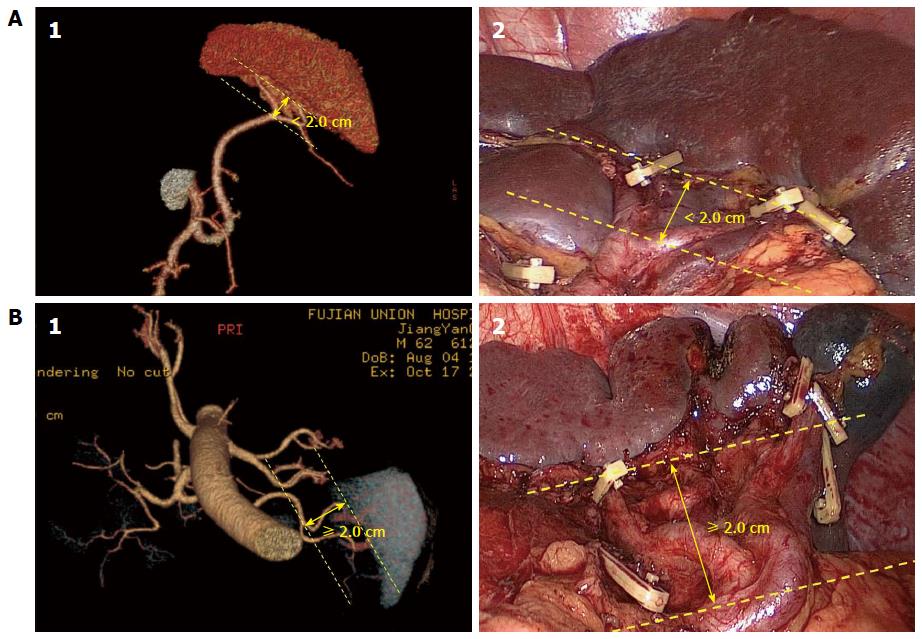
All patients underwent abdominal helical CT (Discovery CT750 HD, GE Healthcare, Waukesha, WI, United States). The scans ranged from the top of the diaphragm to the lower edge of the liver. On average, 100 mL of nonionic contrast agent was infused at a rate of 2 mL/s. CT data acquisition was triggered using the bolus tracking technique. The region of interest was the abdominal aorta just below the diaphragmatic dome. The trigger threshold level was set at a CT value of 100 Hounsfield units. Abdominal CT scans were performed at a slice thickness of 5 mm. Scanning was performed during each phase in a single breath-hold. The 3D images by CT (3DCT) of the splenic vessels were individually reconstructed using the original scanning images. The CT data were downloaded to an offline workstation for image post-processing and analysis. The distance from the SpA’s furcation to the splenic hilar region was measured using the reconstructed images. The distance was then re-measured during surgery by the opening degree of the dissecting forceps, which was 2 cm when opened completely. The results were then compared with the 3DCT. We selected 2 cm as the cut-off point for classification. When the SpA divided into its terminal branches < 2 cm from the splenic hilum, the case was considered to be concentrated type. If the distance was ≥ 2 cm, the case was considered to be the distributed type[11,12]. Patients were then assigned to the concentrated group or the distributed group. TNM stage was defined based on the 7th Edition of the UICC TNM system[13]. The operation time at the splenic hilum was defined as the time for splenic hilar lymphadenectomy. Blood loss at the splenic hilum was defined as the volume of blood lost during dissection of the splenic hilar LNs.
Splenic hilar lymphadenectomy was performed according to the guidelines of the Japanese Classification of Gastric Carcinoma[14]. In our institution, LTGSPL has become highly standardized, with all operations performed by a senior surgeon (Chang-Ming Huang) who had performed > 500 laparoscopic assisted gastrectomies before starting to perform this operation[15,16]. We have summarized an effective procedure called Huang’s 3-step maneuver for performing LTGSPL in clinical practice[17]. The steps are described as follows:
Patient positioning: The patient is placed in a reverse Trendelenburg position with the head elevated about 15-20 degrees and tilted left side up about 20-30 degrees. The surgeon stands between the patient’s legs, and the assistant and camera operator are both on the patient’s right side.
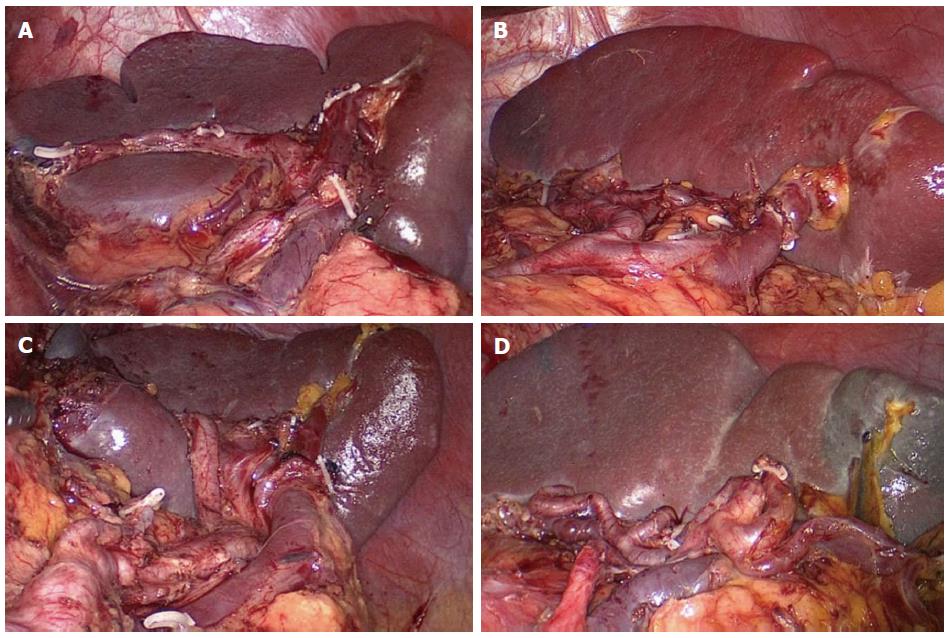
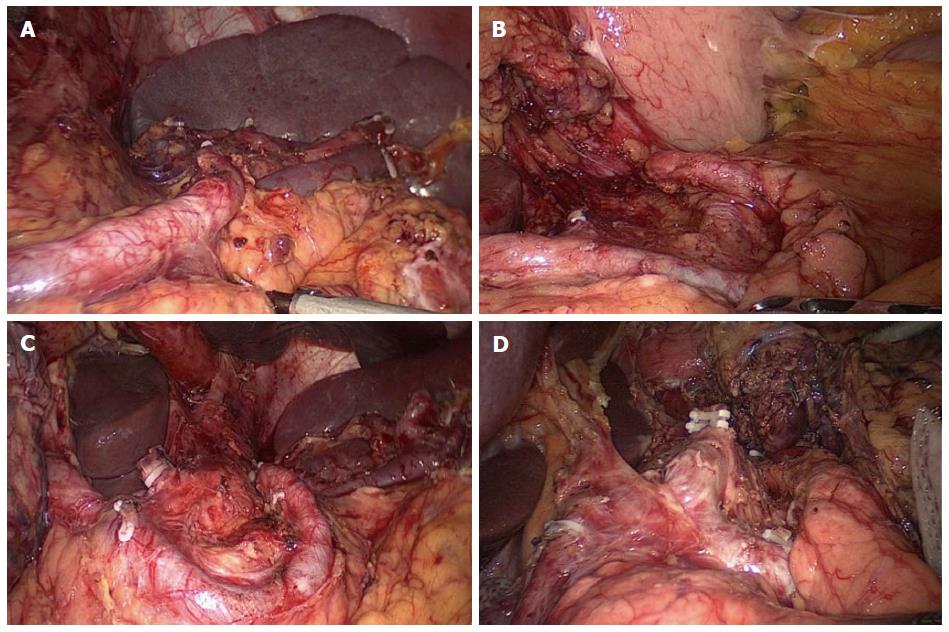
Splenic hilar LNs dissection: The first step is dissection of the LNs in the inferior pole region of the spleen. The surgeon uses an ultrasonic scalpel to separate the greater omentum toward the left up to the splenic flexure of the colon along the superior border of the transverse colon. Next, the anterior pancreatic fascia (APF) is peeled against the anterior inherent pancreatic fascia along the direction of the pancreas to the superior border of the pancreatic tail. Then, the surgeon uses the ultrasonic scalpel to open the APF along the fascia’s continuation, and the retropancreatic space (RPS) is entered at the superior border of the pancreas. Next, the mutually linked potential space in the gastrosplenic ligament (GSL) and the splenorenal ligament can be entered by following the RPS, and the end of the splenic vessel’s trunk can be exposed at the initial segment of the GSL. The dissection is continued along the end of the splenic vessels to expose the lower lobar vessels of the spleen (LLVSs) or the lower pole vessels of the spleen. The assistant uses the right hand to pull up the fatty lymphatic tissue on the surface of the vessels, and the surgeon uses the non-functional face of the ultrasonic scalpel to dissect the lymphatic tissue along the vessels distally up to the splenic hilar. During the dissection, the roots of the left gastroepiploic vein (LGEV) can be revealed at the splenic lobar artery (SLA) or splenic lower pole artery (SLPA) near the lower pole of the spleen. The assistant pulls up the fatty lymphatic tissue at the roots of the LGEVs, and the surgeon uses the ultrasonic scalpel to vascularize the vessels by dissecting the tissue along the anatomical space at the surface of the vessels. Next, the LGEVs are transected at their roots with vascular clamps. The resection of the No. 4sb LNs is then accomplished. At this time, the assistant pulls up the fatty lymphatic tissue on the surface of the LLVSs, while the surgeon uses the ultrasonic scalpel to meticulously dissect this tissue along the anatomical space on the surface of the LLVSs toward the splenic hilar, alternatively using blunt and sharp methods of separation. As the LLVSs are gradually revealed, 1-2 branches of the short gastric vessels (SGVs) issuing from the inferior lobar artery of the spleen may be encountered. If so, the assistant gently pulls up the SGVs, and the surgeon uses the ultrasonic scalpel to meticulously dissect the fatty lymphatic tissue around the vessels to vascularize the vessels, which are divided at their roots with vascular clamps.
The second step is dissection of the LNs in the region of the SpA trunk. The assistant uses the right hand to pull up the isolated fatty lymphatic tissue on the surface of the trunk of SpA. The surgeon uses the ultrasonic scalpel to denude the trunk of the SpA along the latent anatomic space on its surface toward the splenic hilar up to the fork of the splenic lobar arteries. The fatty lymphatic tissue around the end of the SpA is then excised. One or two branches of the posterior gastric artery (PGA), which are derived from the SpA, will always be encountered in this region. At this time, the assistant should clamp the vessels and pull them upward, while the surgeon uses the ultrasonic scalpel to dissect the fatty lymphatic tissue around the PGAs and close to the trunk of the SpA. The PGAs are divided at their roots using vascular clamps. The excision of the No. 11d LNs is then accomplished.
The third step is dissection of the LNs in the superior pole region of the spleen. The assistant gently pulls up the fatty lymphatic tissue on the surface of the splenic vessels’ branches in the GSL, and the surgeon applies the non-functional face of the ultrasonic scalpel along the anatomical space on the surface of the SLA and vein to completely vascularize the vessels in the splenic superior lobar area, using meticulous sharp or blunt pushing, peeling and dissection. At this time, 1-3 branches of the SGVs arising from the SLA and passing through the GSL are usually encountered. The assistant should clamp and pull the SGVs upward, while the surgeon meticulously resects the surrounding fatty lymphatic tissue, proceeding toward the roots of the vessels. Next, the SGVs are divided at their roots with clamps. The last branch of SGVs in the superior pole region of the spleen is often very short, which causes the fundus to lie close against the splenic hilar. The last branch can be easily damaged, causing bleeding, if there is lack of proper traction. At this time, the assistant should pull the fundus toward the lower right side enough to completely expose the vessel, while the surgeon transects the vessel at its root with vascular clamps after carefully separating the surrounding fatty lymphatic tissue. Finally, the splenic hilar lymph nodes are dissected completely.
Statistical analyses were performed using the SPSS 18.0 statistical software package (IBM Corp., Armonk, NY, United States). Means were compared using t-tests. Categorical data were compared with a χ2 test or Fisher’s exact test. P values < 0.05 were considered statistically significant.
Classification of the terminal branches of the SpA: The SpA branches from the celiac artery. It continues toward the left along the upper border of the pancreas and gives off branches to the pancreas along the way. The SpA then divides into the terminal branches in the spleen. The type of terminal branches was classified according to the distance between the artery’s furcation and the splenic hilar region as either a concentrated type or distributed type SpA. The concentrated type SpA divides into its terminal branches < 2 cm from the splenic hilum. The SpA trunk is long, but the branches are short and wide in diameter and reach the spleen as a compact bundle in this type. Conversely, the distributed type SpA generally divides into its terminal branches ≥ 2 cm from the splenic hilum. The SpA trunk is short, but the branches are longer and have a smaller diameter and enter the spleen in a distributed fashion (Figure 1). In the study, the types of the terminal branches of the SpA observed preoperatively by 3DCT were consistent with their intraoperative conditions. The anatomical data showed that 64.7% of the patients had the concentrated type (205 cases) and 35.3% had the distributed type (112 cases).
SLA: The SLAs are the terminal branches of the SpA at the splenic hilum, and there can be one or a number of branches. If the SpA passes tortuously through the splenic hilar without dividing into terminal branches, it is classified as a single-branch type. This type is rare and was observed in 22 cases (6.9%) in our study. When the SpA divides into the superior and inferior lobar arteries, it is classified as a 2-branched type. This type is common and was observed in 250 cases (78.9%). It is called a 3-branched type when the SpA divides into the superior, middle, and inferior lobar arteries of the spleen. This type was present in 43 cases (13.6%) in the study. The SpA rarely branches into 4-7 branches that enter the splenic hilum, and these cases are classified as multiple-branched type. There were only two such cases (0.6%) in our study (Figure 2).
Relationship between the course of the SpA trunk and the pancreas: The course of the SpA trunk is intimately associated with the pancreas, and it is divided into four variations. In Type I, the SpA trunk follows the suprapancreatic course to the splenic hilum after arising from the celiac artery. This type was present in 87 cases (27.4%). In Type II, the middle half of the SPA trunk has either a retro- or intra-pancreatic course. There were 211 Type II cases (66.6%) in our study. In Type III, the distal half of the SPA follows either a retro- or intra-pancreatic course. There were 13 Type III cases (4.1%). In Type IV, the distal three-quarters of the SpA is entirely embedded in the substance of the pancreas or follows a retro-pancreatic course. We observed six Type IV cases (1.9%) in this study (Figure 3).
There is also an existing splenic pole artery (SPoA) that directly enters the upper and/or lower pole of the spleen without passing through the splenic hilum. There is a splenic upper pole artery (SUPA) and a splenic lower pole artery (SLPA). The SPoA may arise from the SpA trunk, SLA or left gastroepiploic artery. The data show that 68 patients had SPoA. The incidence of SUPA was 16.40% (52 cases). The incidence of SLPA was 5.05% (16 cases). The splenic artery also branched into arteries to supply other organs. The short gastric artery (SGA) and left gastroepiploic artery are important vessels related to the splenic hilar lymphadenectomy. The SGA mainly arises from the SLA, the SpA trunk and the left gastroepiploic artery. The mean number of branches of the SGA was 3.62 ± 1.09 (range, 0 to 7) in our study. The detailed data are presented in Table 1.
| Terminal branches of the SpA | P value | ||
| Concentrated type | Distributed type | ||
| Type of SpA | 0.001 | ||
| Single branch SpA | 22 | 0 | |
| 2-branched SpA | 157 | 93 | |
| 3-branched SpA | 26 | 17 | |
| Multiple-branched SpA | 0 | 2 | |
| SpA along the pancrease | 0.561 | ||
| Type I | 61 | 26 | |
| Type II | 133 | 78 | |
| Type III | 7 | 6 | |
| Type IV | 4 | 2 | |
| SUPA | 27 | 23 | 0.085 |
| SLPA | 13 | 3 | 0.154 |
| No. of SGAs | 3.60 ± 1.05 | 3.63 ± 1.17 | 0.821 |
The clinicopathologic characteristics of the 317 patients are presented in Table 2. The series included 244 men and 73 women with a mean age of 60.8 years (range, 11-87 years). Age, sex, body mass index, tumor size, histologic type, tumor depth, N stage and TNM stage did not differ between the groups (all P > 0.05).
| Patient characteristics | Terminal branches of the SpA | P value | |
| Concentrated type | Distributed type | ||
| No. of patients | 205 | 112 | |
| Sex | 0.617 | ||
| Male | 156 | 88 | |
| Female | 49 | 24 | |
| Age (yr) | 60.79 ± 10.30 | 60.79 ± 10.77 | 1.000 |
| BMI (kg/m2) | 22.16 ± 2.62 | 21.68 ± 2.72 | 0.122 |
| Tumor size (cm) | 5.40 ± 2.75 | 5.83 ± 2.63 | 0.178 |
| Histological | 0.072 | ||
| Differentiated | 115 | 51 | |
| Undifferentiated | 90 | 61 | |
| Depth of invasion | 0.501 | ||
| pT1 | 22 | 9 | |
| pT2 | 24 | 16 | |
| pT3 | 84 | 39 | |
| pT4a | 75 | 48 | |
| Lymph node metastasis | 0.978 | ||
| pN0 | 52 | 29 | |
| pN1 | 35 | 21 | |
| pN2 | 39 | 21 | |
| pN3 | 79 | 41 | |
| TNM stage | 0.584 | ||
| I | 30 | 12 | |
| II | 51 | 31 | |
| III | 124 | 69 | |
For all 317 patients, the mean surgical time was 175.41 ± 31.97 min (range, 120-420 min). The mean total blood loss was 53.94 ± 31.77 mL (range, 5-300 mL). The mean number of harvested No. 10 LNs was 2.69 ± 2.16 (range, 0-9). The mean splenic hilar lymphadenectomy time (23.15 ± 8.02 vs 26.21 ± 8.84 min; P = 0.002), mean blood loss resulting from splenic hilar lymphadenectomy (14.78 ± 11.09 vs 17.37 ± 10.62 mL; P = 0.044), number of vascular clamps used at the splenic hilum (9.64 ± 2.88 vs 10.40 ± 3.57; P = 0.040), and mean total number of retrieved LNs (40.36 ± 14.08 vs 44.46 ± 14.80; P = 0.015) were significantly lower in the concentrated group than in the distributed group. The mean total surgical time, mean total blood loss, mean number of retrieved and positive No.10 LNs, and mean number of positive LNs were similar in both groups (all P > 0.05) (Table 3).
| Intraoperative characteristics | Terminal branches of the SpA | P value | |
| Concentrated type | Distributed type | ||
| Operation time (min) | 175.35 ± 32.95 | 175.53 ± 30.24 | 0.963 |
| Operation time at splenic hilum (min) | 23.15 ± 8.02 | 26.21 ± 8.84 | 0.002 |
| Blood loss (mL) | 53.76 ± 33.09 | 54.29 ± 29.33 | 0.887 |
| Blood loss at splenic hilum (mL) | 14.78 ± 11.09 | 17.37 ± 10.62 | 0.044 |
| No. of positive LNs | 7.36 ± 9.04 | 8.22 ± 10.04 | 0.433 |
| No. of retrieved LNs | 40.36 ± 14.08 | 44.46 ± 14.80 | 0.015 |
| No. of positive No. 10 LNs | 0.23 ± 0.81 | 0.11 ± 0.53 | 0.152 |
| No. of retrieved No. 10 LNs | 2.54 ± 2.18 | 2.96 ± 2.11 | 0.103 |
| No. of vascular clamps at splenic hilum | 9.64 ± 2.88 | 10.40 ± 3.57 | 0.040 |
There was no significant difference in the length of hospital stay, time to first flatus, time to a fluid diet, time to a soft diet, or morbidity between the two groups (all P > 0.05) (Table 4).
| Postoperative characteristics | Terminal branches of the SpA | P value | |
| Concentrated type | Distributed type | ||
| Postoperative hospital stay (d) | 12.99 ± 7.94 | 11.54 ± 4.30 | 0.075 |
| Day of first flatus (d) | 4.17 ± 1.06 | 4.10 ± 0.97 | 0.578 |
| Day of first fluid diet (d) Day of first semifluid diet (d) | 4.81 ± 1.73 8.66 ± 5.14 | 4.51 ± 1.54 7.73 ± 2.04 | 0.120 0.068 |
| Postoperative complications | 16 | 6 | 0.412 |
| Pulmonary infection | 7 | 1 | 0.171 |
| Abdominal infection | 3 | 1 | 0.664 |
| Wound problem | 1 | 2 | 0.254 |
| Anastomotic leakage | 3 | 0 | 0.198 |
| Chylous fistula | 1 | 0 | 0.459 |
| Anastomotic bleeding | 1 | 1 | 0.663 |
| Septicemia | 1 | 1 | 0.663 |
The vessels in the splenic hilum are intricate and variable, and are covered with fatty lymphoid tissue. Therefore, careful lymph node dissection in the area is important because uncontrollable bleeding can occur after vessel or spleen injury[18]. Thus, understanding the vessel anatomy in the splenic hilum is important. Most studies of the splenic hilar vessels are performed by dissecting cadavers or postmortem specimens. These studies examine the origin, length and course of the SpA. They also examine the type of terminal branches of the SpA using a sequential injection method involving both radiology and corrosion casting. These types of studies provided the theoretical basis for spleen-preserving treatment in traumatic spleen injuries[11,19,20]. There are also studies that have used multidetector-row CT or digital subtraction angiography to identify the type of SpA, branches of the SpA, and segmental arteries in the spleen. These analyses provided the anatomical imaging data for spleen artery embolotherapy[21-23].
Several scholars have suggested that studies based on cadavers or postmortem specimens do not accurately reflect the splenic hilar vascular anatomy because of tissue degeneration that occurs after the specimens are fixed in formalin. In addition, only the general vessel course in the splenic hilum can be observed by imaging. The distribution of the fatty tissue or the adjacent organs around the vessels cannot be evaluated. Thus, there are limitations in clinical guidance. Recently, laparoscopic splenectomy has been used clinically and is now a standard procedure in hemopathy[24,25]. These studies only investigated surgical feasibility, safety, surgical approach, and surgical complications rather than the vascular anatomy in vivo. Conversely, our study dissected the splenic hilar vessels in vivo using a laparoscope. Therefore, our study was more intuitive and realistic because the normal physiological function existed during the investigation. As a result, our study provides a more reliable and valuable reference for surgeons performing LTGSPL.
The SpA usually passes the upper border of the pancreas in a variety of forms[12]. Identifying the relationship between the SpA and the pancreas helped reduce intraoperative injury. Type I allowed the SpA to be sufficiently exposed and vascularized. Thus, it was easy to dissect the lymphatic fatty tissues around the entire artery. However, in other types, a portion of the SpA ran within the parenchyma of the pancreas. The resection of the lymphatic tissues should be performed more carefully because the pancreatic tissues might be incorrectly regarded to be LNs, which could lead to postoperative complications such as pancreatic fistula. The SLA branches from the SpA at the splenic hilum and is variable[12]. The SLA is tortuous, dissociative and usually identified as the SGV or posterior gastric vessel. Thus, it might be incorrectly severed, leading to splenic ischemia. Therefore, vessels with an unclear course should be separated toward their distal terminal until their orientation can be distinguished. Such manipulation enables a safe division and prevents unnecessary injury caused by a blind dissection. The SGAs were some of the most important structures that required severing during lymphadenectomy. The SGAs were easily injured and resulted in bleeding, which influenced the operation outcome. They originated from the SLA and could be naturally revealed during the process of denuding the SLA. Then, the vessel was severed at its root. There were no branches from the SGAs encountered in this plane and none of the branches had been twisted, which reduced the risk of vascular injury.
The concentrated type SpA was common in this study. The intraoperative characteristics were further compared between the groups, and the results revealed that the mean splenic hilar LN dissection time, mean blood loss due to splenic hilar LN dissection, and number of vascular clamps used at the splenic hilum were significantly lower in the concentrated group because the branches of the distributed type SpA were longer. The length of the branches was 28.25 ± 8.44 mm in the distributed type and 15.29 ± 11.14 mm in the concentrated type in a previous specimen study[26]. Thus, more lymph node tissue must be dissected in the distributed group. The caliber of branches in the distributed type[11,12] was smaller, and these vessels were easily injured, which increased the operative difficulty and risk. As a result, the dissection time and blood loss in the splenic hilum were increased. Moreover, the number of SGA branches was higher in the distributed group, although there was no significant difference between groups. There were more SGAs that needed to be dissected and severed, increasing the surgical difficulty. Thus, the operative risk of LTGSPL was lower in the concentrated type SpA because of the shorter time required and less blood loss. However, the branches of the concentrated type SpA were more centralized and the interval between branches was narrow. The surgeon should also be very attentive when excising the LNs in this area to avoid injuring the vessels. The types of terminal branches of the SpA detected preoperatively using 3DCT were consistent with the intraoperative conditions. Thus, it could be roughly classified according to 3DCT preoperatively. Furthermore, 3DCT also enabled surgeons to know the distribution and variation of the splenic vessels preoperatively[27]. LTGSPL cannot be applied in every center routinely. Thus, it is beneficial for surgeons to master the technique by selecting patients with the concentrated type SpA identified using 3DCT preoperatively. However, some shortcomings of this study need to be noted. First, it is very difficult to perform LTGSPL and cannot be widely applied in other centers. Second, the results were based on a retrospective analysis of the clinical data at a single institution, and needs to be further confirmed in future large-scale, prospective clinical trials.
In conclusion, the vascular anatomy of the splenic hilum is complex and variable, and knowing the anatomical characteristics helps surgeons to perform LTGSPL safely and effectively. New surgeons could choose patients with the concentrated type of splenic artery because it is less difficult to comple the operation in these patients.
We are indebted to all the members of our lab for helpful comments and discussions.
Because the vessels in the splenic hilum are intricate and variable, and the areas adjacent to the splenic hilum are complex and located in a deep narrow operating space, it is difficult to identify the correct vessels accurately, and successfully complete splenic regional lymphadenectomy. Therefore, familiarity with the splenic hilar vascular anatomy is helpful for surgeons to perform laparoscopic total gastrectomy with spleen-preserving splenic hilar lymphadenectomy (LTGSPL) successfully, and may reduce the complications.
In spite of a few case reports and small series, studies on the splenic hilar vascular anatomy in vivo and the impact of splenic artery type on LTGSPL are lacking.
The clinical anatomy data of 317 patients with upper- or middle-third gastric cancer who underwent LTGSPL were collected in vivo. The results of the current study demonstrate that there exists a concentrated type and a distributed type of splenic artery (SpA). The types of splenic lobar artery include 1-branched type, 2-branched type, 3-branched type and multiple-branched type. The types of SpA trunk include Type I, Type II, Type III, and Type IV. Further analysis showed that the mean splenic hilar lymphadenectomy time, mean blood loss resulting from splenic hilar lymphadenectomy, and numbers of vascular clamps used at the splenic hilum were significantly lower in the concentrated group than in the distributed group.
The study dissected the splenic hilar vessels in vivo using laparoscopy. This study was more intuitive and realistic than prior reports because normal physiological function existed during the investigation. Therefore, this research provides a more reliable and valuable reference for surgeons performing LTGSPL.
From our experience in a previous study, the authors selected 2 cm as the cut-off point for SpA classification. When the SpA divided into its terminal branches < 2 cm from the splenic hilum, the case was considered to be concentrated type. If the distance was ≥ 2 cm, the case was considered to be the distributed type.
This is an interesting study in which authors evaluate the anatomy of splenic hilar vessels in vivo and its influence on LTGSPL for patients with upper- or middle-third gastric cancer. This finding has important clinical implications for gastrointestinal surgeons in spleen-preserving splenic hilar lymphadenectomy, especially for the beginners.
| 1. | Mönig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, Dienes HP, Hölscher AH. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89-92. [PubMed] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 3. | Edwards P, Blackshaw GR, Lewis WG, Barry JD, Allison MC, Jones DR. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. Br J Cancer. 2004;90:1888-1892. [PubMed] |
| 4. | Bösing NM, Goretzki PE, Röher HD. Gastric cancer: which patients benefit from systematic lymphadenectomy? Eur J Surg Oncol. 2000;26:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Huscher C, Mingoli A, Sgarzini G, Sansonetti A, Piro F, Ponzano C, Brachini G. Value of extended lymphadenectomy in laparoscopic subtotal gastrectomy for advanced gastric cancer. J Am Coll Surg. 2005;200:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Hur H, Jeon HM, Kim W. Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol. 2008;97:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (3)] |
| 9. | Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007;33:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ssoson-Jaroschewitsch A. Zur chirurgischen Anatomie des Milzhilus. Zeitsch Ges Anat Abt Bd. 1927;84:S218. |
| 12. | Zheng CH, Huang CM, Li P, Xie JW, Wang JB, Lin JX, Lu J. Laparoscopic spleen-preserving hilar lymph nodes dissection based on splenic hilar vascular anatomy. Zhonghua Xiaohua Waike Zazhi. 2012;215-219. [DOI] [Full Text] |
| 13. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Hoboken: Wiley 2009; . |
| 14. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 15. | Jia-Bin W, Chang-Ming H, Chao-Hui Z, Ping L, Jian-Wei X, Jian-Xian L. Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol. 2012;10:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. Learning curve of laparoscopy spleen-preserving splenic hilar lymph node dissection for advanced upper gastric cancer. Hepatogastroenterology. 2013;60:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW. Huang’s three-step maneuver for laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer. Chin J Cancer Res. 2014;26:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 18. | Yüney E, Höbek A, Keskin M, Yilmaz O, Kamali S, Oktay C, Bender O. Laparoscopic splenectomy and LigaSure. Surg Laparosc Endosc Percutan Tech. 2005;15:212-215. [PubMed] |
| 19. | Farag A, Shoukry A, Nasr SE. A new option for splenic preservation in normal sized spleen based on preserved histology and phagocytic function of the upper pole using upper short gastric vessels. Am J Surg. 1994;168:257-261. [PubMed] |
| 20. | Treutner KH, Klosterhalfen B, Winkeltau G, Moench S, Schumpelick V. Vascular Anatomy of the Spleen. Clin Anat. 1993;1-8. |
| 21. | Requarth JA, Miller PR. The splenic artery stump pressure is affected by arterial anatomy after proximal embolotherapy in blunt splenic injury. J Trauma Acute Care Surg. 2012;73:1221-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Dasgupta N, Matsumoto AH, Arslan B, Turba UC, Sabri S, Angle JF. Embolization therapy for traumatic splenic lacerations. Cardiovasc Intervent Radiol. 2012;35:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Pandey SK, Bhattacharya S, Mishra RN, Shukla VK. Anatomical variations of the splenic artery and its clinical implications. Clin Anat. 2004;17:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 24. | Mayberry JC, Sheppard BC, Mullins RJ. Laparoscopic management of an enlarging subcapsular splenic hematoma: case report. J Trauma. 1998;44:565-567. [PubMed] |
| 25. | Hamamci EO, Besim H, Bostanoglu S, Sonişik M, Korkmaz A. Use of laparoscopic splenectomy in developing countries: analysis of cost and strategies for reducing cost. J Laparoendosc Adv Surg Tech A. 2002;12:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Li W, Cui ZX, Dong XT, Kang JS, Liu YL, Zhang XJ. Role of CT angiography in the examinations of splenic artery and its branches. Zhongguo Quanke Yixue. 2010;10:3319-3321. |
| 27. | Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin JX, Lu J. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol. 2014;20:4797-4805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Namikawa T S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Zhang DN