Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8118
Peer-review started: January 22, 2015
First decision: March 10, 2015
Revised: March 27, 2015
Accepted: May 20, 2015
Article in press: May 21, 2015
Published online: July 14, 2015
Processing time: 175 Days and 12.7 Hours
AIM: To evaluate the efficacy of endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) for grading pancreatic neuroendocrine tumors (PNETs).
METHODS: A total of 22 patients were diagnosed with PNET by EUS-FNA between October 2001 and December 2013 at Fukushima Medical University Hospital. Among these cases, we targeted 10 PNET patients who were evaluated according to the World Health Organization (WHO) 2010 classification. Surgery was performed in eight patients, and chemotherapy was performed in two patients due to multiple liver metastases.Specimens obtained by EUS-FNA were first stained with hematoxylin and eosin and then stained with chromogranin, synaptophysin, CD56, and Ki-67. The specimens were graded by the Ki-67 index according to the WHO 2010 classification. Specimens obtained by surgery were graded by the Ki-67 index and mitotic count (WHO 2010 classification). For the eight specimens obtained by EUS-FNA, the Ki-67 index results were compared with those obtained by surgery. In the two cases treated with chemotherapy, the effects and prognoses were evaluated.
RESULTS: The sampling rate for histological diagnosis by EUS-FNA was 100%. No adverse effects were observed. The concordance rate between specimens obtained by EUS-FNA and surgery was 87.5% (7/8). For the two cases treated with chemotherapy, case 1 received somatostatin analog therapy and transcatheter arterial infusion (TAI) targeting multiple liver metastases. Subsequent treatment consisted of everolimus. During chemotherapy, the primary tumor remained unconfirmed, although the multiple liver metastases diminished dramatically. Case 2 was classified as neuroendocrine carcinoma (NEC) according to the Ki-67 index of a specimen obtained by EUS-FNA; therefore, cisplatin and irinotecan therapy was started. However, severe adverse effects, including renal failure and diarrhea, were observed, and the therapy regimen was changed to cisplatin and etoposide. TAI targeting multiple liver metastases was performed. Although the liver metastases diminished, the primary tumor remained unconfirmed. These chemotherapy regimens had immediate effects for both unresectable neuroendocrine tumor (NET) and NEC cases. These two subjects are still alive.
CONCLUSION: EUS-FNA was effective for PNET diagnosis and Ki-67 index grading for WHO 2010 classification, enabling informed decisions on unresectable PNET treatment by identifying NET or NEC.
Core tip: This is a retrospective study to evaluate the efficacy of endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) for grading pancreatic neuroendocrine tumors (PNETs). The concordance rate for grading between specimens obtained by EUS-FNA and surgery using the World Health Organization 2010 classification (Ki-67 indexing) was 87.5% in eight evaluated patients. In the two unresectable cases, chemotherapy was performed after grading was established based on the analysis of specimens obtained by EUS-FNA. Both treatments were adequately effective. EUS-FNA was useful for diagnosing PNET and enabled informed decisions on appropriate treatment plans by identifying neuroendocrine tumor or neuroendocrine carcinoma.
- Citation: Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, Kikuchi H, Konno N, Waragai Y, Asama H, Takasumi M, Watanabe H, Obara K, Ohira H. Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J Gastroenterol 2015; 21(26): 8118-8124
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8118
Neuroendocrine tumors (NETs) develop from neuroendocrine cells distributed throughout the body. Pancreatic NET (PNET) and gastrointestinal NET are classified together into G1 and G2 and neuroendocrine carcinoma (NEC) by the World Health Organization (WHO) 2010 classification, which is based on growth morphology (mitotic count and Ki-67 index)[1]. While PNET is rare, accounting for only 2%-5% of pancreatic tumors[2], more cases have been reported in recent years, correlating with the use of more sophisticated diagnostic imaging methods.
To develop PNET treatment plans, guidelines have been issued from the National Comprehensive Cancer Network (NCCN)[3], North American Neuroendocrine Tumor Society (NANETS)[4], and European Neuroendocrine Tumor Society (ENETS)[5]. Regardless of grade, the first treatment choice for resectable tumors is surgery. PNET spreads to the liver in 10%-50% of cases[6-9], and treatment of liver metastasis includes surgery for the primary lesion, transcatheter arterial embolization, or transcatheter arterial chemoembolization[5]. In case of multiple, unresectable liver metastases or metastases in non-hepatic organs, antihormone or antitumor therapies are applied[3-5].
Antitumor therapy varies between NET and NEC. Everolimus or sunitinib has been reported to be useful for NET G1 or G2[10,11]. Meanwhile, approved chemotherapy regimens for NEC based on large clinical trials do not exist due to scarcity of data. Therefore, chemotherapy for small cell lung carcinoma is applied, as its behavior resembles NEC. It has been reported that the response rate to cisplatin and etoposide therapy for NEC is 42%-67%[12,13]. In addition, cisplatin and irinotecan therapy has also been used in NEC, as this therapy has been reported to be more efficient in small cell lung carcinoma[14].
Thus, it is important to sample sufficiently and accurately establish the grade to enable informed decisions on the treatment of unresectable PNET. Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) has been reported to be useful for diagnosing pancreatic masses, including NET[15]. The accuracy of EUS-FNA has been reported as 80% for diagnosing PNET[16,17]. In this study, we evaluated the efficacy of EUS-FNA for diagnosing the Ki-67 index grade.
A total of 22 patients were diagnosed with PNET by EUS-FNA from October 2001 to December 2013 at Fukushima Medical University Hospital. Among these cases, we targeted 10 patients who were evaluated by the 2010 WHO classification. Surgery was performed in eight patients, and chemotherapy was performed in the remaining two patients due to multiple liver metastases. The Ki-67 indices of the eight surgery cases were evaluated using specimens obtained by EUS-FNA and by surgery; for the two cases where chemotherapy was administered, the prognosis was evaluated. The study was approved by the ethics committee of Fukushima Medical University.
The endoscopic and ultrasonic equipment used in this study included GF-UCT260 or GF-UC240P, and EU-ME1 or EU-ME2 (Olympus Medical Systems, Tokyo, Japan). The biopsy needles were Expect 22G (Boston Scientific, MA, United States), EZ shot 22G (Olympus Medical Systems), or Echotip 19 or 22 or 25G (Cook Medical Inc., NC, United States).
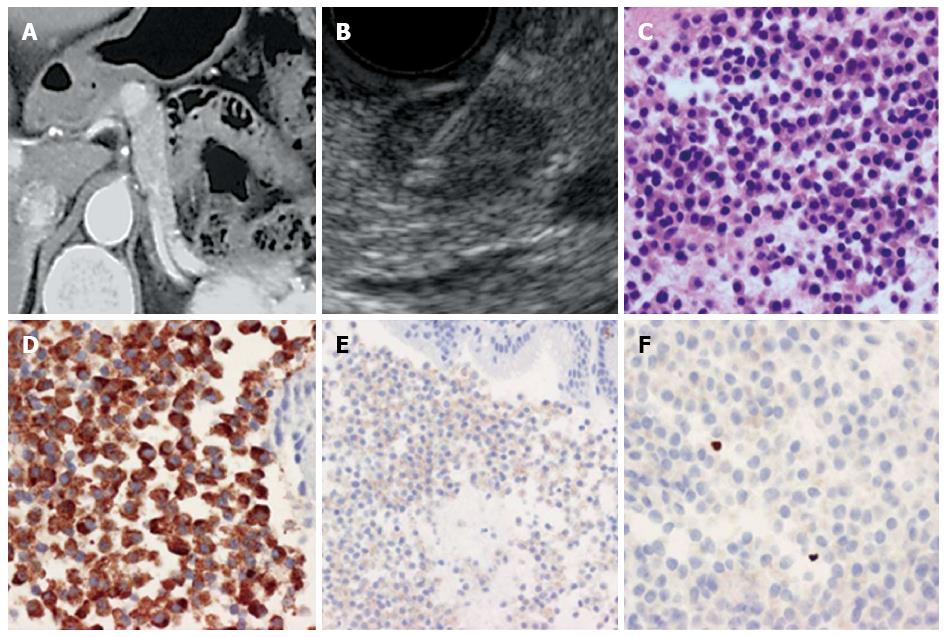
All patients were sedated with midazolam before endoscopy. After the target lesion was visualized on the monitor, a needle biopsy was performed while confirming no blood flow on the puncture line. The needle was inserted through the gastrointestinal wall into the target lesion under EUS guidance with visualization of the needle in real time. After guiding the needle into the target lesion, the stylet was withdrawn, and the needle was moved back and forth within the lesion 20 times while negative pressure was applied using a 20 mL syringe connected to the end of the needle. Twenty multi-direction strokes were performed toward the target lesion for optimal sampling. An additional puncture was performed toward a different part of the tumor than was previously sampled. Suction was released, and the needle was withdrawn from the target lesion. Microscope slides were prepared from the biopsy sample and stained with Cyto-Quick stain. The slides were assessed by Rapid on-site cytological evaluation (ROSE)[18]. If the sample was adequate, the sampling was complete. If the sample was not sufficient, another needle aspiration was performed. After staining specimens with hematoxylin and eosin, chromogranin (DAKO, Glostrup, Denmark), synaptophysin (DAKO, Glostrup, Denmark), CD56 (ZYMED, Carlsbad, United States), and Ki-67 (DAKO, Glostrup, Denmark), the cases were graded according to the WHO 2010 classification based on the Ki-67 index (Figure 1). The specimens obtained by surgery were graded by the Ki-67 index and mitotic count as stipulated by the WHO 2010 classification.
The eight cases for which surgery was performed are summarized in Table 1. The patients were aged 44-79 years and included five males and three females. The major tumor axes ranged from 4.4-40 mm. The pancreatic locations of tumors were the head (n = 4), body (n = 2), and tail (n = 2). The median number of needle passes was 3.5 (2-5). One case was performed using a needle with a diameter of 19G, while the other cases were biopsied with 22G needles. Cases 7 and 8 utilized 22G and 25G needles. The sampling rate for histologic diagnosis was 100%; therefore, sufficient specimens were obtained. No adverse effects were observed. The specimens of EUS-FNA matched those obtained by surgery in eight cases. The concordance rate between EUS-FNA and surgery specimens was 87.5% (7/8) (Table 1). Liver metastases were observed in patient 6; however, tumor metastases or relapses have not been observed in the other surgical cases.
| Sex | Age (yr) | Size (mm) | Location of tumor | No. of needle passes | Needle (G) | EUS-FNA specimen | Surgery specimen | ||||
| Ki-67 index | Grade | Ki-67 index | Mitotic count (/10HPF) | Grade | |||||||
| 1 | M | 71 | 4.4 | Tail | 5 | 19 | < 2.0% | G1 | < 2.0% | 0 | G1 |
| 2 | F | 79 | 19 | Head | 4 | 22 | 0.8% | G1 | < 2.0% | 0 | G1 |
| 3 | M | 44 | 31 | Head | 2 | 22 | 1.8% | G1 | 0.1% | 0 | G1 |
| 4 | F | 51 | 10 | Body | 3 | 22 | 0.4% | G1 | 1.97% | 0 | G1 |
| 5 | M | 75 | 40 | Tail | 3 | 22 | 7.0% | G2 | 7.13% | 2 | G2 |
| 6 | M | 49 | 31 | Body | 3 | 22 | 4.54% | G2 | < 20% | 11 | G2 |
| 7 | F | 46 | 30 | Head | 3 | 22, 25 | < 2.0% | G1 | 7.5% | 15 | G2 |
| 8 | M | 55 | 40 | Head | 2 | 25 | 1.8% | G1 | < 1.0% | 0 | G1 |
Chemotherapy was administered in two unresectable cases (Table 2). Patient 1 was started on somatostatin analog therapy, and transcatheter arterial infusion (TAI) was performed to target multiple liver metastases. After these initial treatments, the patient began taking everolimus. During chemotherapy, the primary tumor remained unconfirmed, though the countless liver metastases diminished dramatically. Patient 2 was classified as NEC based on the Ki-67 index of a specimen obtained by EUS-FNA and was therefore started on cisplatin and irinotecan therapy. Severe adverse effects, including renal failure and diarrhea, were observed; therefore, the regimen was changed to cisplatin and etoposide. TAI targeting multiple liver metastases was performed, resulting in diminished size of the metastases. However, the primary tumor remained unconfirmed. These two cases are still alive.
| Sex | Age (yr) | Size (mm) | Location of tumor | No. of needle passes | Needle (G) | Metastasis | EUS-FNA | Therapy | Prognosis | ||
| Ki-67 index | Grade | ||||||||||
| 1 | M | 64 | 45 | Head | 3 | 22 | Liver | 10.0% | G2 | Everolimus | PR |
| TAI | 15 mo survival antemortem | ||||||||||
| 2 | M | 73 | 30 | Tail | 1 | 22 | Liver | 48.6% | NEC | IP→PE | PR |
| TAI | 12 mo survival antemortem | ||||||||||
When Ki-67 indices of specimens obtained by EUS-FNA are evaluated, it is important to take into account whether such indices reflect the entire tumor. Reports on this topic are scarce, as the WHO classification of NET was revised in 2010, and cases of NET are rare. Here, we evaluated the diagnostic accuracy of PNET grading by EUS-FNA. According to our data, the concordance rate with surgical specimens was 87.5%. Furthermore, the effectiveness was also recognized for patients receiving chemotherapy. Liver metastases after surgery were observed in one case.
Larghi et al[19] compared specimens obtained by EUS-FNA with samples obtained by surgery in 12 PNET cases and found a concordance rate of 83.3% (10/12). One case was diagnosed with NET G1 by the EUS-FNA specimen but with NET G2 by the surgery specimen. An additional case was diagnosed with NET G2 by EUS-FNA but NET G1 by surgery. Hasegawa et al[20] reported that the concordance rate between EUS-FNA specimens and surgery specimens was 74.0% (20/27) when the EUS-FNA specimens were classified by average Ki-67 index, but the concordance rate was 77.8% (21/27) if the EUS-FNA specimens were classified by the highest Ki-67 index. In that report, one case was diagnosed as NEC by surgery but was diagnosed as NET G2 by the EUS-FNA specimen if classified by the average Ki-67 index. However, if classified by the highest Ki-67 index, the two cases diagnosed as NEC by surgery were diagnosed as NEC by EUS-FNA as well. Unno et al[21] compared specimens obtained by EUS-FNA with samples obtained by surgery in 19 PNET cases and found a concordance rate of 89.5% (17/19). One case was diagnosed with NET G2 by the EUS-FNA specimen but with NET G3 by the surgery specimen. Additionally, another case was diagnosed with NET G1 by EUS-FNA but NET G2 by surgery. In our hospital, the only case diagnosed as NET G1 by EUS-FNA was diagnosed as NET G2 by surgery. Moreover, in a report before 2010, Piani et al[22] compared specimens obtained by EUS-FNA and those obtained by surgery in 18 cases. They reported that the concordance rate was 89% if 2% was used as the Ki-67 index cut-off value, and the concordance rate was 78% if a Ki-67 index range of 2% to 10% was used as the cut-off value. Although this cut-off differs from the current cut-off value, a high concordance rate between EUS-FNA specimens and surgery specimens was shown. In these three reports and the present report, concordance rates between grades established by EUS-FNA and surgery were consistently between 70%-80%, and the concordance rate in differentiating lesions as NET or NEC was 98.5% (65/66).
In our study, liver metastases after surgery were observed in one case of NET G2. Pape et al[23] confirmed an increased survival risk for patients with grade 2 or 3 gastroenteropancreatic NET. Jann et al[24] reported that prognoses were statistically worse in patients with grade 2 or 3 NETs in the midgut and hindgut. Therefore, more cautious observation is necessary in patients with NET G2 or G3.
If lesions are unresectable, chemotherapy differs between NET and NEC. Therefore, diagnosing the grade of NET by EUS-FNA must be efficient for determining the chemotherapy choice. The chemotherapy administered in the two cases in this study was sufficiently effective, as evidenced by the fact that the expected progression-free survival of everolimus is 11 mo, and the expected response rate of cisplatin + etoposide therapy ranges from 42% to 67%[12,13]. Although there were only two cases in the present study, both were diagnosed and treated adequately based on conclusions drawn from the EUS-FNA data.
When using EUS-FNA for diagnosing PNETs, there are expected difficulties in obtaining specimens due to bleeding and blood contamination from abundant blood flow. Therefore, methods for obtaining sufficient specimens for immunostaining are required. In two previous reports, Larghi et al[19] used 19G needles, and Hasegawa et al[20] adopted the fanning method reported by Bang et al[25] in 2013. Moreover, Eloubeidi et al[26] performed EUS-FNA without negative pressure and diagnosed 13 cases of PNET. In this study, we applied the fanning method, and Ki-67 indices were evaluable for 10/11 cases for which Ki-67 staining was performed; one case was not evaluable. Therefore, a good concordance rate of grading between EUS-FNA and surgery specimens was observed by this approach.
There are certain limitations to the present study. First, the research was carried out at a single institution with a small number of patients, and it was a retrospective study. Further accumulation of study data is needed for a better comparison of Ki-67 indices between EUS-FNA and surgery specimens. Considering that PNET is relatively rare, it is useful to describe decisions on treatment regimens guided only by EUS-FNA specimens for future medical care. Second, the research evaluated only the Ki-67 index of EUS-FNA specimens. It was reported that K-ras mutations were observed in certain NET patients[27,28], and K-ras status might be correlated with malignancy of NET in the future. The Ki-67 index correlates with the prognosis of NET[24] and is a predictive factor for the effect of chemotherapy. Sorbye et al[29] reported that gastrointestinal NEC patients with Ki-67 < 55% had a lower response rate to platinum-based chemotherapy than patients with Ki-67 ≥ 55% (15% vs 42%). Scoazec et al[30] reported that 20% of gastrointestinal NET and PNET cases diagnosed as NEC by the WHO 2010 classification were well differentiated; therefore, it is thought that well differentiated tumors could be included in NEC cases that do not respond to platinum-based chemotherapy. In this report, the Ki-67 index of the NEC patients was 48.6%; however, the specimens obtained by EUS-FNA showed poor differentiation. Therefore, platinum-based chemotherapy was indicated in this patient, and it was effective.
In conclusion, EUS-FNA is useful for diagnosing the Ki-67 index grade of PNET and for deciding treatment regimens.
Neuroendocrine tumors (NETs) develop from neuroendocrine cells distributed throughout the body. Pancreatic NETs (PNETs) are classified into G1, G2 and neuroendocrine carcinoma (NEC) by the WHO 2010 classification based on growth morphology (mitotic count and Ki-67 index). The first choice in PNET treatment is surgery, though in cases of multiple, unresectable liver metastases or metastases in non-hepatic organs, antihormone or antitumor therapies are applied. Antitumor therapy differs for NET and NEC. Everolimus or sunitinib is useful for NET G1 or G2, while small lung cell carcinoma chemotherapy is used for NEC, as their behaviors are similar. Thus, it is important to sample sufficiently and to accurately establish the grade in order to choose treatments for unresectable PNET. In this study, we evaluated the efficacy of endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) for diagnosing the Ki-67 index grade.
EUS-FNA is important for determining treatment of PNET, a rare tumor. Few prior reports contain analyses of Ki-67 staining, EUS-FNA, and surgery. The results of this study contribute to clarifying the diagnostic potential of EUS-FNA for PNET grading.
In this study, EUS-FNA was a useful tool for diagnosing the Ki-67 index of PNET. The concordance rate between EUS-FNA and surgery was 87.5%. These results are in agreement with previous reports. However, in this report, two patients received chemotherapy based only on the Ki-67 index of EUS-FNA specimens, and the therapeutic values for both PNET and pancreatic NEC were evaluated. This emphasizes the accuracy of EUS-FNA for diagnosing the Ki-67 index in PNET.
This study suggests that EUS-FNA is useful for diagnosing PNET or PNEC. If a patient is diagnosed with unresectable PNET, chemotherapy can be chosen based on the Ki-67 index of the EUS-FNA specimen.
EUS: An endoscopic procedure that enables observation of the chest and abdominal organs in the gastrointestinal tract. EUS-FNA: A technique to obtain specimens of chest and abdominal lesions by EUS guidance by puncturing the gastrointestinal tract.
The author of this paper evaluated the efficacy of EUS-FNA for grading PNET using the Ki-67 index and compared the results with those obtained by surgery from 10 patients. A promising concordance rate (7/8) between the specimens obtained by EUS-FNA and those obtained by surgery was found, and further clinical trials in a large population of PNET patients will be valuable.
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer 2010; . |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3316] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 3. | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Neuroendocrine tumors. 2011. Available from: http://www.nccn.org/. |
| 4. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Steinmüller T, Kianmanesh R, Falconi M, Scarpa A, Taal B, Kwekkeboom DJ, Lopes JM, Perren A, Nikou G, Yao J. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Madeira I, Terris B, Voss M, Denys A, Sauvanet A, Flejou JF, Vilgrain V, Belghiti J, Bernades P, Ruszniewski P. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422-427. [PubMed] |
| 7. | Mignon M. Natural history of neuroendocrine enteropancreatic tumors. Digestion. 2000;62 Suppl 1:51-58. [PubMed] |
| 8. | Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, Pitt HA. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776-783; discussion 783-785. [PubMed] |
| 9. | Zerbi A, Falconi M, Rindi G, Delle Fave G, Tomassetti P, Pasquali C, Capitanio V, Boninsegna L, Di Carlo V. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am J Gastroenterol. 2010;105:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2161] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 11. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1877] [Article Influence: 125.1] [Reference Citation Analysis (0)] |
| 12. | Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufié P, Aparicio T, Aparicio T, Lasser P, Elias D, Duvillard P. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 350] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227-232. [PubMed] |
| 14. | Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 953] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 15. | Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Ardengh JC, de Paulo GA, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378-384. [PubMed] |
| 17. | Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: a study of 48 cases. Cancer. 2008;114:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Larghi A, Capurso G, Carnuccio A, Ricci R, Alfieri S, Galasso D, Lugli F, Bianchi A, Panzuto F, De Marinis L. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc. 2012;76:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, Imaoka H, Niwa Y, Tajika M, Kondo S. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Unno J, Kanno A, Masamune A, Kasajima A, Fujishima F, Ishida K, Hamada S, Kume K, Kikuta K, Hirota M. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol. 2014;49:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Piani C, Franchi GM, Cappelletti C, Scavini M, Albarello L, Zerbi A, Giorgio Arcidiacono P, Bosi E, Manzoni MF. Cytological Ki-67 in pancreatic endocrine tumours: an opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G, Wiedenmann B. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Jann H, Roll S, Couvelard A, Hentic O, Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski P. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Eloubeidi MA, Tamhane AR, Buxbaum JL. Unusual, metastatic, or neuroendocrine tumor of the pancreas: a diagnosis with endoscopic ultrasound-guided fine-needle aspiration and immunohistochemistry. Saudi J Gastroenterol. 2012;18:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, Yatabe Y. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int. 2010;60:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Ogura T, Yamao K, Sawaki A, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Kondo S, Shimizu Y. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 740] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 30. | Scoazec JY, Couvelard A, Monges G, Leteurtre E, Belleannee G, Guyetant S, Duvillard P, Danjoux M, Parot X, Lepage C. Well-differentiated grade 3 digestive neuroendocrine tumors: Myth or reality? The PRONET study group (abstract). J Clin Oncol. 2012;30:abstract 4129. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gangl A, Ker CG, Li SD S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM