Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8110
Peer-review started: February 10, 2015
First decision: March 10, 2015
Revised: March 25, 2015
Accepted: May 2, 2015
Article in press: May 4, 2015
Published online: July 14, 2015
Processing time: 168 Days and 4 Hours
AIM: To assess the effect of technical parameters on outcomes of transjugular intrahepatic portosystemic shunt (TIPS) created using a stent graft.
METHODS: The medical records of 68 patients who underwent TIPS placement with a stent graft from 2008 to 2014 were reviewed by two radiologists blinded to the patient outcomes. Digital Subtraction Angiographic images with a measuring catheter in two orthogonal planes was used to determine the TIPS stent-to-inferior vena cava distance (SIVCD), hepatic vein to parenchymal tract angle (HVTA), portal vein to parenchymal tract angle (PVTA), and the accessed portal vein. The length and diameter of the TIPS stent and the use of concurrent variceal embolization were recorded by review of the patient’s procedure note. Data on re-intervention within 30 d of TIPS placement, recurrence of symptoms, and survival were collected through the patient’s chart. Cox proportional regression analysis was performed to assess the effect of these technical parameters on primary patency of TIPS, time to recurrence of symptoms, and all-cause mortality.
RESULTS: There was no significant association between the SIVCD and primary patency (P = 0.23), time to recurrence of symptoms (P = 0.83), or all-cause mortality (P = 0.18). The 3, 6, and 12-mo primary patency rates for a SIVCD ≥ 1.5 cm were 82.4%, 64.7%, and 50.3% compared to 89.3%, 83.8%, and 60.6% for a SIVCD of < 1.5 cm (P = 0.29). The median time to stenosis for a SIVCD of ≥ 1.5 cm was 19.1 mo vs 15.1 mo for a SIVCD of < 1.5 cm (P = 0.48). There was no significant association between the following factors and primary patency: HVTA (P = 0.99), PVTA (P = 0.65), accessed portal vein (P = 0.35), TIPS stent diameter (P = 0.93), TIPS stent length (P = 0.48), concurrent variceal embolization (P = 0.13) and reinterventions within 30 d (P = 0.24). Furthermore, there was no correlation between these technical parameters and time to recurrence of symptoms or all-cause mortality. Recurrence of symptoms was associated with stent graft stenosis (P = 0.03).
CONCLUSION: TIPS stent-to-caval distance and other parameters have no significant effect on primary patency, time to recurrence of symptoms, or all-cause mortality following TIPS with a stent-graft.
Core tip: Current knowledge on the technical factors influencing the patency of transjugular intrahepatic portosystemic shunts (TIPS) is limited to the published data on TIPS created using bare-metal stents. However, stent grafts have replaced bare metal stents in TIPS creation. In this paper, we rigorously analyzed the effects of various technical factors on patency of TIPS created with stent grafts and also demonstrated how these factors influenced time to recurrence of symptoms and all-cause mortality. Our results challenge the accepted assumption that placement of the hepatic venous end of the stent beyond 1.5 cm of the hepatocaval confluence decreases primary shunt patency rates.
- Citation: Andring B, Kalva SP, Sutphin P, Srinivasa R, Anene A, Burrell M, Xi Y, Pillai AK. Effect of technical parameters on transjugular intrahepatic portosystemic shunts utilizing stent grafts. World J Gastroenterol 2015; 21(26): 8110-8117
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8110
The transjugular intrahepatic portosystemic shunt (TIPS) is widely used in the management of portal hypertension and supplanted the use of surgically created shunts due to its equivalent efficacy, decreased cost, and improved outcomes with future orthotropic liver transplantation[1-4]. Though originally used in the context of variceal bleeding[5-7], the indications for a TIPS quickly expanded to include refractory ascites[8-11], Budd-Chiari syndrome[12,13], and hepatic hydrothorax[14-17]. TIPS were initially created using bare-metal stents, which had poor primary patency rates requiring stent revision up to a rate of 50% within 1 year of creation[18-20]. There has been many theorized causes for the poor primary patency rates: (1) early acute thrombosis, often caused by technical failure (e.g., stent shortening or migration) or a biliary-stent fistula; (2) parenchymal stenosis resulting from a fibrotic healing response to the trauma of shunt creation; and (3) late “pseudointimal” hyperplasia of the hepatic vein occurring between 3 mo and 1 year after TIPS placement[21-25].
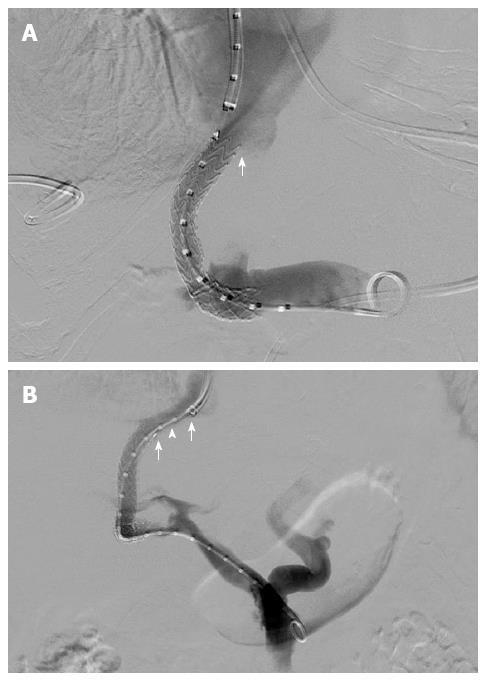
Stent grafts including the Viatorr® stent graft (Gore® Flagstaff, Arizona) have shown superior efficacy in terms of primary patency and clinical outcomes compared to bare metallic stents and have, therefore, replaced bare metal stents for TIPS[26-29]. The effect of various technical parameters influencing primary patency of TIPS performed with bare metal stents has been previously studied to varying degrees. A distance greater than 2 cm between the hepatic venous end of the stent and the hepatic vein-inferior vena cava confluence - stent to inferior vena cava distance (SIVCD) (Figure 1), has been identified as one of the main technical factors affecting the patency of TIPS created with a bare-metal stent[30]. Late stenosis (after 3 mo) of a bare metal stent is secondary to pseudo-intimal hyperplasia[21-25]. Prior studies on stent grafts have suggested that they are less predisposed to pseudo-intimal hyperplasia as compared to the bare metal stents[31,32]. The effect of SIVCD, the hepatic vein-parenchymal tract angle (HVTA, Figure 2) and portal vein-parenchymal tract angle (PVTA, Figure 2), stent graft diameter, stent graft length, and the accessed portal vein on the outcomes of TIPS have not been well studied after the introduction of stent grafts for TIPS. The purpose of this study is to evaluate the effect of these selected technical factors on the primary shunt patency, time to recurrence of symptoms and survival following TIPS created with a Viatorr® stent graft (Gore® Flagstaff, Arizona).
The institutional review boards of two institutions from which the data were gathered approved this retrospective study and waived the requirement for an informed consent. A database of patients who had TIPS preformed from 2008 to 2014 was generated. Patients lost to follow up were censored from the analysis. Sixty-eight patients with appropriate imaging studies and follow up were included in the study. The demographic information of the patients including the indication for TIPS is presented in Table 1. The etiology of cirrhosis included alcoholic (55%), hepatitis C (32%), non-alcoholic steatohepatitis (8%) and other (5%, hepatitis B, autoimmune, primary biliary cirrhosis, and cryptogenic). TIPS was placed according to the latest 2009 American Association for the Study of Liver Diseases 2009 Guidelines[33]. The indications for the procedure included: refractory variceal bleeding, refractory ascites, hepatic hydrothorax, hepatorenal syndrome, or a combination of these factors.
| Demographic factor | n (%) |
| Male | 35 (51.5) |
| Female | 33 (48.5) |
| Average age, yr | 55.5 |
| Child Pugh Class | |
| A | 10 (14.7) |
| B | 44 (64.7) |
| C | 14 (20.6) |
| Indication1 | |
| Variceal bleeding | 38 (55.9) |
| Ascites | 38 (55.9) |
| Hepatic hydrothorax | 8 (11.8) |
| Hepatorenal syndrome | 1 (1.5) |
Two radiologists blinded to the outcomes of this study independently reviewed the angiographic images obtained during TIPS placement. Digital subtraction angiographic images were utilized for the measurement of the SIVCD, HVTA, and PVTA. A measuring catheter positioned within the stent graft was used to calculate the SIVCD. All patients included in this study had a TIPS completion venogram that depicted the confluence of the IVC and the hepatic vein. The HVTA and PVTA were measured in right anterior oblique view. The two observers had excellent correlation with the interclass correlation coefficients for the measured variables of SIVCD, HVTA, and PVTA of 0.98, 0.91, and 0.93, respectively. No measurement had more than a 10% discrepancy and, therefore, no measurements were discarded for analysis. The accessed portal vein was also determined by review of angiographic images. The TIPS stent graft diameter, TIPS stent graft length, and final TIPS balloon angioplasty diameter s were determined by review of the procedure note. Electronic chart review was performed to review interventions within first 30 d after TIPS creation, the time to recurrence of symptoms, United States Doppler studies, repeat venography and/or TIPS interventions, and mortality.
The median pre-TIPS and post-TIPS portosystemic gradient for this cohort was 18 (Range 7-42) mmHg and 6 (Range 1-10) mmHg, respectively. In 64% of patients, a large physiological portosystemic shunt was observed, which could explain the low median pre-TIPS portosystemic gradient. All patients included in this study achieved a portosystemic gradient of < 12 following TIPS. All stents placed were Viatorr® stent grafts (Gore® Flagstaff, Arizona).
The SIVCD distance ranged from 0.0 to 3.8 cm. The HVTA and PVTA ranged from 117°-180° and 71°-176°, respectively. The accessed portal vein varied and included the main portal vein (n = 4, 6.0%), right portal vein (n = 31, 46.3%), first order right portal vein (n = 25, 37.3%), left portal vein (n = 5, 7.5%), and first order left portal vein (n = 2, 3.0%). The diameter of the stent graft used included 8 mm (n = 45, 66.2%) and 10 mm (n = 23, 33.8%). The stent graft length varied based on the patient’s anatomy and included 6 cm (n = 10, 15.2%), 7 cm (n = 28, 42.5%), 8 cm (n = 16, 24.2%), 9 cm (n = 6, 9.1%), and 10 cm (n = 6, 9.1%). An 8 mm balloon was used initially for 10 mm stent grafts with further dilation with a 10 mm balloon if needed. The maximum balloon dilation diameter included either 8 mm (n = 45, 66.2%) or 10 mm (n = 23, 33.8%). Variceal embolization was performed following TIPS if any significant gastroesophageal varices were still visualized following TIPS.
The median follow up time was 11.2 mo. Patients were regularly followed with clinical and imaging follow-up performed at interval time points using TIPS ultrasound or when the patient presented with recurrence of symptoms. The determination of TIPS stenosis using ultrasound was based on established velocity thresholds of approximately 90-190 cm/s[34,35]. However, given these reference ranges can vary significantly with respiration and Doppler angle, all identified stenosis were confirmed with follow up venography. A stenosis on venography was defined as a portosystemic gradient greater than 12 mmHg and/or 50% narrowing on angiographic images (confirmed on two orthogonal views). A stenosis identified on venography was treated with angioplasty +/- additional stenting. In a few select patients (n-3), venography was performed without preceding ultrasound due to high pre-test probability of stenosis given recurrence of clinical symptoms. Patients lost to follow up were censored at the time of the last known imaging of the shunt (either duplex ultrasound or shunt venography). Patients who underwent liver transplantation were also censored at the time of transplantation (n = 3).
Cox proportional hazard regression analysis was performed using SIVCD as a continuous variable for its effect on primary patency, time to recurrence of symptoms, and all-cause mortality. Using 1.5 cm as the cutoff, Kaplan-Meier survival analyses were used to test any difference in primary patency, time to recurrence of symptoms, and all-cause mortality between SIVCD ≤ 1.5 cm or > 1.5 cm. The cutoff of 1.5 cm was chosen as it was similar to the 2.0 cm cutoff used in a prior study[30]. Then, cox proportional hazard multivariate regression analysis was performed on the simultaneous effect of all eight technical parameters (SIVCD, HVTA, PVTA, accessed portal vein, TIPS stent diameter, TIPS stent length, final balloon diameter, and reintervention within 30 d) on primary patency, time to recurrence of symptoms, and all-cause mortality. The association between recurrence of symptoms and stent graft stenosis was also evaluated via a χ2 test. Effects with P value less than 0.05 were considered as statistically significant. All analyses were conducted in statistical software package SAS® 9.3. The statistical methods of this study were reviewed by Yin Xi from the University of Texas Southwestern Medical Center.
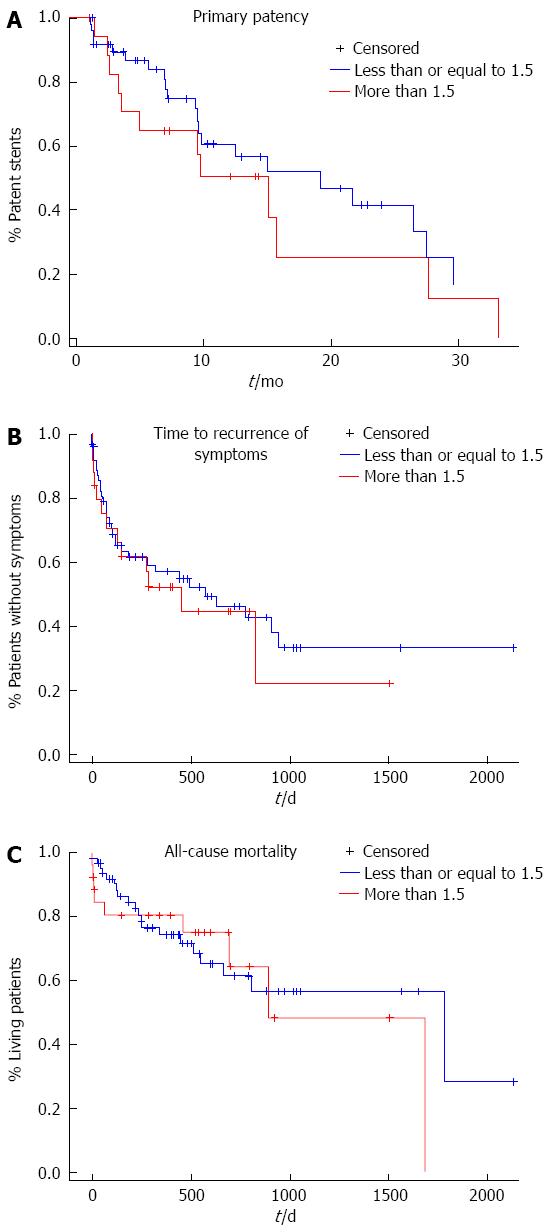
Cox proportional regression analysis did not show any association between the TIPS stent to IVC distance (SIVCD) and primary patency (P = 0.23), time to recurrence of symptoms (P = 0.83), and all-cause mortality (P = 0.18). The data was also split into two groups for Kaplan-Meier analysis using 1.5 cm as the cutoff (N greater than 1.5 cm- 19, N less than or equal to 1.5 cm- 49). There was no statistically significant difference between the two groups in terms of age, sex, indication for procedure, etiology of cirrhosis, or Child-Pugh class. The 3, 6, and 12-mo primary patency rates for a SIVCD ≥ 1.5 cm were 82.4% (SE = 9.3%), 64.7% (SE = 11.6%), and 50.3% (SE = 12.7%) compared to 89.3% (SE = 4.5%), 83.8% (SE = 5.7%), and 60.6% (SE = 8.6%) for a SIVCD of < 1.5 cm as shown in Figure 3. The median time to stenosis for a SIVCD of ≥ 1.5 cm was 19.1 mo (SE = 4.0 mo) compared to 15.1 mo (with SE = 2.2 mo) for an SIVCD < 1.5 cm (Kaplan-Meier analysis, P = 0.29). After one year, 57% (SE = 6.6%) of the patients with a SIVCD less than 1.5 cm were without symptoms vs 53% (with SE = 10.5%) of the patients with a SIVCD greater than 1.5 cm. (Kaplan-Meier analysis, P = 0.64) In the cohort with a SIVCD of greater than 1.5 cm, the patients who had recurrence of symptoms all recurred with the same symptom that served as the primary indication for the procedure. In the cohort with a SIVCD of less than 1.5 cm, there were three patients who had recurrence of symptoms that differed from their primary indication as two patients had a TIPS placed for recurrent ascites who recurred with variceal bleeding and one patient had a TIPS placed for variceal bleeding who recurred with recurrent ascites. After one year, the survival rate of the cohort of patients with a SIVCD less than 1.5 cm were 74% (with SE = 6.0%) vs 81% (with SE = 7.8%) for the cohort of patients with a stent graft distance greater than 1.5 cm. Figure 3 shows the primary patency, time to recurrence of symptoms, and all-cause mortality as a function of time. As expected, recurrence of symptoms was associated with stent graft stenosis with P value 0.0275 (χ2 test).
Furthermore, cox proportional hazard regression analysis did not show any association between the HVTA and primary patency (P = 0.99), recurrence of symptoms (P = 0.62), and all-cause mortality (P = 0.16). Similarly, cox proportional hazard regression analysis did not show any association between the PVTA and primary patency (P = 0.65), recurrence of symptoms (P = 0.25), and all-cause mortality (P = 0.17).
Finally, the accessed portal vein, TIPS stent graft diameter, TIPS stent graft length, final TIPS balloon angioplasty diameter, presence of concurrent variceal embolization or reintervention within first 30 d had no statistically significant effect on primary stent patency, time to recurrence of symptoms, or all-cause mortality (Table 2).
| Primary patency | Time to recurrence of symptoms | All-cause mortality | |
| P value | P value | P value | |
| SIVCD | 0.23 | 0.83 | 0.18 |
| HVTA | 0.99 | 0.62 | 0.16 |
| PVTA | 0.65 | 0.25 | 0.17 |
| Accessed portal vein | 0.35 | 0.93 | 0.67 |
| TIPS stent diameter | 0.93 | 0.69 | 0.37 |
| TIPS stent length | 0.48 | 0.58 | 0.69 |
| Final balloon diameter | 0.99 | 0.43 | 0.22 |
| Variceal Embolization | 0.13 | 0.43 | 0.30 |
| < 30 d of intervention | 0.24 | 0.33 | 0.54 |
The standard for positioning the hepatic venous end of the stent graft during TIPS creation is based on the study by Clark et al[30] that reported results from TIPS created using bare-metal stents. In this study, authors reported superior primary patency of TIPS when the hepatic venous end of the bare metal stent used for TIPS creation was positioned within 2 cm of the confluence between the hepatic vein and the IVC. Though early stenosis can be attributed to procedural complications (i.e., biliary-stent fistulas), late stenosis of a bare metal stent (especially after 3 mo) is mainly secondary to pseudo-intimal hyperplasia[21-25]. Prior research on stent grafts has suggested that they are less predisposed to pseudo-intimal hyperplasia as compared to the bare metal stents[31,32]. For instance, Huang et al[31] found a statistically significant decrease in the incidence of intimal hyperplasia with stent grafts compared to that with bare metal stents (33% vs 3%, respectively, P < 0.01) in a study involving 60 patients who underwent TIPS.
Our results support the hypothesis that stent grafts are less predisposed to pseudo-intimal hyperplasia as there was no significant effect of SIVCD on primary patency of TIPS. This was further substantiated by the finding that there was no difference in clinical outcomes such as time to recurrence of symptoms or all-cause mortality. Our study shows that positioning the hepatic venous end of the stent graft within 1.5 cm of hepatocaval junction during TIPS creation may not be necessary, unlike what was reported with bare metallic stents.
Stent graft geometry including the HVTA and PVTA was not well studied with bare metal stents or stent grafts. Despite early thoughts that shear stress from stent angulation may contribute to stenosis, two series presented only in abstract form (Perry et al[36] and Weeks et al[37]) with 19 and 61 patients, respectively, had shown that stent geometry (i.e., HVTA and PVTA) had no effect on primary patency. Our data further supports there is no significant correlation between HVTA/PVTA and primary patency. Furthermore, our data further showed that stent graft angulation did not affect time to recurrence of symptoms or all-cause mortality.
Our results showed that there was no statistically significant effect of stent graft length, stent graft diameter, and final balloon angioplasty diameter on primary patency, time to recurrence of symptoms, or all-cause mortality. Therefore, during TIPS stent creation, focus should be on the combined effect of these technical factors on the pressure gradient and anatomic considerations as opposed to focusing on any one of the individual above parameters alone for the purposes of increasing primary stent patency.
The accessed portal vein also had no statistically effect on primary patency, time to recurrence of symptoms, or all-cause mortality. The right hepatic vein is usually chosen to access the right portal vein as the anterior approach reduces the likelihood of extra capsular puncture. Our data shows there is no indication to change this choice of hepatic vein to portal vein based on any gain in the primary patency of the stent.
Our data does not show any statistical correlation between reintervention within the first 30 d and more long term primary patency, time to recurrence of symptoms, or all-cause mortality. Therefore, short term patency may not be a good predictor of long term patency. This is consistent with prior reports which showed that the factors resulting in early thrombosis (i.e., stent migration, stent shortening, biliary-stent fistulas) differ from those affecting long term patency (i.e., pseudo-intimal hyperplasia).
This study has limitations. First, it was a retrospective study from only two institutions and included a moderate number of patients. A prospective, large cohort, multi-institutional study is desirable; however, we believe that between the two institutions included in this study, there was a wide range of patient populations. This study is also limited as it only had a moderate follow up time interval. However, as above, prior research has shown that pseudo-intimal hyperplasia typically occurs within 3 mo to one year following placement of the TIPS, which is within the follow up interval. Long term follow up is desirable. Given the similar patient characteristics including gender, age, MELD score, and indication for procedure across the two groups of SIVCD, confounding factors were minimized.
In conclusion, the positioning of hepatic venous end of TIPS stent graft in relation to hepatic vein - inferior vena cava confluence has little effect on the primary patency rate, time to recurrence of symptoms, and all-cause mortality following TIPS. Similarly, other technical factors such as hepatic vein to parenchymal tract angle, portal vein to parenchymal tract angle, TIPS stent graft diameter, TIPS stent length, concurrent variceal embolization and early re-intervention have no effect on primary patency, recurrence of symptoms, or all-cause mortality.
Patients with cirrhosis have a liver replaced with fibrous tissue that impedes portal venous blood flow to the liver, resulting in ascites and/or gastrointestinal bleeding. A transjugular intrahepatic portosystemic shunt (TIPS) diverts this blood flow through the liver and has been proven to alleviate symptoms. TIPS were originally placed using a bare-metal stents but now stent grafts are used due to their improved patency rates. The current literature on the technical factors to consider when creating a TIPS is based on the data from TIPS created with bare-metal stents.
This paper reevaluates the effect of various technical parameters on primary shunt patency, time to recurrence of symptoms and all-cause mortality following TIPS created with stent grafts.
Previous studies based on TIPS created with bare metallic stents have suggested that the positioning of the hepatic venous end of the stent within 2 cm of the hepatic vein - inferior vena cava junction improves primary patency of TIPS. The study shows that this may no longer be necessary when TIPS is created with a stent-graft given that it has no effect on primary TIPS patency, time to recurrence of symptoms and all-cause mortality. Other technical factors, previously studied to varying degrees, including the angle the stent makes with the hepatic and portal vein, the length and diameter of TIPS stent, concurrent variceal embolization, and reinterventions within 30 d have little effect on TIPS patency, time to recurrence of symptoms, or all-cause mortality.
The results of this study can be applied during TIPS creation using a stent graft. Given that the technical parameters have little effect on final outcomes of TIPS, one should focus on physiological end points to improve clinical outcomes.
A bare metal stent is a hollow tube lined with a lattice of metal (either stainless steel or Nitinol alloy). A stent-graft is a stent with an external (sometimes internal and external) covering on the metal lattice, typically made of a polymer of expanded polytetrafluoroethylene.
This manuscript retrospectively re-analyzed the gathered clinical data of of the transjugular intrahepatic portosystemic shunt used for patients with portal hypertension.
| 1. | D’Amico G, Luca A. TIPS is a cost effective alternative to surgical shunt as a rescue therapy for prevention of recurrent bleeding from esophageal varices. J Hepatol. 2008;48:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, Abu-Elmagd K, Connor J. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Menegaux F, Keeffe EB, Baker E, Egawa H, Concepcion W, Russell TR, Esquivel CO. Comparison of transjugular and surgical portosystemic shunts on the outcome of liver transplantation. Arch Surg. 1994;129:1018-1023; discussion 1023-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Rösch J, Hanafee W, Snow H, Barenfus M, Gray R. Transjugular intrahepatic portacaval shunt. An experimental work. Am J Surg. 1971;121:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Cabrera J, Maynar M, Granados R, Gorriz E, Reyes R, Pulido-Duque JM, Rodriguez SanRoman JL, Guerra C, Kravetz D. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology. 1996;110:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 155] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Rössle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, Hauenstein KH, Geiger R, Stiepak C, Keller W. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1997;349:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Sanyal AJ, Freedman AM, Luketic VA, Purdum PP, Shiffman ML, Cole PE, Tisnado J, Simmons S. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Ann Intern Med. 1997;126:849-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Albillos A, Bañares R, González M, Catalina MV, Molinero LM. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | D’Amico G, Luca A, Morabito A, Miraglia R, D’Amico M. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology. 2005;129:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Deltenre P, Mathurin P, Dharancy S, Moreau R, Bulois P, Henrion J, Pruvot FR, Ernst O, Paris JC, Lebrec D. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 385] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 12. | Michl P, Bilzer M, Waggershauser T, Gülberg V, Rau HG, Reiser M, Gerbes AL. Successful treatment of chronic Budd-Chiari syndrome with a transjugular intrahepatic portosystemic shunt. J Hepatol. 2000;32:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Perelló A, García-Pagán JC, Gilabert R, Suárez Y, Moitinho E, Cervantes F, Reverter JC, Escorsell A, Bosch J, Rodés J. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Chalasani N, Clark WS, Martin LG, Kamean J, Khan MA, Patel NH, Boyer TD. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Haskal ZJ, Zuckerman J. Resolution of hepatic hydrothorax after transjugular intrahepatic portosystemic shunt (TIPS) placement. Chest. 1994;106:1293-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Strauss RM, Martin LG, Kaufman SL, Boyer TD. Transjugular intrahepatic portal systemic shunt for the management of symptomatic cirrhotic hydrothorax. Am J Gastroenterol. 1994;89:1520-1522. [PubMed] |
| 18. | LaBerge JM, Somberg KA, Lake JR, Gordon RL, Kerlan RK, Ascher NL, Roberts JP, Simor MM, Doherty CA, Hahn J. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 214] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Luca A, D’Amico G, La Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: meta-analysis of randomized clinical trials. Radiology. 1999;212:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Sahagun G, Benner KG, Saxon R, Barton RE, Rabkin J, Keller FS, Rosch J. Outcome of 100 patients after transjugular intrahepatic portosystemic shunt for variceal hemorrhage. Am J Gastroenterol. 1997;92:1444-1452. [PubMed] |
| 21. | Saxon RR, Mendel-Hartvig J, Corless CL, Rabkin J, Uchida BT, Nishimine K, Keller FS. Bile duct injury as a major cause of stenosis and occlusion in transjugular intrahepatic portosystemic shunts: comparative histopathologic analysis in humans and swine. J Vasc Interv Radiol. 1996;7:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sze DY, Vestring T, Liddell RP, Kato N, Semba CP, Razavi MK, Kee ST, Dake MD. Recurrent TIPS failure associated with biliary fistulae: treatment with PTFE-covered stents. Cardiovasc Intervent Radiol. 1999;22:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Terayama N, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Miyayama S, Takashima T, Kobayashi K, Nakanishi I, Nakanuma Y. Transjugular intrahepatic portosystemic shunt: histologic and immunohistochemical study of autopsy cases. Cardiovasc Intervent Radiol. 1997;20:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Ducoin H, El-Khoury J, Rousseau H, Barange K, Peron JM, Pierragi MT, Rumeau JL, Pascal JP, Vinel JP, Joffre F. Histopathologic analysis of transjugular intrahepatic portosystemic shunts. Hepatology. 1997;25:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Teng GJ, Bettmann MA, Hoopes PJ, Ermeling BL, Yang L, Wagner RJ. Transjugular intrahepatic portosystemic shunt in a porcine model: histologic characteristics at the early stage. Acad Radiol. 1998;5:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology. 2001;221:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Saxon RR. A new era for transjugular intrahepatic portosystemic shunts? J Vasc Interv Radiol. 2004;15:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2004;15:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Huang Q, Wu X, Fan X, Cao J, Han J, Xu L, Li N. Comparison study of Doppler ultrasound surveillance of expanded polytetrafluoroethylene-covered stent versus bare stent in transjugular intrahepatic portosystemic shunt. J Clin Ultrasound. 2010;38:353-360. [PubMed] |
| 32. | Marin ML, Veith FJ, Cynamon J, Parsons RE, Lyon RT, Suggs WD, Bakal CW, Waahl S, Sanchez LA, Yuan JG. Effect of polytetrafluoroethylene covering of Palmaz stents on the development of intimal hyperplasia in human iliac arteries. J Vasc Interv Radiol. 1996;7:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [PubMed] |
| 34. | Ferguson JM, Jalan R, Redhead DN, Hayes PC, Allan PL. The role of duplex and colour Doppler ultrasound in the follow-up evaluation of transjugular intrahepatic portosystemic stent shunt (TIPSS). Br J Radiol. 1995;68:587-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Perry LJAP, Brophy DP, Lang EV. Does the geometry of TIPS predict the likelihood of subsequent shunt dysfunction [abstract]? J Vascular Intervent Radiol. 2000;11:297. |
| 37. | Weeks SMRK, Sandhu J, Shrestha R, Jaques PF, Mauro MA. TIPS: does shunt geometry affect patency [abstract]? J Vascular Intervent Radiol. 2001;12:138. |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: He ST S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN