Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8096
Peer-review started: February 1, 2015
First decision: March 10, 2015
Revised: April 2, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: July 14, 2015
Processing time: 162 Days and 21.9 Hours
AIM: To analyze the relationship between the serum lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) levels and clinical and histopathological features of biopsy-confirmed nonalcoholic fatty liver disease (NAFLD) patients.
METHODS: Fifty-three consecutive, biopsy-proven NAFLD patients (31 males and 22 females, mean age 42.5 ± 9.6 years) and 26 age- and gender-matched, healthy controls (14 males and 12 females, mean age 39 ± 10.7 years) were included. The patients with NAFLD were consecutive patients who had been admitted to the hepatology outpatient clinic within the last year and had been diagnosed with NAFLD as the result of liver biopsy. The healthy controls were individuals who attended the outpatient clinic for routine health control and had no known chronic illnesses. The histological evaluation was conducted according to the NAFLD activity scoring system recommended by The National Institute of Diabetes and Digestive and Kidney Diseases Nonalcoholic Steatohepatitis Clinical Research Network. The serum LOX-1 levels were measured using an ELISA kit (Life Science Inc. USCN. Wuhan, Catalog No. E1859Hu) in both patients and healthy controls. A receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff value of LOX-1 and thereby distinguish between patients with nonalcoholic steatohepatitis (NASH) and healthy controls. A P-value < 0.05 was considered statistically significant.
RESULTS: NAFLD and healthy control groups were similar in terms of age and sex. NAFLD patients consisted of 8 patients with simple steatosis (15%), 27 with borderline NASH (51%) and 18 with definitive NASH (34%). Metabolic syndrome was found in 62.2% of the patients with NAFLD. The mean serum LOX-1 level in biopsy-proven NAFLD patients was 8.49 ± 6.43 ng/mL compared to 4.08 ± 4.32 ng/mL in healthy controls (P = 0.001). The LOX-1 levels were significantly different between controls, simple steatosis and NASH (borderline+definite) cases (4.08 ± 4.32 ng/mL, 6.1 ± 6.16 ng/mL, 8.92 ± 6.45 ng/mL, respectively, P = 0.004). When the cut-off value for the serum LOX-1 level was set at 5.35 ng/mL, and a ROC curve analysis was performed to distinguish between steatohepatitis patients and controls; the sensitivity and specificity of the serum LOX-1 level were 69.8% and 69.2%, respectively.
CONCLUSION: The serum LOX-1 levels were significantly higher in NAFLD patients than in healthy controls. Additionally, the serum LOX-1 levels could differentiate between steatohepatitis patients and healthy controls.
Core tip: Lipoprotein receptor-1 (LOX-1) is a biomarker that has been demonstrated to be related to atherosclerosis, insulin resistance, obesity and diabetes. Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome. To date, no studies have investigated the association between serum LOX-1 and liver inflammation in biopsy-proven NAFLD patients. In this study, we have shown that the serum LOX-1 levels are correlated with the NAFLD histology scores, which might decrease the need for performing liver biopsy in NAFLD patients.
- Citation: Ozturk O, Colak Y, Senates E, Yilmaz Y, Ulasoglu C, Doganay L, Ozkanli S, Oltulu YM, Coskunpinar E, Tuncer I. Increased serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels in patients with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol 2015; 21(26): 8096-8102
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8096.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8096
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, and its prevalence is continuously increasing[1,2]. The disease may present in different clinical forms. Though simple steatosis usually has a benign course, nonalcoholic steatohepatitis (NASH) may progress to liver fibrosis, cirrhosis and hepatocellular carcinoma due to ongoing necroinflammation[3,4].
Currently, liver biopsy is the gold standard for diagnosing NAFLD and NASH and for evaluating liver fibrosis[5]. However, liver biopsy is an invasive technique that is associated with several complications. Therefore, alternative non-invasive methods are under investigation for diagnosing this common disease[6].
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a protein coded by the oxidized low density lipoprotein (oxLDL) receptor-1 gene, and it is expressed by endothelial cells, vascular smooth muscle cells, macrophages, and adipocytes[7,8]. LOX-1 is a membrane glycoprotein that binds, internalizes, and degrades oxLDL. LOX-1 is activated by oxLDL and leads to endothelial dysfunction, apoptosis and atherosclerotic process via intracellular signal transduction. Additionally, LOX-1 has multiligand receptor features, and the defined ligands of LOX-1 are aged red blood cells, apoptotic cells, activated platelets, leukocytes, bacteria, phosphatidyl serine, advanced glycation endproducts, C reactive protein, and heat shock protein 70[9].
In addition to its role in the process of atherosclerotic, LOX-1 is active in inflammatory processes. Proinflammatory cytokines that are shown to upregulate LOX-1, such as transforming growth factor-beta, interleukin-6, interleukin-1α, interleukin-1β, and tumor necrosis factor-α, are also involved in the pathogenesis of NAFLD[9,10]. Furthermore, recent studies have reported an increase in the LOX-1 levels in diabetes mellitus, metabolic syndrome, and coronary artery disease[11,12].
In this study, we investigated the relationship between LOX-1 and histopathological changes and inflammation as well as clinical and biochemical parameters in liver biopsy-proven NAFLD (simple steatosis and borderline and definitive NASH) patients. To address these questions, we evaluated the extracellular soluble component of LOX-1 in NAFLD cases and healthy controls.
A total of 53 patients diagnosed with NAFLD (31 males and 22 females, mean age 42.5 ± 9.6 years) and 26 healthy control subjects (14 males and 12 females, mean age 39 ± 10.7 years) were included in the study. The patients with NAFLD were consecutive patients who had been admitted to the hepatology outpatient clinic within the last year and had been diagnosed with NAFLD based on liver biopsy. The healthy controls were individuals who attended the outpatient clinic for routine health control and had no known chronic illnesses. All participants in the study were recruited from Department of Gastroenterology of Istanbul Medeniyet University Göztepe Education and Research Hospital. To avoid selection bias for recruiting the patients and controls, all participants had to be residents of Istanbul for a minimum of 5 years as a prerequisite. The study was reviewed and approved by Istanbul Medeniyet University Goztepe Education and Research Hospital Institutional Review Board (document No. 8-B/28.12.2010) and written informed consent was obtained from all participants.
All NAFLD patients had elevated serum ALT levels for at least 6 mo, and none of the patients had alcohol consumption greater than 20 g/d. All patients were negative for viral hepatitis serology. Hemochromatosis, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis and alpha-1 antitrypsin deficiency were ruled out. All tests/procedures for the aforementioned excluded conditions were performed for research. Patients with biliary strictures and malignancies were excluded. Additionally, the patients had no history of using hepatotoxic medicines, such as estrogens, amiodarone, steroids, tamoxifen, methotrexate, valproic acid and herbal drugs. The data regarding the hepatotoxic drug history were obtained from both the patient’s medical records and their interviews. The data for the hepatotoxic drug history were obtained by interview for controls. All serological and biochemical tests of the participants were performed in the same laboratory, the Central laboratory of Göztepe Education and Research Hospital. All individuals in the control group were healthy on physical examination and had normal liver parenchyma in the sonographic liver examination. Their serological and biochemical parameters were all in the normal ranges.
Physical examinations, anthropometric and biochemical measurements, and body mass index (BMI) calculations were performed in all study participants. Blood pressure measurements were performed in a quiet room with a sphygmomanometer after 10 min of resting. After a 12-h overnight fasting period, blood samples, both from patients with NAFLD and controls, from the antecubital veins were collected between 8:00-9:00 am. After 2 h, which allowed the blood to clot, at room temperature, the samples were centrifuged at 1000 G for 20 min. The sera obtained from the cases were stored at -80 °C until further analysis.
Diabetes mellitus was diagnosed according to the American Diabetes Association criteria[13]. Metabolic syndrome was diagnosed using the Adult Treatment Panel III criteria[14]. Homeostasis Model of Assessment - Insulin Resistance (HOMA - IR) was calculated using the following equation: insulin resistance (IR) = fasting plasma glucose (mmol/L) × fasting plasma insulin (mU/L)/22.5 (IR was accepted as normal if IR < 2.5 and present if insulin resistance IR ≥ 2.5).
Duplicate measurements of the LOX-1 serum levels were performed using an ELISA kit (Life Science Inc. USCN. Wuhan, Catalog No. E1859Hu) according to the manufacturer’s instructions. The standard curve concentrations used for the ELISA were 10 ng/mL, 5 ng/mL, 2.5 ng/mL, 1.25 ng/mL, 0.625 ng/mL, 0.312 ng/mL, and 0.156 ng/mL. The minimum detectable level of human LOX-1 is less than 0.03 ng/mL.
A liver biopsy was performed under local anesthesia using a Hepafix 16-gauge needle (Braun Melsungen AG, Melsungen, Germany) with ultrasound guidance. All biopsy samples were fixed with 10% formaldehyde and then embedded in paraffin blocks. The liver specimens were stained with hematoxylin-eosin, Masson’s trichrome and reticulin silver stains. An experienced hepatopathologist scored and evaluated the tissue specimens. The pathologist was blinded to all patient data. The histological evaluation was conducted according to the NAFLD activity scoring system (NAS) recommended by The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NASH Clinical Research Network[15]. In this scoring system, hepatic steatosis was graded from 1 to 3 according to the steatosis ratio, with 5%-33%, 33%-66% and > 66% representing scores of 1, 2 and 3, respectively. Lobular inflammation was defined as an overall assessment of all inflammation; no foci was scored as 0, < 2 foci per × 200 field scored as 1, two-four foci per × 200 field scored as 2, and more than 4 foci per × 200 field scored as 3. Ballooning scoring is defined as a score of 0 if there is no ballooning of hepatocytes, 1 if there are few ballooning hepatocytes, and 2 if there are numerous ballooning hepatocytes. Fibrosis was staged as follows: stage 0, no liver fibrosis; stage 1, perisinusoidal or periportal fibrosis; stage 2, perisinusoidal and portal/periportal fibrosis; stage 3, bridging fibrosis and stage 4, cirrhosis. Histologically, the total NAS scores were calculated as a sum of the steatosis (1-3), lobular inflammation (0-3) and ballooning (0-2) scores. Based on this scoring system, patients with a total NAS score of 0-2 were diagnosed with simple steatosis, 3-4 borderline NASH, and 5 or greater definitive NASH[15].
StatMate 2.0 (GraphPad Inc., San Diego, CA, United States) was used for the power calculation of the study. The data were analyzed using SPSS 16.0 (IL United States SPSS Inc., Chicago, IL, United States). Normally distributed continuous variables are presented as the mean ± SD. Student’s t-test was used to evaluate the difference between the independent groups. Differences in the levels of LOX-1 among the NAFLD subgroups (simple steatosis and borderline NASH+definitive NASH) and control group were determined by one-way analysis of variance (ANOVA) followed by a Bonferroni multiple-comparison post hoc test. Categorical data were analyzed using the χ2 test. A Spearman rank correlation was used to examine the relationship between variables. A receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff value of LOX-1 for distinguishing between patients with NASH and healthy controls. A P-value < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Recep Minga, biomedical statistician of İkon Research and Consultancy co.
The clinical and biochemical characteristics of the healthy controls and NAFLD patients are presented in Table 1. The age and gender distribution were similar in both groups. The following characteristics were significantly higher in the NAFLD patients: waist circumference, diastolic blood pressure, HOMA-IR, triglyceride level and transaminase and ferritin levels. Eight patients (15%) had simple steatosis, whereas 27 (51%) had borderline NASH and 18 (34%) were diagnosed with definite NASH. Metabolic syndrome was found in 62.2% of the patients with NAFLD.
| Characteristic | Control | NAFLD | Simple steatosis | NASH (borderline + definite) | P value2 | P value3 |
| (n = 26) | (n = 53) | (n = 8) | (n = 45) | |||
| Gender (males/females) | 14/12 | 31/22 | 4/4 | 27/18 | 0.690 | 0.800 |
| Age (yr) | 39 ± 10.7 | 42.5 ± 9.6 | 42.8 ± 13.2 | 4 1 ± 8.6 | 0.190 | 0.310 |
| BMI (kg/m²) | 28.7 ± 5.1 | 31.6 ± 5.3 | 29.9 ± 4.31 | 32.07 ± 5.5 | 0.030 | 0.040 |
| Smoking | 5 (19.2) | 11 (20.7) | 3 (37.5) | 8 (17.7) | 0.900 | 0.080 |
| Waist circumference (cm) | 85.2 ± 7.3 | 102.2 ± 9.1 | 100.2 ± 9.9 | 102.6 ± 9 | 0.002 | 0.002 |
| Diabetes Mellitus | No | 11 (20.8) | 3 (37.5) | 8 (17.7) | 0.012 | 0.050 |
| Metabolic syndrome | No | 33 (62.2) | 3 (37.5) | 30 (66.6) | < 0.001 | < 0.001 |
| Hypertension | No | 12 (22.6) | 3 (37.5) | 9 (20) | 0.008 | 0.010 |
| Systolic blood pressure (mmHg) | 116 ± 17 | 121 ± 16 | 115 ± 15 | 122 ± 17 | 0.310 | 0.270 |
| Diastolic blood pressure (mmHg) | 74 ± 12 | 82 ± 10 | 79 ± 8 | 82 ± 10 | 0.006 | 0.010 |
| ESR median (min-max, mm/h) | 21 (6-38) | 11.5 (3-40) | 16 (4-40) | 11 (3-38) | 0.001 | 0.110 |
| CRP median (min-max, mg/L) | 0.4 (0.2-1.5) | 0.4 (0.1-2) | 0.5 (0.1-1.4) | 0.4 (0.1-2) | 0.720 | 0.670 |
| Hemoglobin A1c (%) | 5.7 ± 0.3 | 5.9 ± 0.9 | 5.5 ± 0.4 | 5.9 ± 0.9 | 0.730 | 0.310 |
| HOMA-IR median (min-max) | 2 (0.6-3.2) | 2.5 (0.3-11.8) | 1.6 (0.3-4.2) | 2.7 (0.5 ± 11.8) | 0.001 | 0.010 |
| AST median (min-max, U/L) | 19 (9-60) | 35 (16-147) | 36 (19-91) | 35 (16-147) | < 0.001 | < 0.001 |
| ALT median(min-max, U/L) | 18 (5-50) | 52 (17-196) | 43 (18-97) | 53 (17-196) | < 0.001 | < 0.001 |
| Total cholesterol (mmol/L) | 5.15 ± 1 | 5.43 ± 1.39 | 5.46 ± 1.13 | 5.43 ± 1.45 | 0.450 | 0.670 |
| HDL-cholesterol (mmol/L) | 1.21 ± 0.2 | 1.19 ± 0.2 | 1.26 ± 0.2 | 1.16 ± 0.2 | 0.290 | 0.310 |
| LDL-cholesterol (mmol/L) | 3.52 ± 0.8 | 3.67 ± 0.9 | 3.67 ± 0.8 | 3.67 ± 0.9 | 0.520 | 0.770 |
| Triglycerides (mmol/L) | 1.4 ± 0.6 | 2.5 ± 2.12 | 2.2 ± 1.08 | 2.5 ± 2.27 | 0.005 | 0.420 |
| Ferritin median (min-max, pmol/L) | 20.7 (2.8-101) | 91 (6.5-326) | 141.5 (7.8-300) | 88.5 (6.5-326) | < 0.001 | < 0.001 |
| LOX-1 (ng/mL) | 4.08 ± 4.32 | 8.49 ± 6.43 | 6.1 ± 6.16 | 8.92 ± 6.45 | 0.001 | 0.004 |
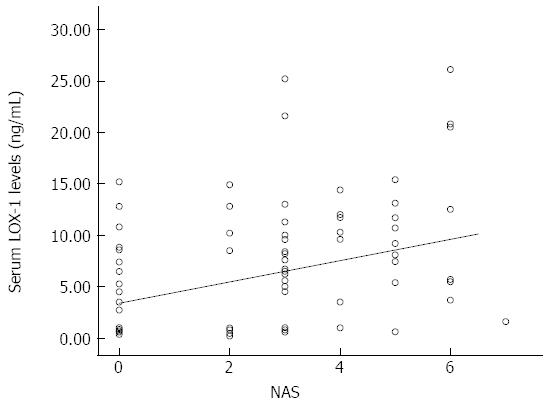
The LOX-1 level was significantly higher in the NAFLD group compared to healthy controls (8.49 ± 6.43 ng/mL vs 4.08 ± 4.32 ng/mL, respectively, P = 0.001). The LOX-1 levels were significantly different between the control, simple steatosis and NASH (borderline + definite) cases (4.08 ± 4.32 ng/mL, 6.1 ± 6.16 ng/mL, 8.92 ± 6.45 ng/mL, respectively, P = 0.004). The distribution of the serum LOX-1 levels in the study subgroups (controls and patients) is shown in a scatterplot figure (Figure 1). In addition, the LOX-1 serum levels were significantly higher in the NASH group than in the healthy subjects (8.92 ± 6.45 ng/mL vs 4.08 ± 4.32 ng/mL, P = 0.003). The LOX-1 levels were not significantly different in the NAFLD subgroups based on histological data. As a result, multiple linear regression analysis was not performed. In the NAFLD group, cases with (33) or without (20) metabolic syndrome had no significant difference in LOX-1 (7.27 ± 5.32 ng/mL vs 10.63 ± 7.84 ng/mL, respectively, P = 0.849).
The LOX-1 cut-off value that was used to distinguish healthy controls and NASH (borderline and definite) was 5.35 ng/mL. The area under ROC (AUROC) according to this cut-off level was 72.5% (SE = 0.06, Mann Whitney U-test, P = 0.001). With this cut-off value, the LOX-1 measurement could distinguish NASH patients from healthy controls with a sensitivity of 69.8%, specificity of 69.2%, negative predictive value of 69.6%, and positive predictive value of 69.4%.
In this study, we demonstrated, for the first time, a significant difference in the LOX-1 levels between biopsy-proven NAFLD and healthy controls. The LOX-1 levels were significantly higher in NASH patients compared with healthy controls. Therefore, LOX-1 may distinguish NASH cases from healthy subjects. There was no difference between the LOX-1 levels in the simple steatosis subgroup and the healthy controls or NASH cases.
Previous studies have reported that diabetes, obesity, and hypertension cases had high serum LOX-1 levels[9,16,17]. The elevated LOX-1 levels in NAFLD patients are in parallel with these metabolic disorders. This result may be due to insulin resistance, which is an underlying common pathophysiologic mechanism of these disorders[16,18].
In this study, the LOX-1 levels in patients with NAFLD were higher than healthy controls. Although there is a gradual increase in the progression from healthy control to simple steatosis and then to NASH (borderline + definite) subgroup cases, the only significant difference was obtained between controls and NASH patients. However, we did not find any significant difference between simple steatosis and the NASH subgroups. Lubrano et al[19] reported that the serum LOX-1 levels were correlated with other inflammatory markers and with the severity of coronary artery disease. Additionally, in an endothelial dysfunction study, Sakurai et al[20] showed that the LOX-1 level is linearly correlated with increasing oxidative stress.
The high LOX-1 levels in coronary artery disease, endothelial dysfunction and NAFLD patients suggest that inflammatory processes and oxidative stress are common pathophysiologic mechanisms. In addition, coronary artery disease and endothelial dysfunction were present in NAFLD patients[21-23]. One important result of this study is the identification of high LOX-1 levels in patients with steatohepatitis.
Liver biopsy is still the gold standard method for establishing NASH, which is the progressive form of NAFLD. However, due to several complications of this invasive procedure, a number of alternative methods and non-invasive diagnostic modalities are in development[15,24,25]. Increased LOX-1 serum levels in patients with NASH may simplify the selection of cases for differentiating between NAFLD subgroups prior to liver biopsy. However, these data must be confirmed by studies with large patient samples.
There is no relationship between the LOX-1 level and degree of fibrosis in our study. Kelly et al[26] reported a relationship between the LOX-1 level and renal function and fibrosis in obese and diabetic rats. Injections of anti-LOX-1 antibodies into the rats improved renal function and reduced fibrosis. In a study examining the relationship between LOX-1 and angiotensin II (which has roles in fibrotic processes) in human coronary artery endothelial cell culture, the activation of angiotensin II type 1 receptors was shown to increase the LOX-1 levels[27,28]. The angiotensin II type 1 receptor is also involved in the development of liver fibrosis[29]. Although these findings show a relationship between LOX-1 and kidney fibrosis, they do not suggest a causal relationship with liver fibrosis. There is a need for large-scale studies on LOX-1 expression at the cellular level to determine the role of LOX-1 in the pathogenesis of fibrosis. Based on our data, we concluded that in the natural course of NAFLD, inflammation is associated with elevated LOX-1 levels but that fibrosis is not.
There are some limitations to this study. The first limitation is the small number of cases. Therefore, the results must be verified in additional large-scale studies. Second, the study is a cross-sectional, case-control study; therefore, it does not provide information on the pathophysiologic and causal relationship for the disease course. The measurement of only the serum LOX-1 levels and not the liver tissue levels is also a limitation. Additionally, there is no evidence available at the tissue level in patients with NAFLD. Fourth, the patients enrolled in this study were only of Turkish descent; therefore, additional research is needed to assess the role of LOX-1 in different ethnic populations. Finally, the measured and unmeasured differences between the studied groups could have accounted for the findings.
In conclusion, this study showed an association, but not casuality, between serum LOX-1 levels and both NAFLD and NASH. As a result, the serum LOX-1 levels may be a useful marker for differentiating patients with NAFLD and NASH from healthy individuals, but our results must be verified in large-scale randomized trials.
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease. It is the hepatic manifestation of metabolic syndrome. Sensitive non-invasive test is needed to diagnose patients.
Serum Lipoprotein receptor-1 (LOX-1) is a novel biomarker of atherosclerosis and associated to diabetes, hypertension and metabolic syndrome. As NAFLD is related to these entities, LOX-1 might have a role in NAFLD pathogenesis.
Serum LOX-1 levels are increased in correlation with NAFLD activity scores and might improve to differentiate healthy people from definite nonalcoholic steatohepatitis (NASH).
Histopathological evaluation of the liver is needed for a definitive diagnosis of NASH. In this study serum LOX-1 was able to discriminate NASH from healthy controls. The increased levels might indicate the patients who will need histologic evaluation.
NAFLD (non-alcoholic fatty liver) is a spectrum of chronic liver diseases where lipid accumulation exceeds 5% in the hepatocytes. Simple steatosis usually has a benign course but NASH may progress to liver fibrosis, cirrhosis and hepatocellular carcinoma due to ongoing necroinflammation. The degree of severity is determined by histopathological scoring systems.
The inclusion and exclusion criteria are well defined in the study. Small sample size is a limitation of this study. It might have a role in the non-invasive diagnosis of NAFLD.
| 1. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1938] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 2. | Rafiq N, Younossi ZM. Nonalcoholic fatty liver disease: a practical approach to evaluation and management. Clin Liver Dis. 2009;13:249-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (2)] |
| 5. | Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50 Suppl:S412-S416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Baršić N, Lerotić I, Smirčić-Duvnjak L, Tomašić V, Duvnjak M. Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3945-3954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Novelli G, Mango R, Vecchione L, Mariotti E, Borgiani P, Mehta JL, Romeo F. [New insights in atherosclerosis research: LOX-1, leading actor of cardiovascular diseases]. Clin Ter. 2007;158:239-248. [PubMed] |
| 8. | Yan M, Mehta JL, Hu C. LOX-1 and obesity. Cardiovasc Drugs Ther. 2011;25:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Navarra T, Del Turco S, Berti S, Basta G. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J Atheroscler Thromb. 2010;17:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Gusdon AM, Song KX, Qu S. Nonalcoholic Fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective. Oxid Med Cell Longev. 2014;2014:637027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Md Sayed AS, Zhao Z, Guo L, Li F, Deng X, Deng H, Xia K, Yang T. Serum lectin-like oxidized-low density lipoprotein receptor-1 and adiponectin levels are associated with coronary artery disease accompanied with metabolic syndrome. Iran Red Crescent Med J. 2014;16:e12106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Yan M, Mehta JL, Zhang W, Hu C. LOX-1, oxidative stress and inflammation: a novel mechanism for diabetic cardiovascular complications. Cardiovasc Drugs Ther. 2011;25:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4360] [Article Influence: 272.5] [Reference Citation Analysis (0)] |
| 14. | Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3603] [Cited by in RCA: 3715] [Article Influence: 168.9] [Reference Citation Analysis (0)] |
| 15. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8557] [Article Influence: 407.5] [Reference Citation Analysis (8)] |
| 16. | Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J Lipid Res. 2008;49:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring). 2008;16:1454-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110-3117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Sakurai K, Sawamura T. Stress and vascular responses: endothelial dysfunction via lectin-like oxidized low-density lipoprotein receptor-1: close relationships with oxidative stress. J Pharmacol Sci. 2003;91:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Yilmaz Y, Kurt R, Gurdal A, Alahdab YO, Yonal O, Senates E, Polat N, Eren F, Imeryuz N, Oflaz H. Circulating vaspin levels and epicardial adipose tissue thickness are associated with impaired coronary flow reserve in patients with nonalcoholic fatty liver disease. Atherosclerosis. 2011;217:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, Coskunpinar E, Kahraman OT, Mesci B, Ulasoglu C. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Colak Y, Senates E, Ozturk O, Doganay HL, Coskunpinar E, Oltulu YM, Eren A, Sahin O, Ozkanli S, Enc FY. Association of serum lipoprotein-associated phospholipase A2 level with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2012;10:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Colak Y, Senates E, Coskunpinar E, Oltulu YM, Zemheri E, Ozturk O, Doganay L, Mesci B, Yilmaz Y, Enc FY. Concentrations of connective tissue growth factor in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Dis Markers. 2012;33:77-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Kelly KJ, Wu P, Patterson CE, Temm C, Dominguez JH. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am J Physiol Renal Physiol. 2008;294:F1136-F1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res. 1999;84:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314-322. [PubMed] |
| 29. | Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, Iida H, Kato S, Fujita K, Takahashi H. Association between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 2009;29:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Baclig MO, Torabizadeh Z S- Editor: Qi Y L- Editor: A E- Editor: Liu XM