Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8021
Peer-review started: January 16, 2015
First decision: February 10, 2015
Revised: February 25, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: July 14, 2015
Processing time: 180 Days and 11.1 Hours
AIM: To investigate a possible association between losartan and sirtuin 1 (SIRT1) in reduced-size orthotopic liver transplantation (ROLT) in rats.
METHODS: Livers of male Sprague-Dawley rats (200-250 g) were preserved in University of Wisconsin preservation solution for 1 h at 4 °C prior to ROLT. In an additional group, an antagonist of angiotensin II type 1 receptor (AT1R), losartan, was orally administered (5 mg/kg) 24 h and 1 h before the surgical procedure to both the donors and the recipients. Transaminase (as an indicator of liver injury), SIRT1 activity, and nicotinamide adenine dinucleotide (NAD+, a co-factor necessary for SIRT1 activity) levels were determined by biochemical methods. Protein expression of SIRT1, acetylated FoxO1 (ac-FoxO1), NAMPT (the precursor of NAD+), heat shock proteins (HSP70, HO-1) expression, endoplasmic reticulum stress (GRP78, IRE1α, p-eIF2) and apoptosis (caspase 12 and caspase 3) parameters were determined by Western blot. Possible alterations in protein expression of mitogen activated protein kinases (MAPK), such as p-p38 and p-ERK, were also evaluated. Furthermore, the SIRT3 protein expression and mRNA levels were examined.
RESULTS: The present study demonstrated that losartan administration led to diminished liver injury when compared to ROLT group, as evidenced by the significant decreases in alanine aminotransferase (358.3 ± 133.44 vs 206 ± 33.61, P < 0.05) and aspartate aminotransferase levels (893.57 ± 397.69 vs 500.85 ± 118.07, P < 0.05). The lessened hepatic injury in case of losartan was associated with enhanced SIRT1 protein expression and activity (5.27 ± 0.32 vs 6.08 ± 0.30, P < 0.05). This was concomitant with increased levels of NAD+ (0.87 ± 0.22 vs 1.195 ± 0.144, P < 0.05) the co-factor necessary for SIRT1 activity, as well as with decreases in ac-FoxO1 expression. Losartan treatment also provoked significant attenuation of endoplasmic reticulum stress parameters (GRP78, IRE1α, p-eIF2) which was consistent with reduced levels of both caspase 12 and caspase 3. Furthermore, losartan administration stimulated HSP70 protein expression and attenuated HO-1 expression. However, no changes were observed in protein or mRNA expression of SIRT3. Finally, the protein expression pattern of p-ERK and p-p38 were not altered upon losartan administration.
CONCLUSION: The present study reports that losartan induces SIRT1 expression and activity, and that it reduces hepatic injury in a ROLT model.
Core tip: Losartan is an angiotensin II type 1 receptor (AT1R) antagonist known to protect livers against ischemia-reperfusion injury (IRI). However, the mechanisms underlying this hepatoprotective effect are not fully understood, especially in case of reduced-size orthotopic liver transplantation (ROLT). SIRT1 has recently emerged as an important target to modulate for alleviating IRI. In our study, we describe that AT1R antagonism enhances SIRT1 activity and prevents endoplasmic reticulum stress and liver apoptosis in a rat model of ROLT. Consequently, losartan increases the resistance of ROLT grafts against IRI.
- Citation: Pantazi E, Bejaoui M, Zaouali MA, Folch-Puy E, Pinto Rolo A, Panisello A, Palmeira CM, Roselló-Catafau J. Losartan activates sirtuin 1 in rat reduced-size orthotopic liver transplantation. World J Gastroenterol 2015; 21(26): 8021-8031
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8021
Ischemia-reperfusion injury (IRI) is an important obstacle during liver transplantation, contributing to a significant loss of graft function. It is characterized by a cascade of deleterious cellular responses that lead to inflammation, cell death, and ultimately, organ failure[1]. These complications are increased in case of reduced-size liver grafts compared with standard liver transplant operations[2,3]. Thus, further investigation is required to explore new therapeutic strategies to counteract IRI.
Various reports have associated the renin-angiotensin system (RAS) with liver IRI[4,5]. The main efector of RAS is angiotensin II, which is produced via angiotensin converting enzyme (ACE) from angiotensin I. It exerts its biological actions through two receptor subtypes: angiotensin II type I receptor (AT1R) and angiotensin II type II receptor[6]. Angiotensin II has been associated with increased inflammation and oxidative stress in liver IRI, and various studies have evidenced that AT1R antagonists, such as losartan, efficiently protected livers against IRI in both warm ischemia and transplantation models[7-10].
Sirtuins are deacetylases dependent on nicotinamide adenine dinucleotide (NAD)+ that either activate or suppress various proteins. Thus, they are implicated in various cellular pathways, including metabolic processes, apoptosis and oxidative stress[11]. Sirtuin 1 (SIRT1) and the mitochondrial sirtuin 3 (SIRT3) are the most studied sirtuins and represent interesting targets for counteracting IRI in various organs[12,13]. SIRT1 has been shown to be involved in a wide range of cellular processes related to cell cycle and the cellular response to stresses, including the endoplasmic reticulum stress (ERS)[14-17].
IRI is known to promote ERS which finally induces cellular death[18]. In addition, we have previously shown that inhibiting ERS can be a useful strategy against IRI[19]. In a model of partial hepatectomy with ischemia-reperfusion in steatotic and non-steatotic rat livers, ERS inhibition ameliorated hepatic damage by reducing inflammation and apoptosis[19]. Therefore, we may hypothesize that preventing ERS might be useful for ameliorating the negative outcomes of reduced-size orthotopic liver transplantation (ROLT).
There is little evidence about a potential relationship between SIRT1 and angiotensin II antagonists. Miyazaki et al[20] have reported that SIRT1 overexpression suppresses AT1R in cultured vascular smooth muscle cells. In addition, a recent study in primary cultures of adipocytes evidenced a mutual interaction between RAS and SIRT1, with an association with metabolic homeostasis[21]. Conversely, there are no reports concerning a relationship between SIRT1 and angiotensin II antagonists in liver transplantation. Given that both are involved in common processes related to IRI, ERS, and apoptosis[22,23], we hypothesized that SIRT1 may be implicated in the protective effects of an AT1R antagonist against hepatic IRI following ROLT.
The present study therefore aimed to assess whether an AT1R antagonist, losartan, could be effective in protecting reduced-size liver grafts from IRI and to examine the possible underlying mechanisms involved. Furthermore, a potential relationship between losartan and SIRT1 was explored.
Male Sprague-Dawley rats (200-250 g) were used as donors and recipients. Animals were housed in conventional temperature- and humidity-controlled facilities with a 12-h light/dark cycle. All animals had free access to water and a standard laboratory diet. All procedures were performed under isoflurane inhalation anesthesia. Animal experiments were approved by the Ethics Committees for Animal Experimentation (CEEA, Directive 400/12), University of Barcelona and all procedures complied with European Union regulations for animal experiments (EU guideline 86/609/EEC). Rats were randomly distributed into groups as described below.
The following three experimental groups were created: (1) Sham (n = 6): Animals were subjected to transverse laparotomy and silk ligatures were located in the right suprarenal vein, diaphragmatic vein, and hepatic artery. After 24 h, animals were sacrificed and blood and liver samples were collected and stored at -20 °C and -80 °C respectively, for further investigation; (2) ROLT (n = 12, 6 transplants): ROLT was performed according to the Kamada’s cuff technique, without hepatic artery reconstruction[24]. During the donor surgery, the right suprarenal vein, diaphragmatic vein, and hepatic artery were ligated and the bile duct was cannulated. Then, the reduction of the liver was carried out. Liver reduction was achieved by removing the left lateral lobe and the two caudate lobes just before harvesting the liver, resulting in a 40% reduction of the liver mass. The pedicle of the left lateral lobe was ligated with 5.0 silk ligature, and the lobe was removed. The two caudate lobes were removed separately with the ligation[25]. Then, the donor livers were flushed and preserved with cold (4 °C) University of Wisconsin (UW) solution for 1 h and then implanted to the receptor. Receptors were killed 24 h after transplantation and blood and liver samples were collected and stored at -20 °C and -80 °C respectively for further investigation; and (3) Losartan + ROLT (n = 12, 6 transplants): We used the same protocol as for group 2, but an AT1R antagonist (losartan) was orally administered (5 mg/kg) at 24 h and 1 h before the donor and the recipient surgery[9].
Hepatic injury was assessed in terms of transaminase levels with commercial kits from RAL (Barcelona, Spain). Briefly, plasma extracts were collected before liver extraction and centrifuged at 4 °C for 10 min at 3000 rpm. Then, 200 μL of the supernatant were added to the substrate provided by the commercial kit. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined at 365 nm with an ultraviolet spectrometer and calculated according to the manufacturer’s instructions[26].
Liver NAD+/NADH levels were quantified with a commercially available kit (MAK037, Sigma Chemical, St. Louis, MO, United States) according to the manufacturer’s instructions.
Liver tissue was homogenized in a HEPES ((N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer as previously described[27]. Then, 50 μg of proteins were separated on 8%-15% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gels and trans-blotted on PVDF (polyvinylidene difluoride) membranes (Bio-rad Laboratories). Membranes were then blocked for one hour with 5% (w/v) non-fat milk in T-TBS (tween-tris-buffered saline) and incubated overnight at 4 °C with the corresponding primary antibody: SIRT1 (07-131), purchased from Merck Millipore, Billerica, MA; ac-FoxO1 (D-19, sc-49437) and GRP78 (GRP78, H-129, sc-13968), both purchased from Santa Cruz Biotechnology Inc, CA, United States); SIRT3 (2627), cleaved caspase-3 (Asp175, 9664), phosphorylated-eukaryotic translation initiation factor 2 (p-eIF2a) (Ser51, 9721), inositol-requiring enzyme 1α (IRE1α) (3294), caspase-12 (2202), p-p38 Thr180/Tyr182, 9211), p-p44/42 (Erk1/2, Thr202/Tyr204, 9101) purchased from Cell Signaling, Danvers, MA; HSP70 (610607, Transduction Laboratories, Lexington, KY); Heme Oxygenase-1 (H4535), NAMPT (AP22021SU, Acris Antibodies GmbH, Germany); and b-actin (A5316, Sigma Chemical, St. Louis, MO, United States). Membranes were then incubated for 1 h at room temperature with the corresponding secondary antibody linked to horseradish peroxidase. Bound complexes were detected using WesternBright ECL-HRP substrate (Advansta, Barcelona, Spain) and quantified via the Quantity One software for image analysis. Results were expressed as the densitometric ratio between the protein of interest and the loading control (β-actin).
Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed. Total liver RNA was isolated using a TRIzol reagent (Invitrogen). Reverse transcription was realized on a 1 μg RNA sample using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). The reaction included incubation at 25 °C (5 min), at 42 °C (30 min) and 85 °C (5 min) and then cDNA was stored at -80 °C. Subsequent PCR amplification was conducted in an iCycler iQ Multi-Color Real-Time PCR device (Bio-Rad Laboratories) using SsoAdvancedTM Universal SYBR Green Supermix (Bio-rad Laboratories) and the following rat primers for SIRT3: forward, 5′-TAGTCCAGGGTGTGGAAAGG-3′ and reverse, 3′-CCGCAGGTGAAGAAGTAAGC-5′. Reactions were performed in duplicate and threshold cycle values were normalized to GAPDH gene expression. The ratio of SIRT3 relative expression to GAPDH was calculated by the ΔCt formula.
Data are expressed as mean ± SE. Statistical comparison was performed by variance analysis, followed by the Student-Newman-Keuls test, using the GraphPad Prism software. P value < 0.05 was considered statistically significant.
We first examined whether treatment with losartan affected hepatic injury in our experimental model. As shown in Table 1, increased ALT and AST levels were observed when rats were submitted to ROLT in comparison with the sham group. However, treatment with losartan significantly reduced the transaminase levels in the ROLT group.
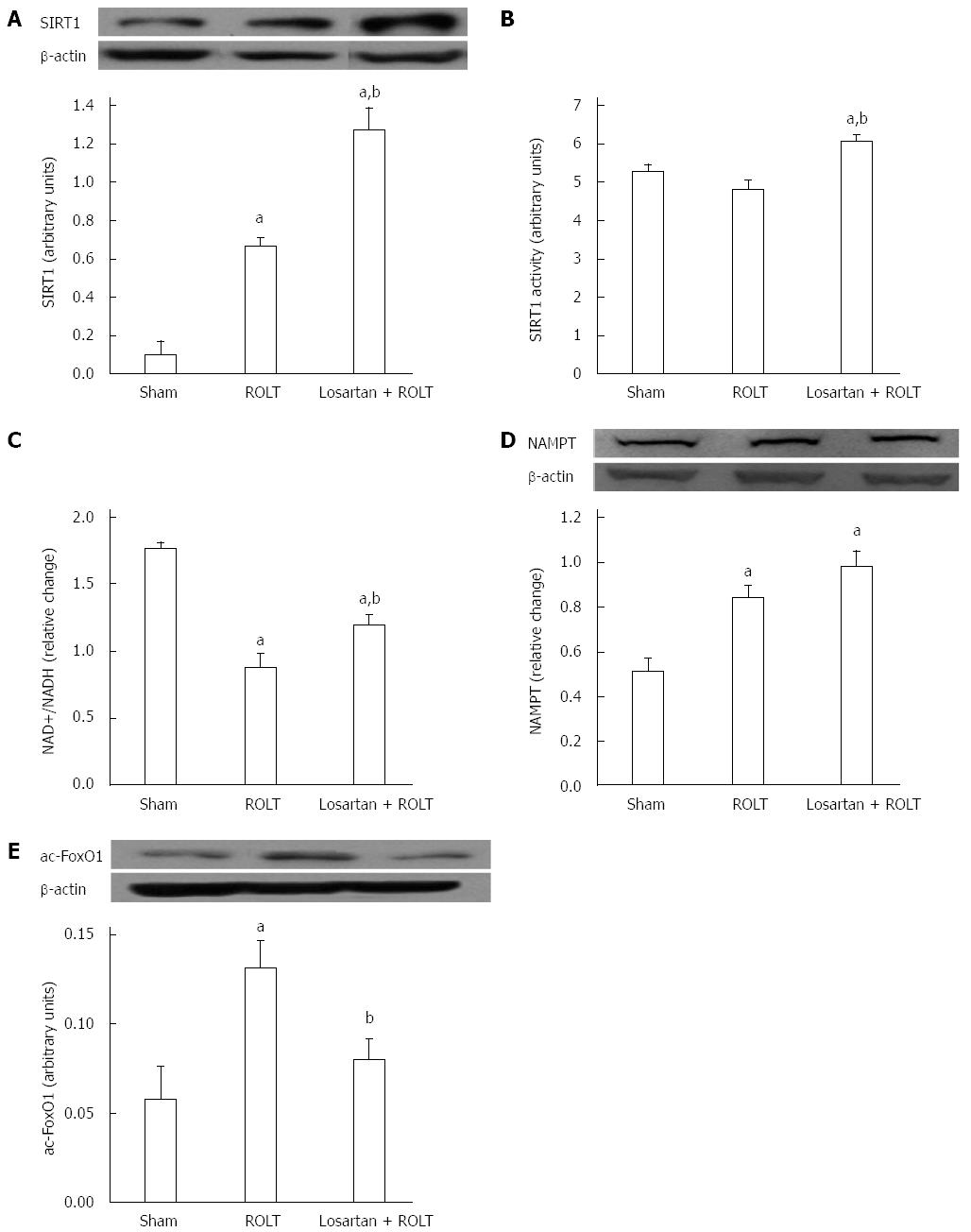
To investigate the possible interaction of SIRT1 with angiotensin II, we investigated the activity and the protein expression pattern of SIRT1. Animals subjected to ROLT showed augmented SIRT1 protein expression levels, which were further enhanced when losartan was administered (Figure 1A). In addition, losartan administration prior to the ROLT procedure significantly increased SIRT1 activity compared with both the ROLT and sham groups (Figure 1B). However, no significant differences were observed between the sham and ROLT groups.
In addition, we examined the levels of NAD+, the co-factor necessary for SIRT1 activity and nicotinamide phosphoribosyltransferase (NAMPT) protein expression, which is the major precursor for NAD+ biosynthesis. Figure 1C demonstrates that NAD+ levels were high in the sham group, but decreased in the ROLT and losartan + ROLT groups; however, losartan pre-treatment contributed to elevated NAD+ levels compared with ROLT alone. NAMPT protein was significantly augmented in both the ROLT and losartan + ROLT group in comparison to sham (Figure 1D).
Further, the forkheadbox (FoxO) transcription factors subfamily have been shown to mediate some of the effects of sirtuins. Given that FoxO1 is a direct substrate of SIRT1, we therefore determined its acetylation (Figure 1E). Animals subjected to ROLT showed elevated ac-FoxO1 protein levels compared with the sham group. By contrast, the augmented SIRT1 activity found when losartan was administered was consistent with a decrease in the ac-FoxO1 protein levels.
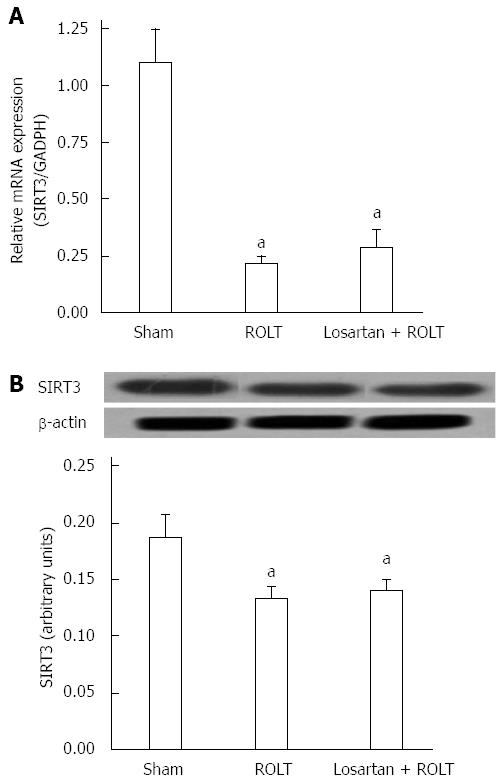
Because SIRT1 appeared to be modulated, we explored the role of SIRT3. We observed that SIRT3 mRNA levels were significantly downregulated in both ROLT and losartan + ROLT groups when compared with the sham group (Figure 2A). The same pattern was observed for SIRT3 protein levels, with significant decreases in animals subjected to ROLT and losartan + ROLT (Figure 2B).
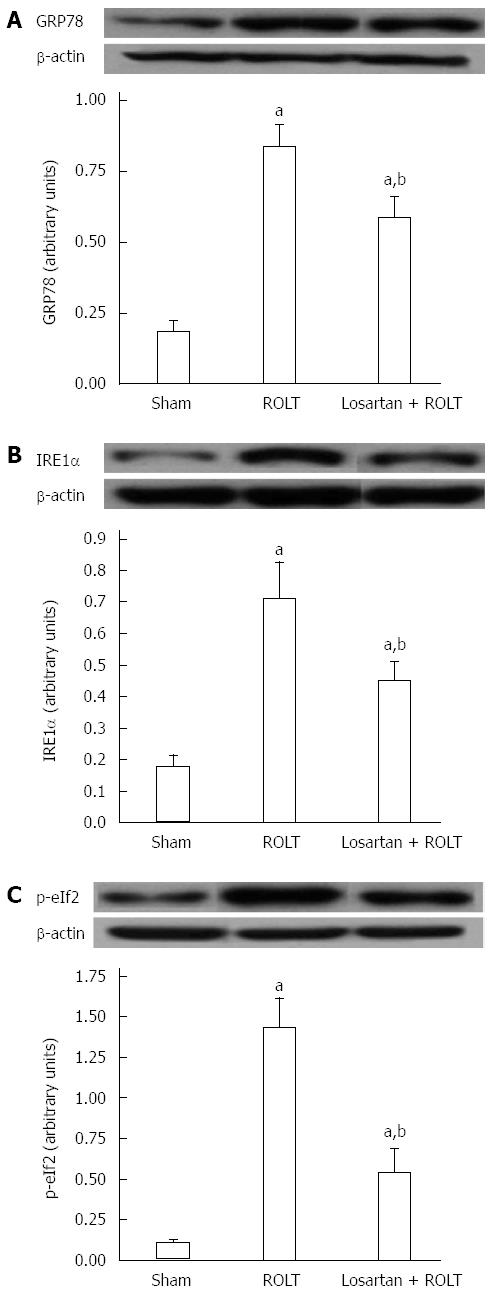
To identify other potential molecular mechanisms involved in the hepatoprotective effect of losartan against IRI, we examined different ERS parameters, including GRP78, IRE1α, and p-eIF2. As indicated in Figure 3, important increases of all ERS parameters occurred following ROLT but not the sham operation. Losartan pre-treatment also restored the ERS parameters.
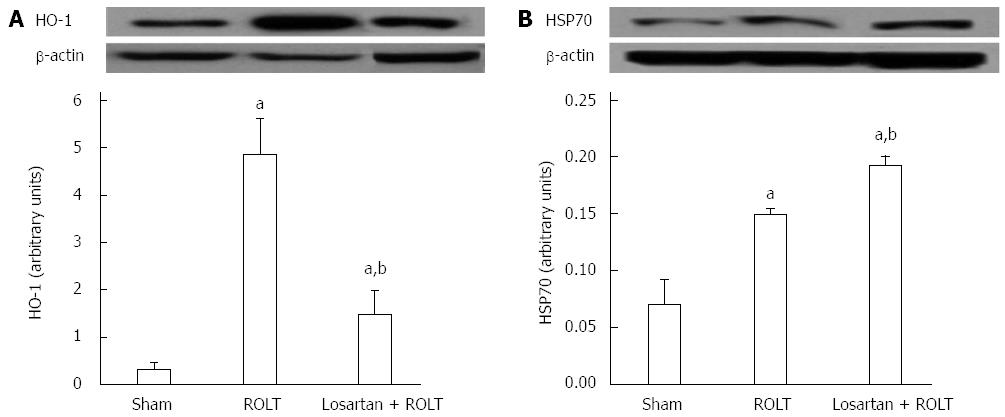
Because heat shock proteins are implicated in liver IRI, we determined the protein expression pattern of heme oxygenase 1 (HO-1) and of the heat shock protein 70 (HSP70). As it is shown in Figure 4, enhanced HO-1 and HSP70 protein levels were found in animals subjected to ROLT. However, Losartan treatment decreased HO-1 protein levels and increased HSP70 protein levels.
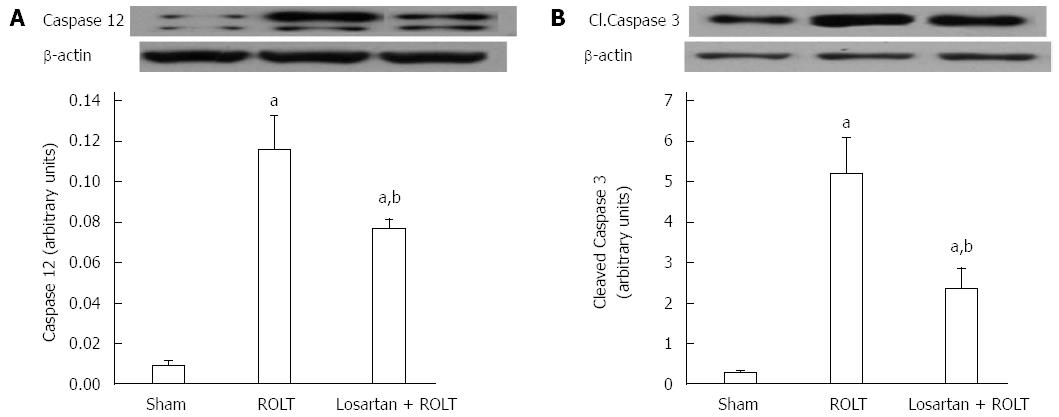
Liver IRI is characterized by increased hepatic apoptosis, so we determined the protein levels of caspase-12 and caspase-3, which are known to promote apoptosis. Figure 5 shows that increased levels of both proteins in animals undergoing ROLT were diminished by losartan pre-treatment.
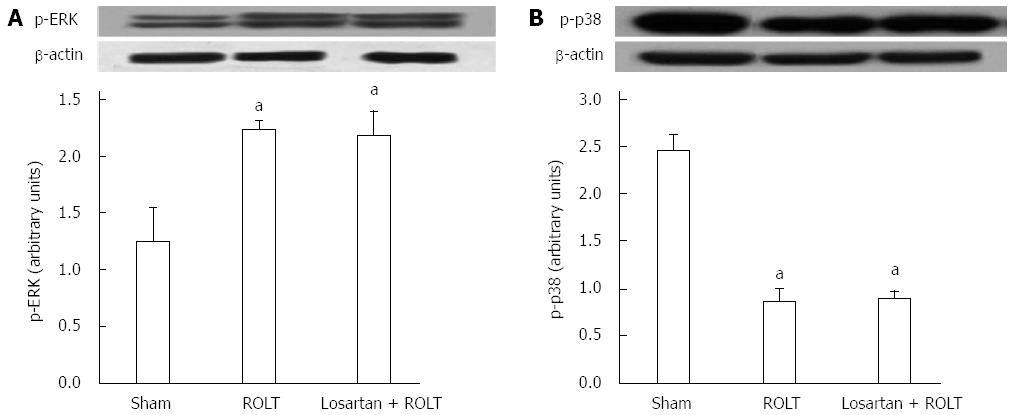
The mitogen activated protein kinases (MAPKs) are serine/threonine protein kinases that mediate intracellular signal transduction events associated with IRI. Therefore, we determined the activation of extracellular signal-regulated kinase (ERK) and p38. Figure 6A shows that animals undergoing ROLT had increased levels of p-ERK, but that losartan pre-treatment did not enhance ERK activation compared with ROLT alone. Moreover, the content of p-p38 was decreased in both the ROLT and losartan + ROLT groups. Losartan pre-treatment did not alter p-p38 content when compared to ROLT alone (Figure 6B).
This study demonstrated that inhibition of AT1R lessens hepatic injury in ROLT. Specifically, we provide new insights into losartan-mediated hepatoprotection in rats undergoing ROLT, including the induction of SIRT1 and the attenuation of ERS.
The protective effects of losartan against IRI were associated with increased SIRT1 activity and protein expression. SIRT1 up-regulation and angiotensin II blockade have been separately reported as therapeutic strategies against IRI in various organs[5,12,28,29]. Enhancement of SIRT1 has also been associated with decreased hepatic injury in rat orthotopic liver transplantation[30]. In our experimental rat ROLT model, SIRT1 protein expression was upregulated, but we observed no differences in its activity. Furthermore, FoxO1 deacetylation was inhibited in the ROLT group. SIRT1 overexpression and failure to augment its activity during IRI has also been reported in a recent work by our group[27]. In addition, losartan administration not only enhanced SIRT1 expression but also significantly increased both SIRT1 activity and FoxO1 deacetylation in comparison with the ROLT group. Further, losartan-induced increases in SIRT1 activity can be attributed to the enhanced NAD+ levels, which are indispensable for sirtuin activity. In turn, the NAD+ levels may be attributed to the NAMPT levels, which were slightly, but not significantly, increased after losartan treatment. Moreover, enhanced deacetylation of FoxO1 was related with NAMPT and NAD+ increases in rat orthotopic liver transplantation[30]. The present data demonstrate the existence of an angiotensin II/SIRT1 axis in liver transplantation, and that the benefits of angiotensin II inhibition against liver IRI are mediated, at least in part, through SIRT1 activation. This is consistent with a recent study in rat skeletal muscle, in which angiotensin II administration decreased SIRT1 expression[31].
Next, we speculated that SIRT3 might be affected by ROLT and losartan treatment. Real-time qRT-PCR and Western blot analysis revealed that SIRT3 mRNA and protein levels were significantly decreased in both the ROLT and losartan + ROLT groups compared with the sham group. This may be attributed to the mitochondrial disturbances that commonly take place during IRI[32]. SIRT3 is the major mitochondrial deacetylase implicated in metabolism, oxidative stress responses, and cardiac IRI[13,33-35]. The fact that SIRT3 mRNA and protein levels were comparable between the ROLT and losartan + ROLT groups suggests that the protective effect of losartan was independent of the SIRT3 pathway.
The endoplasmic reticulum is an organelle responsible for protein folding. Under stress conditions, the homeostasis of the endoplasmic reticulum is disturbed, leading to accumulation of unfolded proteins. In this case, an adaptive unfolded protein response (UPR) is activated to lessen the effects of ERS; however, when the insult is exaggerated in IRI, the ERS response can lead to cell death[36]. The UPR has three core branches: an IRE1α that induces the cleavage of the mRNA encoding X-box-binding protein 1 (XBP-1); a PKR-like endoplasmic reticulum kinase (PERK) that phosphorylates the eIF2a; and an activating transcription factor (ATF6). Under stress conditions, IRE1α, PERK, and ATF6 are released from their binding with the 78-kD glucose-regulated/binding immunoglobulin protein (GRP78) and become activated[37]. In a liver transplantation model, we have previously seen that activation of these UPR branches is associated with cell death and is a determinant factor of liver injury[18]. In this study, we observed that ROLT triggered the activation of GRP78 and the subsequent activation of the IRE1α and p-eIF2 pathways. Moreover, losartan pre-treatment abolished the activation of all ERS parameters. This is consistent with a recent study in human islets, which revealed that losartan exerted its protective effects against glucotoxicity by reducing ERS[38].
Losartan treatment was also accompanied by significant regulation of HSP70 and HO-1. The chaperone activity of HSP70 has been associated with cellular attempts to maintain proteins in an accurately folded state[36]. In our study, losartan pre-treatment induced HSP70 overexpression, which could have contributed to a decreased accumulation of unfolded proteins and therefore less ERS. Furthermore, because a direct relationship has previously been reported between SIRT1 and HSP70 in hepatic IRI, SIRT1 might contribute to HSP70 enhancement[27]. The increased ERS levels observed in the ROLT group were consistent with enhanced HO-1 protein expression that probably occurred due to an adaptive cell mechanism to prevent stress, as previously proposed by Liu et al[39]. In this sense, HO-1 expression was decreased when losartan pre-treatment diminished ERS.
Apoptosis is one of the most significant events in the pathophysiology of liver IRI. Aiming to mitigate the effects of ERS-mediated apoptosis could be an effective strategy for minimize IRI. It is known that IRE1α provokes caspase 12 cleavage, which in turn activates caspase 9 and then caspase 3 to stimulate apoptosis[40,41]. In our study, the induction of ERS in the ROLT group led to increased cell death, as reflected by the enhanced caspase 12 and caspase 3 protein levels. Further, the decrease in ERS in the losartan + ROLT group coincided with decreases in the levels of these caspases.
MAPKs are linked with cell cycle, liver regeneration, apoptosis, and oxidative stress pathways. The ERK cascade is closely connected with the regulation of cell growth and differentiation, whereas p38 is involved in cellular responses to environmental stress[42]. It has been reported that active p38 MAPK is present in the quiescent liver, and that it is dephosphorylated in the regenerating liver[43,44]. ERK phosphorylation is also involved in the signaling pathways of liver regeneration[45]. Therefore, the lowered p-p38 and increased p-ERK levels observed in the ROLT and losartan + ROLT groups could be associated with enhanced liver regeneration. In a previous study, our group reported that losartan pre-treatment did not enhance liver regeneration after ROLT[46]. Thus, losartan pre-treatment did not provide an additional increase in liver regeneration, resulting in no differences in p-p38/ERK activation between the two ROLT groups. Consequently, we can assume that SIRT1 activation by losartan treatment is not associated with liver regeneration in a ROLT model. Losartan administration decreased significantly hepatic injury and affected signaling processes related to IRI, such ERS and apoptosis. However, it could not further enhance liver regeneration, an essential processes for the success of transplantation with reduced-size liver grafts. Further studies will be required to elucidate the mechanisms by which losartan improves hepatic injury after ROLT.
Furthermore, angiotensin II is known to exert vasoconstrictor effects[47-49] and angiotensin II blockers, such as losartan, have been reported to decrease arterial pressure and act as effective antihypertensive agents[50,51]. A potential hypotensive effect of losartan was out of the scope of the present study, whereas prolonged time treatments with losartan are usually applied in order to evaluate blood pressure changes[52].
In conclusion, the present results indicate that SIRT1 is implicated in the protective effects of AT1R inhibition by losartan against IRI following ROLT. Losartan pre-treatment markedly attenuates liver injury by regulating signaling pathways that are involved in the pathophysiology of IRI, including heat shock protein, ERS, and liver apoptosis pathways. Moreover, it is evidenced that SIRT1 is a downstream target of angiotensin II in a rat ROLT model. Further studies are required to identify whether other angiotensin peptides (i.e., 1-7) can also modulate SIRT1.
The authors would like to thank Robert Sykes and Michael Maudsley at the Language Advisory Service of the University of Barcelona for revising the English text.
Ischemia-reperfusion injury (IRI) is a complex pathophysiological process inherent to liver transplantation. Endoplasmic reticulum stress (ERS) and apoptosis are common features of liver IRI in this context. Angiotensin II is a basic constituent of the renin-angiotensin system and has been shown to worsen IRI. Angiotensin II acts by binding to angiotensin II type I receptors (AT1R) and angiotensin II type II receptors. Of note, antagonists of these receptors have been found to protect against liver IRI. In addition, sirtuin 1 (SIRT1) is a NAD+-dependent deacetylase that modulates various cellular pathways associated to IRI, but its relationship with angiotensin II in liver IRI has not been studied. In this study, the authors demonstrate that administration of losartan, an antagonist of AT1R, significantly reduced liver injury in a rat model of reduced-size orthotopic liver transplantation (ROLT) by activating SIRT1 and decreasing ERS and liver apoptosis.
Angiotensin II has been associated with inflammatory responses and oxidative stress in liver IRI. Inhibition of its action with AT1R antagonists, such as losartan, results in decreased hepatic injury by attenuating pro-inflammatory responses, activating HIF-1α and peroxisome proliferator-activated receptor gamma in various hepatic IRI models. The present study report that the hepatoprotective effects of losartan against IRI associated with ROLT are mediated through SIRT1 enhancement, HSP70 overexpression, and attenuation of ERS and liver apoptosis.
The role of SIRT1 in a ROLT model has not yet been determined, nor has the potential link between angiotensin II and SIRT1 or ERS in liver IRI. The present study evaluated the potential role of losartan administration on SIRT1 expression and activity and on ERS activation in a rat ROLT model. The present study demonstrated that angiotensin II inhibition led to SIRT1 up-regulation and a subsequent decrease in ERS that contributed to reduced hepatic injury following ROLT.
Pharmacological activation of SIRT1 by losartan might be a promising therapeutic tool for ameliorating the detrimental effects of IRI following ROLT in rat models.
The manuscript is well written and data presented are detailed as well as the figures.
| 1. | Quesnelle KM, Bystrom PV, Toledo-Pereyra LH. Molecular responses to ischemia and reperfusion in the liver. Arch Toxicol. 2015;89:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 2. | Du Z, Wei C, Cheng K, Han B, Yan J, Zhang M, Peng C, Liu Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J Surg Res. 2013;183:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 482] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Ramalho FS, Alfany-Fernandez I, Casillas-Ramirez A, Massip-Salcedo M, Serafín A, Rimola A, Arroyo V, Rodés J, Roselló-Catafau J, Peralta C. Are angiotensin II receptor antagonists useful strategies in steatotic and nonsteatotic livers in conditions of partial hepatectomy under ischemia-reperfusion? J Pharmacol Exp Ther. 2009;329:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Casillas-Ramirez A, Amine-Zaouali M, Massip-Salcedo M, Padrissa-Altés S, Bintanel-Morcillo M, Ramalho F, Serafín A, Rimola A, Arroyo V, Rodés J. Inhibition of angiotensin II action protects rat steatotic livers against ischemia-reperfusion injury. Crit Care Med. 2008;36:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1267] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 7. | Yang YY, Lee PC, Huang YT, Lee WP, Kuo YJ, Lee KC, Hsieh YC, Lee TY, Lin HC. Involvement of the HIF-1α and Wnt/β-catenin pathways in the protective effects of losartan on fatty liver graft with ischaemia/reperfusion injury. Clin Sci (Lond). 2014;126:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Koh EJ, Yoon SJ, Lee SM. Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation. Br J Pharmacol. 2013;169:1404-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Guo L, Richardson KS, Tucker LM, Doll MA, Hein DW, Arteel GE. Role of the renin-angiotensin system in hepatic ischemia reperfusion injury in rats. Hepatology. 2004;40:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Alfany-Fernandez I, Casillas-Ramirez A, Bintanel-Morcillo M, Brosnihan KB, Ferrario CM, Serafin A, Rimola A, Rodés J, Roselló-Catafau J, Peralta C. Therapeutic targets in liver transplantation: angiotensin II in nonsteatotic grafts and angiotensin-(1-7) in steatotic grafts. Am J Transplant. 2009;9:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479-1514. [PubMed] |
| 12. | Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, Roselló-Catafau J. Role of sirtuins in ischemia-reperfusion injury. World J Gastroenterol. 2013;19:7594-7602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol. 2014;306:H1602-H1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416-430. [PubMed] |
| 15. | Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003-1019. [PubMed] |
| 16. | Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2498] [Cited by in RCA: 2618] [Article Influence: 119.0] [Reference Citation Analysis (1)] |
| 18. | Mosbah IB, Zaouali MA, Martel C, Bjaoui M, Abdennebi HB, Hotter G, Brenner C, Roselló-Catafau J. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death Dis. 2012;3:e279. [PubMed] |
| 19. | Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodés J, Brenner C, Roselló-Catafau J, Peralta C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263-1269. [PubMed] |
| 21. | Oliveira Andrade JM, Paraíso AF, Garcia ZM, Ferreira AV, Sinisterra RD, Sousa FB, Guimarães AL, de Paula AM, Campagnole-Santos MJ, dos Santos RA. Cross talk between angiotensin-(1-7)/Mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides. 2014;55:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Uhal BD, Nguyen H, Dang M, Gopallawa I, Jiang J, Dang V, Ono S, Morimoto K. Abrogation of ER stress-induced apoptosis of alveolar epithelial cells by angiotensin 1-7. Am J Physiol Lung Cell Mol Physiol. 2013;305:L33-L41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun. 2012;422:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [PubMed] |
| 25. | Xia R, Emond JC. Orthotopic partial liver transplantation in the rat: a model of 70% hepatectomy and reduced size liver transplantation. Transplantation. 1993;56:1041-1043. [PubMed] |
| 26. | Bejaoui M, Zaouali MA, Folch-Puy E, Pantazi E, Bardag-Gorce F, Carbonell T, Oliva J, Rimola A, Abdennebi HB, Roselló-Catafau J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J Pharm Pharmacol. 2014;66:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Pantazi E, Zaouali MA, Bejaoui M, Serafin A, Folch-Puy E, Petegnief V, De Vera N, Ben Abdennebi H, Rimola A, Roselló-Catafau J. Silent information regulator 1 protects the liver against ischemia-reperfusion injury: implications in steatotic liver ischemic preconditioning. Transpl Int. 2014;27:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lutz J, Risch K, Liu S, Antus B, Schmaderer C, Roos M, Ouyang N, Lehmann M, Heemann U. Angiotensin type 1 and type 2 receptor blockade in chronic allograft nephropathy. Kidney Int. 2006;70:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, Varela AT, Rolo AP, Palmeira CM, Roselló-Catafau J. Sirtuin 1 in rat orthotopic liver transplantation: an IGL-1 preservation solution approach. World J Gastroenterol. 2015;21:1765-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kackstein K, Teren A, Matsumoto Y, Mangner N, Möbius-Winkler S, Linke A, Schuler G, Punkt K, Adams V. Impact of angiotensin II on skeletal muscle metabolism and function in mice: contribution of IGF-1, Sirtuin-1 and PGC-1α. Acta Histochem. 2013;115:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 870] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 33. | Teodoro JS, Duarte FV, Gomes AP, Varela AT, Peixoto FM, Rolo AP, Palmeira CM. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion. 2013;13:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 35. | Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 926] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 36. | Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1501] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 37. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 976] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 38. | Madec AM, Cassel R, Dubois S, Ducreux S, Vial G, Chauvin MA, Mesnier A, Chikh K, Bosco D, Rieusset J. Losartan, an angiotensin II type 1 receptor blocker, protects human islets from glucotoxicity through the phospholipase C pathway. FASEB J. 2013;27:5122-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem. 2005;280:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186-194. [PubMed] |
| 41. | Badiola N, Penas C, Miñano-Molina A, Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J, Zhu C, Blomgren K. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem. 2004;279:43107-43116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Campbell JS, Argast GM, Yuen SY, Hayes B, Fausto N. Inactivation of p38 MAPK during liver regeneration. Int J Biochem Cell Biol. 2011;43:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Padrissa-Altés S, Franco-Gou R, Boillot O, Serafín A, Rimola A, Arroyo V, Rodés J, Peralta C, Roselló-Catafau J. Effect of angiotensin II and bradykinin inhibition in rat reduced-size liver transplantation. Liver Transpl. 2009;15:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Morton JJ, Beattie EC, MacPherson F. Angiotensin II receptor antagonist losartan has persistent effects on blood pressure in the young spontaneously hypertensive rat: lack of relation to vascular structure. J Vasc Res. 1992;29:264-269. [PubMed] |
| 49. | Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension. 2011;58:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Park CG, Youn HJ, Chae SC, Yang JY, Kim MH, Hong TJ, Kim CH, Kim JJ, Hong BK, Jeong JW. Evaluation of the dose-response relationship of amlodipine and losartan combination in patients with essential hypertension: an 8-week, randomized, double-blind, factorial, phase II, multicenter study. Am J Cardiovasc Drugs. 2012;12:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Agasti AK, Mahajan AU, Phadke AY, Nathani PJ, Sawant P. Comparative randomized study on efficacy of losartan versus propranolol in lowering portal pressure in decompensated chronic liver disease. J Dig Dis. 2013;14:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Kwon HM, Shin JW, Lim JS, Hong YH, Lee YS, Nam H. Comparison of the effects of amlodipine and losartan on blood pressure and diurnal variation in hypertensive stroke patients: a prospective, randomized, double-blind, comparative parallel study. Clin Ther. 2013;35:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Feier FH, Piardi T, Wang XP S- Editor: Yu J L- Editor: A E- Editor: Ma S