Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8014
Peer-review started: March 19, 2015
First decision: April 13, 2015
Revised: April 30, 2015
Accepted: June 9, 2015
Article in press: June 10, 2015
Published online: July 14, 2015
Processing time: 117 Days and 21.6 Hours
Since Isaacson and Wright first reported on the extra-nodal marginal zone B-cell lymphoma of the stomach in 1983, following studies have clarified many aspects of this disease. We now know that the stomach is the most affected organ by this disease, and approximately 90% of gastric mucosa-associated lymphoid tissue (MALT) lymphomas are related to Helicobacter pylori (H. pylori) infection. This implies that approximately 10% of gastric MALT lymphomas occur independent of H. pylori infection. The pathogenesis of these H. pylori-negative gastric MALT lymphomas remains unclear. To date, there have been several speculations. One possibility is that genetic alterations result in nuclear factor-kappa B (NF-κB) activation. Among these alterations, t(11;18)(q21;q21) is more frequently observed in H. pylori-negative gastric MALT lymphomas, and such translocation results in the synthesis of fusion protein API2-MALT1, which causes canonical and noncanonical NF-κB activation. Another possibility is infection with bacteria other than H. pylori. This could explain why H. pylori eradication therapy can cure some proportions of H. pylori-negative gastric MALT lymphoma patients, although the bacteria responsible for MALT lymphomagenesis are yet to be defined. Recent advances in endoscopy suggest magnifying endoscopy with narrow band imaging as a useful tool for both detecting gastric MALT lymphoma lesions and judging the response to treatment. A certain proportion of H. pylori-negative gastric MALT lymphoma patients respond to eradication therapy; hence, H. pylori eradication therapy could be considered as a first-line treatment for gastric MALT lymphomas regardless of their H. pylori infection status.
Core tip: Although the majority of gastric mucosa-associated lymphoid tissue (MALT) lymphoma patients are infected with Helicobacter pylori (H. pylori), approximately 10% of patients do not have H. pylori infection. Recent studies have demonstrated that eradication therapy for H. pylori is effective not only for H. pylori-positive but also for H. pylori-negative gastric MALT lymphoma patients.
-
Citation: Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T.
Helicobacter pylori -negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol 2015; 21(26): 8014-8020 - URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8014
Mucosa-associated lymphoid tissue (MALT) lymphoma is an extra-nodal B-cell lymphoma that arises in MALT, which was first reported by Isaacson and Wright in 1983[1]. The following studies have elucidated many aspects of this disease.
The stomach is one of the most affected organs by this disease, and approximately 90% of the affected stomachs are infected with Helicobacter pylori (H. pylori)[2].
This implies that approximately 10% of gastric MALT lymphomas occur independent of H. pylori infection. These gastric MALT lymphomas were considered to be resistant to H. pylori eradication. Although there were reports of H. pylori-negative gastric MALT lymphomas that were successfully treated with antibiotic therapy[3,4], some assumed that those were only false negative cases[5]. We now know that some proportions of gastric MALT lymphoma in H. pylori-uninfected stomachs can be successfully treated with antibiotic therapy[3]. However, there are not as many reports on H. pylori-uninfected MALT lymphomas compared with the infected ones. Therefore, in this review, we will summarize the current knowledge of H. pylori-negative gastric MALT lymphomas.
The pathogen in H. pylori-infected cases of gastric MALT lymphomas is clearly H. pylori. This statement is supported by the fact that approximately 75% of H. pylori-positive gastric MALT lymphomas achieve complete remission (CR) by eradication of this bacterium alone[6,7] (Table 1). Chronic H. pylori infection attracts lymphoid cells to the gastric MALT, where these cells are continuously stimulated by H. pylori and give rise to MALT lymphomas. Previous studies have demonstrated that not only B cells, but also T cells and macrophages play an important role in this lymphomagenesis[8-10].
| H. pylori-positive | H. pylori-negative | |
| Pathogenesis | H. pylori infection | Genetic alterations |
| Other bacterial infection | ||
| Autoimmune diseases | ||
| First-line therapy | Antibiotic therapy | Antibiotic therapy |
| Response rate for antibiotic therapy | 75% | 28% |
Because H. pylori-negative gastric MALT lymphoma patients are not infected with H. pylori, this bacterium cannot be responsible for the lymphomagenesis. To date, there have been several opinions regarding the pathogenesis of H. pylori-negative gastric MALT lymphomas.
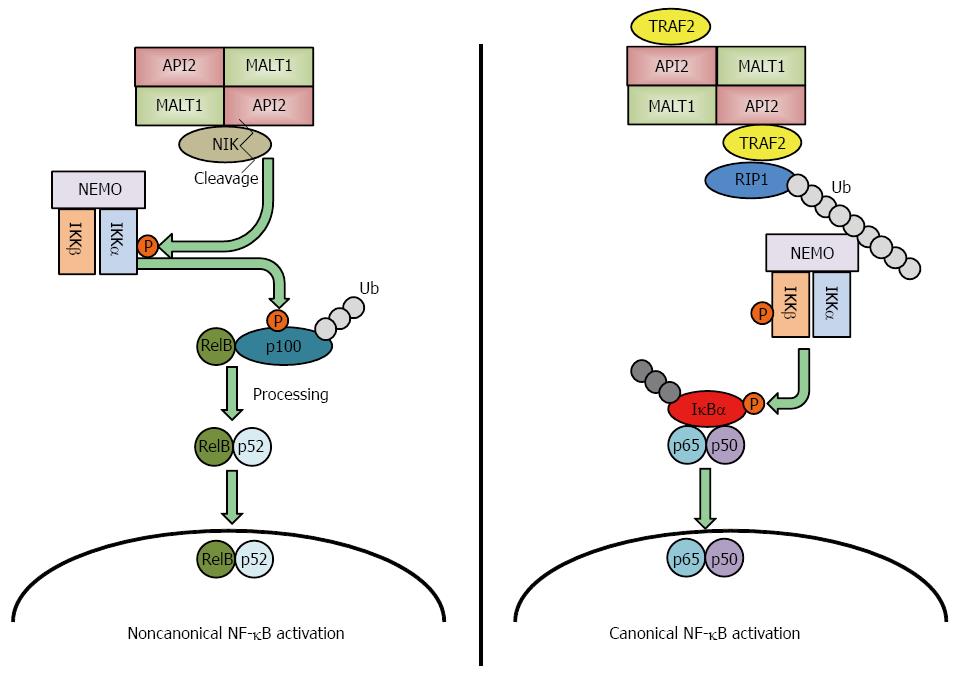
Several genetic alterations have been identified in gastric MALT lymphomas: t(11;18)(q21;q21), t(1;14)(p22;q32), t(14;18)(q32;q21) and t(3;14)(p13;q32)[11,12]. Among these genetic abnormalities, t(11;18)(q21;q21) is the most frequently detected translocation in gastric MALT lymphomas[11-14] and is more frequently observed in H. pylori-negative gastric MALT lymphomas. This translocation fuses the N-terminus of the API2 gene to C-terminus of the MALT1 gene, which results in the synthesis of an API2-MALT1 fusion protein[15]. MALT1 was first reported in 1999 by two groups, and they both suggested that API2-MALT1 is important to the pathogenesis of gastric MALT lymphomas[16,17]. The following studies revealed that API2-MALT1 fusion protein activates nuclear factor-kappa B (NF-κB) through noncanonical pathway by inducing the proteolytic cleavage of NF-κB-inducing kinase (NIK), resulting in deregulated NIK activity and noncanonical NF-κB activation[18] (Figure 1 left panel). Conversely, Zhou et al[19] reported that API2-MALT1 chimeric protein causes canonical NF-κB activation via deregulated ubiquitin ligase activity, which increases K63-polyubiquitination of NEMO, and Lucas et al[20] reported that heterotopic API2-MALT1 oligomerization and binding of TNF receptor associated factor 2 (TRAF2) are required for maximal NF-κB activation. Recent report detailed the role of RIP1 ubiquitination, resulting from TRAF2 recruitment to API2-MAT1, as necessary for full NF-κB activation[21] (Figure 1 right panel). This deregulated activation of NF-κB induces tumorigenesis[22]; therefore, this genetic alteration could be a cause of H. pylori-negative gastric MALT lymphoma.
Some groups suspected the involvement of a bacterium other than H. pylori. Indeed, there are several bacteria and viruses identified to have a correlation between marginal zone B cell lymphomas (Campylobacter jejuni and immunoproliferative small intestinal disease, Borellia burgdorferi and primary cutaneous B-cell lymphoma, Chlamydophila psittaci and ocular adnexal lymphoma, hepatitis C virus and splenic marginal zone lymphoma)[23], and it is possible that bacteria other than H. pylori are causing chronic inflammation in the stomachs of H. pylori-negative gastric MALT lymphoma patients. Morgner et al[24] reported 5 cases of H. pylori-negative but H. heilmannii-positive gastric MALT lymphomas. These patients were treated with 40 mg omeprazole and 750 mg amoxicillin 3 times per day for 14 d, which is a similar treatment to H. pylori eradication, and the eradication of this bacterium resulted in CR in all 5 patients. H. heilmannii infection causes gastric B-cell lymphomas in mice[25], hence gastric infection by this bacterium may be a cause of H. pylori-negative gastric MALT lymphomas. Nonetheless, bacteria that have not yet been identified may be involved in this MALT lymphomagenesis, and further studies are warranted to clarify the details.
Another possibility is the involvement of autoimmune diseases. Sjögren’s syndrome is associated with an increased risk for parotid gland MALT lymphomas[26], and a meta-analysis reported that the odds ratio of MALT lymphoma development for Sjögren’s syndrome was 18.8 (95%CI: 9.5-37.3)[27]. Hashimoto’s thyroiditis is also a known risk factor for thyroid lymphoma[28]. Therefore, we cannot deny the possibility that autoimmune diseases are also involved in gastric MALT lymphomagenesis, and further investigations are warranted.
Because the clinical symptoms of gastric MALT lymphomas are usually non-specific[29], gastric MALT lymphoma lesions are traditionally detected by screening esophagogastroduodenoscopy. Gastric MALT lymphoma lesions of H. pylori-negative subjects are similar to those observed in H. pylori-positive subjects[30]. Their endoscopic findings are classified as the superficial-spreading type, mass-forming type, diffuse infiltrating type and unclassified[31,32]. Many gastric MALT lymphoma lesions have similar endoscopic findings to early gastric cancers, but the discrimination between them is critical because the treatment for these diseases is different. Among these, the superficial-spreading type is most sensitive to antibiotic therapy[6].
The depth of the affected lesion is evaluated by endoscopic ultrasonography. Depth evaluation is important because gastric MALT lymphomas with deep submucosal invasions respond less to eradication therapy[33].
A definitive diagnosis for gastric MALT lymphomas is made by histopathology. Histological scoring of lymphoid infiltrations in the stomach according to Wotherspoon et al[34] has been broadly used for histopathological evaluation. It is important to perform an adequate number of biopsies from the lesions both for making an accurate diagnosis and ruling out the possibility of diffuse large B cell lymphomas, and Fischbach proposed that at least ten biopsies are required[35]. In addition to histopathology, immunostaining for B-cell markers can aid in diagnosis[36].
Genetic alterations that are observed in MALT lymphomas can be helpful for diagnosing the disease and for predicting the response to antibiotic therapy as mentioned below. The presence or the absence of these alterations can be diagnosed by reverse transcription polymerase chain reaction or by fluorescence in situ hybridization[36].
Clinical staging of gastric MALT lymphomas is defined according to either the Lugano international conference classification[37] or the modified Ann Arbor staging system[36]. The former is mainly based on radiological findings, whereas the latter takes the depth of gastric wall infiltration into consideration. Because depth of the affected lesion correlates with antibiotic therapy responsiveness[33], the latter may reflect the prognosis better than the former, but validation by prospective studies are warranted.
There are a number of methods for the diagnosis of H. pylori infection[38]. It is always important to combine different methods together, usually combining a non-invasive and an invasive method (e.g., urea breath test and histopathology), to exclude the possibility of a false-negative result. We must also be aware of the possibility of a pseudo-negative result in patients with extreme gastric mucosal atrophy and patients taking proton-pump inhibitors (PPI).
Recently, the usefulness of magnifying endoscopy in detecting gastrointestinal lesions has been given attention[39], and magnifying endoscopy with narrow-band imaging (MNBI) is proposed to be a useful technique to diagnose gastric MALT lymphomas[32,40,41]. Interestingly, Nonaka et al[41] reported that MNBI was useful in not only detecting gastric MALT lymphomas but also in evaluating their response to eradication therapy. This additional information is very helpful because it is often not easy to judge whether the patient should undergo second-line therapy after H. pylori eradication therapy in H. pylori-negative gastric MALT lymphomas.
All of the gastric MALT lymphoma patients are considered to have an indication for antibiotic treatment regardless of their clinical stages[36,42]. Standard antibiotic therapy consists of a combination of amoxicillin, clarithromycin and PPI[43]. According to previous reports, approximately 75% of H. pylori-positive gastric MALT lymphomas are successfully treated by the eradication of this bacterium[6,7]. However, due to the increasing drug resistance of H. pylori, many alternative therapies are proposed, such as the therapy adopting metronidazole instead of clarithromycin[44].
The first-line treatment for H. pylori-negative gastric MALT lymphomas is also antibiotic therapy[36]. Even in the absence of H. pylori infection, several studies have reported that certain proportions of patients responded to this antibiotic therapy[3,4,14,30,45-52] (Table 2).
| Responding patients/treated patients (responding rate) | |
| Steinbach et al[45] (1999) | 0/6 (0) |
| Ruskoné-Fourmestraux et al[30] (2001) | 0/10 (0) |
| Ye et al[14] (2003) | 0/5 (0) |
| Nakamura et al[3] (2006) | 2/7 (29) |
| Raderer et al[4] (2006) | 5/6 (83) |
| Akamatsu et al[46] (2006) | 1/9 (11) |
| Terai et al[47] (2008) | 1/4 (25) |
| Park et al[48] (2010) | 3/4 (75) |
| Asano et al[49] (2012) | 5/17 (29) |
| Choi et al[50] (2013) | 2/5 (40) |
| Raderer et al[52] (2015) | 5/13 (46) |
Raderer et al[4] reported that five out of six H. pylori-negative gastric MALT lymphoma patients responded to antibiotic therapy (one had partial remission and four had complete remission), which corresponds to an excellent response rate of 83%. This was the highest reported response rate among H. pylori-negative gastric MALT lymphomas. The response rate decreased to 46% in their later study[52], but this was still relatively high compared with other reports[3,46-50]. Contrary to these reports, earlier studies reported that H. pylori-negative gastric MALT lymphomas did not respond to antibiotic therapy[14,30,45]. One possible explanation for this discrepancy could be the insufficient time span between antibiotic therapy and the judgment of the treatment. For example, Ye et al[14] judged the antibiotic treatment as not effective at the median time of 7.5 mo (range: 4-12 mo). However, the median time span to reach complete remission after antibiotic therapy was 6 mo in our study, and we experienced a number of cases that took 24 mo or longer to reach remission[47]. Raderer et al[52] also noted that, although most of the patients responded to antibiotic therapy in 3-9 mo, they did experience a case that took 36 mo to achieve complete remission. Nonetheless, further studies are warranted to clarify the cause underlying this discrepancy.
Regarding the predictive factor for responsiveness, multiple lesions[49], lesions in both proximal and distal parts of the stomach[50], and the presence of t(11;18)(q21;q21)[50] were predictive factors for non-responsiveness to antibiotic therapy. We have previously reported that 5 out of 17 H. pylori-negative gastric MALT lymphoma patients treated with antibiotics and PPI responded to the therapy, and 3 out of 5 responders had single lesions, while all non-responders had multiple lesions[49]. Similarly, Choi et al[50] reported that non-responders had lesions in both proximal and distal parts of the stomach, although they did not distinguish patients’H. pylori infection status (2 out of 40 in responders, 4 out of 15 in non-responders). Regarding the t(11;18)(q21;q21) translocation, 2 out of 3 H. pylori-negative patients without this translocation were indicated to have responded to antibiotic therapy, while the remaining patient with this translocation did not[50]. Other studies also reported that gastric MALT lymphoma patients with t(11;18)(q21;q21) are more resistant to antibiotic therapy[6,12]. Because this translocation is more frequently observed in H pylori-negative gastric MALT lymphoma patients[12,14], this needs to be taken into consideration.
The reason why H. pylori-negative gastric MALT lymphomas respond to antibiotic therapy is not clear, but there are several possible speculations.
One speculation, as mentioned above, is that these patients were infected with bacteria other than H. pylori, and the antibiotic eradication therapy for H. pylori was able to eradicate this non-H. pylori bacteria[24].
Another explanation is that clarithromycin (included in the eradication medication) affected the patients’ immune system through its immunomodulatory effect[53,54]. Nevertheless, further studies are warranted to elucidate the mechanisms involved in this reaction.
Previously, when antibiotic therapy was considered ineffective, radiotherapy was used as the first-line therapy for H. pylori-negative gastric MALT lymphomas[55]. Gastric MALT lymphomas are sensitive to radiotherapy, and, according to Zullo et al[56], 97.8% of patients who were resistant to antibiotic therapy responded to radiotherapy. Chemotherapy efficiency for these patients has also been described[56], and a combination of anti-CD20 monoclonal antibodies with chemotherapy reportedly gives promising results[57,58].
However, currently there is no consensus for the patients who did not respond to antibiotic therapy. Because the disease is indolent by nature, and because complications are possible[59,60], a “watch-and-wait” strategy may be efficient for these patients if they do not have progressive disease[2,36,52].
Recent studies on H. pylori-negative gastric MALT lymphomas have suggested that H. pylori eradication therapy is effective in some proportion of patients with this disease and could be considered as a first-line treatment.
| 1. | Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410-1416. [PubMed] |
| 2. | Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol. 2013;19:8181-8187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Matsumoto T, Ye H, Nakamura S, Suekane H, Matsumoto H, Yao T, Tsuneyoshi M, Du MQ, Iida M. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 2006;107:2770-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Raderer M, Streubel B, Wöhrer S, Häfner M, Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut. 2006;55:616-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 5. | Lahner E, Milione M, Delle Fave G, Annibale B. H. pylori negative MALT lymphoma patients successfully treated with antibiotics: doubts about their H. pylori negativity. Gut. 2006;55:1669; author reply 1669. [PubMed] |
| 6. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 7. | Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014;27:27-33. [PubMed] |
| 8. | Greiner A, Knörr C, Qin Y, Sebald W, Schimpl A, Banchereau J, Müller-Hermelink HK. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol. 1997;150:1583-1593. [PubMed] |
| 9. | Craig VJ, Arnold I, Gerke C, Huynh MQ, Wündisch T, Neubauer A, Renner C, Falkow S, Müller A. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood. 2010;115:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Munari F, Lonardi S, Cassatella MA, Doglioni C, Cangi MG, Amedei A, Facchetti F, Eishi Y, Rugge M, Fassan M. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood. 2011;117:6612-6616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Sagaert X, De Wolf-Peeters C, Noels H, Baens M. The pathogenesis of MALT lymphomas: where do we stand? Leukemia. 2007;21:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Nakamura S, Ye H, Bacon CM, Goatly A, Liu H, Banham AH, Ventura R, Matsumoto T, Iida M, Ohji Y. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007;56:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Nakamura S, Matsumoto T, Nakamura S, Jo Y, Fujisawa K, Suekane H, Yao T, Tsuneyoshi M, Iida M. Chromosomal translocation t(11; 18)(q21; q21) in gastrointestinal mucosa associated lymphoid tissue lymphoma. J Clin Pathol. 2003;56:36-42. [PubMed] |
| 14. | Ye H, Liu H, Raderer M, Chott A, Ruskone-Fourmestraux A, Wotherspoon A, Dyer MJ, Chuang SS, Dogan A, Isaacson PG. High incidence of t(11; 18)(q21; q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101:2547-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Motegi M, Yonezumi M, Suzuki H, Suzuki R, Hosokawa Y, Hosaka S, Kodera Y, Morishima Y, Nakamura S, Seto M. API2-MALT1 chimeric transcripts involved in mucosa-associated lymphoid tissue type lymphoma predict heterogeneous products. Am J Pathol. 2000;156:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Akagi T, Motegi M, Tamura A, Suzuki R, Hosokawa Y, Suzuki H, Ota H, Nakamura S, Morishima Y, Taniwaki M. A novel gene, MALT1 at 18q21, is involved in t(11; 18) (q21; q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene. 1999;18:5785-5794. [PubMed] |
| 17. | Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11; 18)(q21; q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93:3601-3609. [PubMed] |
| 18. | Rosebeck S, Madden L, Jin X, Gu S, Apel IJ, Appert A, Hamoudi RA, Noels H, Sagaert X, Van Loo P. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science. 2011;331:468-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Zhou H, Du MQ, Dixit VM. Constitutive NF-kappaB activation by the t(11; 18)(q21; q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005;7:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Lucas PC, Kuffa P, Gu S, Kohrt D, Kim DS, Siu K, Jin X, Swenson J, McAllister-Lucas LM. A dual role for the API2 moiety in API2-MALT1-dependent NF-kappaB activation: heterotypic oligomerization and TRAF2 recruitment. Oncogene. 2007;26:5643-5654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Rosebeck S, Rehman AO, Apel IJ, Kohrt D, Appert A, O’Donnell MA, Ting AT, Du MQ, Baens M, Lucas PC. The API2-MALT1 fusion exploits TNFR pathway-associated RIP1 ubiquitination to promote oncogenic NF-κB signaling. Oncogene. 2014;33:2520-2530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 498] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 23. | Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10:1419-1432. [PubMed] |
| 24. | Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdörffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821-828. [PubMed] |
| 25. | O’Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J Pathol. 2004;203:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Nocturne G, Mariette X. Sjögren Syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol. 2015;168:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 545] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | Aozasa K. Hashimoto’s thyroiditis as a risk factor of thyroid lymphoma. Acta Pathol Jpn. 1990;40:459-468. [PubMed] |
| 29. | Hu C, Yi C, Dai X. Clinical study of 31 patients with primary gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2006;21:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, Molina T, Rambaud JL. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297-303. [PubMed] |
| 31. | Nakamura S, Yao T, Aoyagi K, Iida M, Fujishima M, Tsuneyoshi M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer. 1997;79:3-11. [PubMed] |
| 32. | Isomoto H, Matsushima K, Hayashi T, Imaizumi Y, Shiota J, Ishii H, Minami H, Ohnita K, Takeshima F, Shikuwa S. Endocytoscopic findings of lymphomas of the stomach. BMC Gastroenterol. 2013;13:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [PubMed] |
| 34. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [PubMed] |
| 35. | Fischbach W. Gastric MALT lymphoma - update on diagnosis and treatment. Best Pract Res Clin Gastroenterol. 2014;28:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 37. | Rohatiner A, d’Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [PubMed] |
| 38. | Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol. 2014;20:9299-9313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 39. | Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, Stoian M, Dobru D. Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc. 2015;7:110-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Nonaka K, Ishikawa K, Arai S, Nakao M, Shimizu M, Sakurai T, Nagata K, Nishimura M, Togawa O, Ochiai Y. A case of gastric mucosa-associated lymphoid tissue lymphoma in which magnified endoscopy with narrow band imaging was useful in the diagnosis. World J Gastrointest Endosc. 2012;4:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 41. | Nonaka K, Ohata K, Matsuhashi N, Shimizu M, Arai S, Hiejima Y, Kita H. Is narrow-band imaging useful for histological evaluation of gastric mucosa-associated lymphoid tissue lymphoma after treatment? Dig Endosc. 2014;26:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Fischbach W. MALT lymphoma: forget surgery? Dig Dis. 2013;31:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Zullo A, Hassan C, Ridola L, De Francesco V, Vaira D. Standard triple and sequential therapies for Helicobacter pylori eradication: an update. Eur J Intern Med. 2013;24:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Miftahussurur M, Yamaoka Y. Appropriate first-line regimens to combat Helicobacter pylori antibiotic resistance: an Asian perspective. Molecules. 2015;20:6068-6092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Steinbach G, Ford R, Glober G, Sample D, Hagemeister FB, Lynch PM, McLaughlin PW, Rodriguez MA, Romaguera JE, Sarris AH. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88-95. [PubMed] |
| 46. | Akamatsu T, Mochizuki T, Okiyama Y, Matsumoto A, Miyabayashi H, Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Terai S, Iijima K, Kato K, Dairaku N, Suzuki T, Yoshida M, Koike T, Kitagawa Y, Imatani A, Sekine H. Long-term outcomes of gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. Tohoku J Exp Med. 2008;214:79-87. [PubMed] |
| 48. | Park HS, Kim YJ, Yang WI, Suh CO, Lee YC. Treatment outcome of localized Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J Gastroenterol. 2010;16:2158-2162. [PubMed] |
| 49. | Asano N, Iijima K, Terai S, Jin X, Ara N, Chiba T, Fushiya J, Koike T, Imatani A, Shimosegawa T. Eradication therapy is effective for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. Tohoku J Exp Med. 2012;228:223-227. [PubMed] |
| 50. | Choi YJ, Kim N, Paik JH, Kim JM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH. Characteristics of Helicobacter pylori-positive and Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma and their influence on clinical outcome. Helicobacter. 2013;18:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Zullo A, Hassan C, Ridola L, De Francesco V, Rossi L, Tomao S, Vaira D, Genta RM. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. J Clin Gastroenterol. 2013;47:824-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Raderer M, Wöhrer S, Kiesewetter B, Dolak W, Lagler H, Wotherspoon A, Muellauer L, Chott A. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol. 2015;94:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Govi S, Dognini GP, Licata G, Crocchiolo R, Resti AG, Ponzoni M, Ferreri AJ. Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: final results of a single-centre phase II trial. Br J Haematol. 2010;150:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Kiesewetter B, Raderer M. Antibiotic therapy in nongastrointestinal MALT lymphoma: a review of the literature. Blood. 2013;122:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Kim SW, Lim do H, Ahn YC, Kim WS, Kim SJ, Ko YH, Kim KM. Clinical outcomes of radiation therapy for early-stage gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol. 2013;19:6062-6068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Zullo A, Hassan C, Andriani A, Cristofari F, Bassanelli C, Spinelli GP, Tomao S, Morini S. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: a pooled-data analysis. Med Oncol. 2010;27:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Zucca E, Conconi A, Laszlo D, López-Guillermo A, Bouabdallah R, Coiffier B, Sebban C, Jardin F, Vitolo U, Morschhauser F. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol. 2013;31:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Raderer M, Wohrer S, Streubel B, Drach J, Jager U, Turetschek K, Troch M, Puspok A, Zielinski CC, Chott A. Activity of rituximab plus cyclophosphamide, doxorubicin/mitoxantrone, vincristine and prednisone in patients with relapsed MALT lymphoma. Oncology. 2006;70:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Otsuka T, Noda T, Yokoo M, Ibaraki K. Recurrent gastric perforation as a late complication of radiotherapy for mucosa-associated lymphoid tissue lymphoma of the stomach. Intern Med. 2008;47:1407-1409. [PubMed] |
| 60. | Asano N, Iijima K, Terai S, Uno K, Endo H, Koike T, Iwai W, Iwabuchi T, Hatta W, Abe Y. Signet Ring Cell Gastric Cancer Occurring after Radiation Therapy for Helicobacter pylori-Uninfected Mucosa-Associated Lymphoid Tissue Lymphoma. Case Rep Gastroenterol. 2011;5:325-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abadi ATB, Biernat MM, Kuo CH, Leja M S- Editor: Wang JL L- Editor: A E- Editor: Liu XM