Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7225
Peer-review started: January 6, 2015
First decision: February 10, 2015
Revised: March 13, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: June 21, 2015
Processing time: 165 Days and 17.6 Hours
AIM: To compare the clinicopathological features of patients with non-schistosomal rectosigmoid cancer and schistosomal rectosigmoid cancer.
METHODS: All the patients with rectosigmoid carcinoma who underwent laparoscopic radical surgical resection in the Shanghai Minimally Invasive Surgical Center at Ruijin Hospital affiliated to Shanghai Jiao-Tong University between October 2009 and October 2013 were included in this study. Twenty-six cases of colonic schistosomiasis diagnosed through colonoscopy and pathological examinations were collected. Symptoms, endoscopic findings and clinicopathological characteristics were evaluated retrospectively.
RESULTS: There were no significant differences between patients with and without schistosomiasis in gender, age, CEA, CA19-9, preoperative biopsy findings or postoperative pathology. Patients with rectosigmoid schistosomiasis had a significantly higher CA-125 level and a larger proportion of these patients were at an early tumor stage (P = 0.003). Various morphological characteristics of schistosomiasis combined with rectosigmoid cancer could be found by colonoscopic examination: 46% were fungating mass polyps, 23% were congestive and ulcerative polyps, 23% were cauliflower-like masses, 8% were annular masses. Only 27% of the patients were diagnosed with rectal carcinoma preoperatively after the biopsy. Computed tomography (CT) scans showed thickened intestinal walls combined with linear and tram-track calcifications in 26 patients.
CONCLUSION: Rectosigmoid carcinoma combined with schistosomiasis is associated with higher CA-125 values and early tumor stages. CA-125 and CT scans have a reasonable sensitivity for the accurate diagnosis.
Core tip: The association between schistosomiasis and colorectal malignancy has long been suggested in the literature. This study aimed to improve our understanding of the relationship between Schistosoma japonicum-related enteropathy and rectosigmoid carcinoma, with a particular focus on laboratory examination, endoscopic findings and clinicopathological characteristics of rectosigmoid schistosomiasis.
- Citation: Feng H, Lu AG, Zhao XW, Han DP, Zhao JK, Shi L, Schiergens TS, Lee SM, Zhang WP, Thasler WE. Comparison of non-schistosomal rectosigmoid cancer and schistosomal rectosigmoid cancer. World J Gastroenterol 2015; 21(23): 7225-7232
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7225
Human schistosomiasis is a prevalent parasitic disease caused by trematode flukes of the genus Schistosoma, of which Schistosoma mansoni, Schistosoma japonicum (S. japonicum) and Schistosoma haematobium are the three major species. By conservative estimates, at least 230 million people worldwide are infected with Schistosoma spp, and it is important to acknowledge that schistosomiasis is now becoming a cause for concern in Europe, especially in southern Europe, because of climate change as well as infected travelers who return from endemic areas[1]. A number of epidemiological data has suggested a close etiological relationship between colorectal cancer and schistosomiasis, especially S. japonica[2,3]. The microenvironmental changes and inflammation may form a causal link between schistosome chronic infection and colorectal carcinogenesis[4,5]. However, as the symptoms of colonic schistosomiasis are nonspecific and may mimic other gastrointestinal problems, this condition could be under diagnosed[6], and there is little relevant clinical data in the medical literature, mostly limited to case reports[7-9]. On the other hand, in some schistosoma-endemic areas, colonic schistosomiasis can be correctly diagnosed while colorectal cancer may be missed, especially when CEA or CA19-9 levels are within the normal range.
Detailed knowledge about schistosomiasis is necessary to improve the accuracy of clinical diagnosis. At present, colorectal neoplasia associated with schistosoma has only been reported on few occasions.
This research was conducted retrospectively, based on the recent data of schistosomal rectosigmoid cancer, including surgical findings and clinicopathological characteristics, to find a sensitive biomarker that might improve the accuracy of clinical diagnosis, and discuss the probable etiological role of chronic schistosomal infestation in rectosigmoid cancer.
In this study, retrospective analysis was conducted for 26 consecutive cases in patients diagnosed with rectosigmoid carcinoma combined with colonic schistosomiasis between 01-10-2009 and 01-11-2013, who underwent surgical resection at the Shanghai Minimally Invasive Surgical Center of Ruijin Hospital, which is affiliated to Shanghai Jiao-Tong University. Those patients were admitted to hospital because of liquid or pasty diarrhea, abdominal pain, pain on colon palpation or hematochezia. Colonoscopies were performed in the outpatient service of our department 1-14 d before hospitalization. Two to three biopsies were obtained and sent to the department of pathology. CT scans, magnetic resonance imaging and laboratory examinations were performed in the outpatient service or on the first day of hospitalization. Patients who were diagnosed with rectosigmoid carcinoma in the same time period without schistosomiasis were selected as a control group. After surgical resection, all specimens were reviewed histopathologically, and the pathological TNM stages were determined according to the classification established by the American Joint Committee on Cancer (AJCC, 7th edition). The gold standard for diagnosis of schistosomiasis depends on finding ova by microscopy in the colon, rectum or stool.
Abdominal ultrasonography, laboratory profiles, urine and stool tests were acquired after being admitted to hospital. Data on clinicopathological characteristics and treatments were collected routinely from the hospital records by trained registrars.
Analyses were performed using Stat View 5.0 for Windows (SAS Institute Inc., Cary, NC, United States). The χ2 test or Fisher’s exact test were applied to analyze the categorical variables. The results were subjected to a nonparametric Mann-Whitney U test. A Student’s t-test was also used to analyze the intragroup differences. P < 0.05 was regarded as statistically significant. The statistical methods of this study were reviewed by Yi-Fei Zhang from the Institute for Stroke and Dementia Research Hospital of the University of Munich.
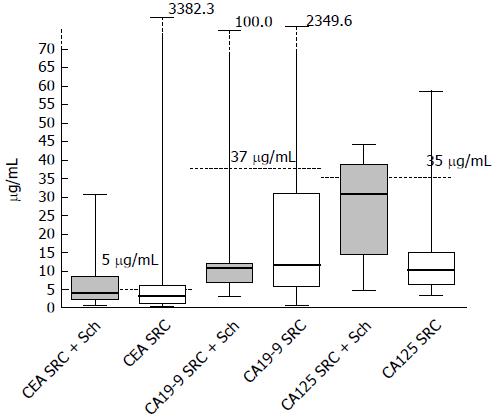
In this study, 26 patients were diagnosed with sigmoid or rectal carcinoma combined with rectosigmoid schistosomiasis. Of these patients, 69.2% were male, while 31% out of the patients were female. 12 patients (46%) had elevated CEA values (> 5 μg/mL) and 47% patients (7/15) had abnormal CA-125 values (> 35 U/mL). The distribution of these biomarkers is shown in Figure 1. It worth noting that only 1 patient (9%, 1/11) in this series had an abnormal CA19-9 value (> 37.0 U/mL). In addition, there were no significant differences in gender, age, CEA value, CA19-9 value or findings from preoperative biopsy when these two groups were compared, based on characteristics and colonoscopic findings. Instead, patients with rectosigmoid schistosomiasis had significantly higher CA-125 values than those without (P = 0.0001) (Table 1). 85% of the patients who were diagnosed with sigmoid or rectal carcinoma combined with rectosigmoid schistosomiasis were at early tumor stages (stage I or stage II), compared to 47% of patients without schistosomiasis (P = 0.003).
| With schistosomiasis | Without schistosomiasis | ||
| (n = 26) | (n = 34) | P value | |
| Gender male/female | 18/8 | 22/12 | |
| Age (yr) | 60.7 ± 10.6 | 63 ± 8.7 | 0.6100 |
| CEA | 4.2 ± 8.8 | 82.9 ± 428.1 | 0.3800 |
| CA19-9 | 10.9 ± 306.5 | 35.1 ± 65.1 | 0.1300 |
| CA-125 | 27.4 ± 3.3 | 12.7 ± 10.0 | 0.0001 |
| Preoperative biopsy | |||
| Carcinoma | 7 (26.9) | 16 (47.1) | |
| Hyperplastic polyps | 5 (19.2) | 8 (23.5) | |
| Villous adenoma | 3 (11.5) | 1 (2.9) | |
| Tubular adenoma | 3 (11.5) | 1 (2.9) | 0.3400 |
| Others | 8 (30.7) | 8 (23.5) | |
| Morphology | |||
| Congestive, Ulcerative | 6 (23.1) | 13 (38.2) | |
| Fungating mass | 12 (46.2) | 8 (23.5) | 0.1600 |
| Cauliflower-like mass | 6 (23.1) | 6 (17.6) | |
| Annular | 2 (7.6) | 7 (20.6) | |
| Tumor stage | |||
| I | 16 (61.5) | 6 (17.6) | |
| II | 6 (23.1) | 10 (29.4) | 0.0030 |
| III | 4 (15.4) | 17 (50) | |
| IV | 0 | 1 (2.9) | |
| Differentiation | |||
| Well | 16 (61.5) | 15 (44.1) | |
| Moderate | 7 (26.9) | 16 (47.1) | 0.4000 |
| Poor | 3 (11.5) | 3 (8.8) | |
| Postoperative pathology | |||
| Adenocarcinoma | 16 (61.5) | 30 (88.2) | |
| Signet-ring cell carcinoma | 7 (7.7) | 0 | 0.1300 |
| Mucinous adenocarcinoma | 3 (30.8) | 4 (11.8) |
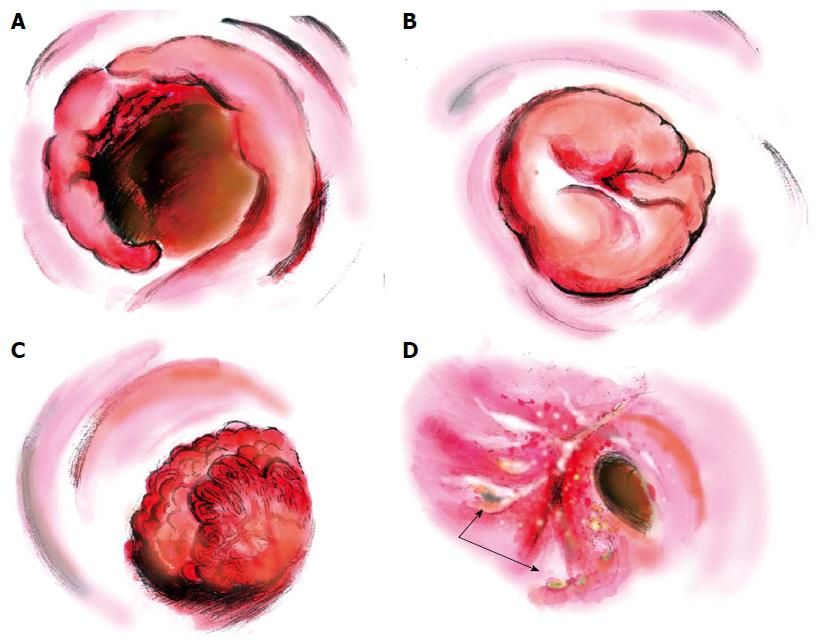
Various morphological characteristics of schistosomiasis combined with rectosigmoid cancer were found in colonoscopic examinations (Figure 2). The fungating mass polyp was the major morphological type, being present in around 46% of all 26 patients. For the remaining patients, six (23%) had congestive and ulcerative polyps, six (23%) had cauliflower-like masses and two (8%) were annular type. Preoperative rectosigmoid biopsy provides an efficient but insensitive way of visualizing eggs, especially for those with low worm burdens. In this study, only seven patients (27%) were diagnosed with rectosigmoid carcinoma preoperatively; 19% of the biopsies showed hyperplastic polyps, and 8% and 23% revealed intraepithelial neoplastic changes (Table 1).
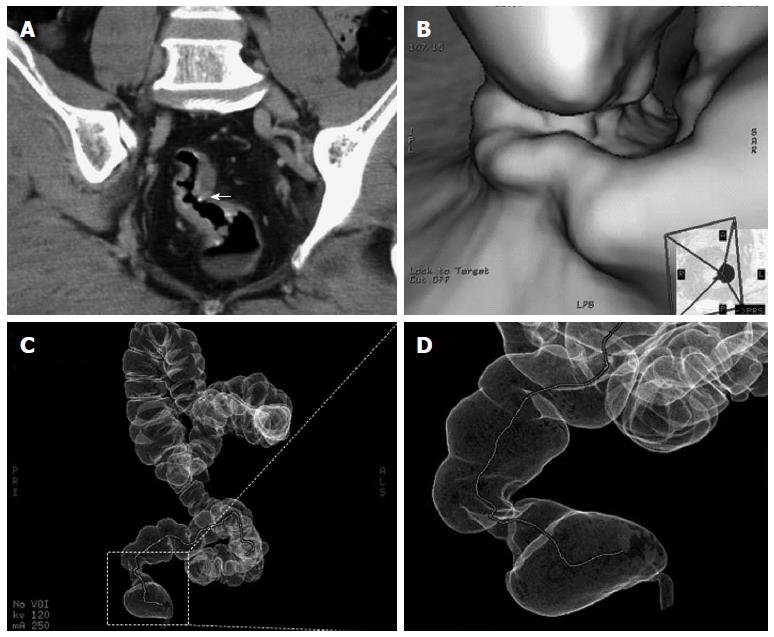
Abdominal CT enhanced dynamic scans (CTA) demonstrated evenly thickened intestinal walls combined with linear and tram-track calcifications in 26 patients, with (8%) or without (92%) perirectal fatty infiltration, the rectal lumen were locally narrowed in the primary lesions. Calcified ova could be found in 22 patients (85%). No significant lymphadenopathy was demonstrated. For those who had intestinal stenosis and for whom colonoscopic examinations were not recommended, virtual colonoscopy and virtual dissection were used to assess the condition and situation (Figure 3B-D).
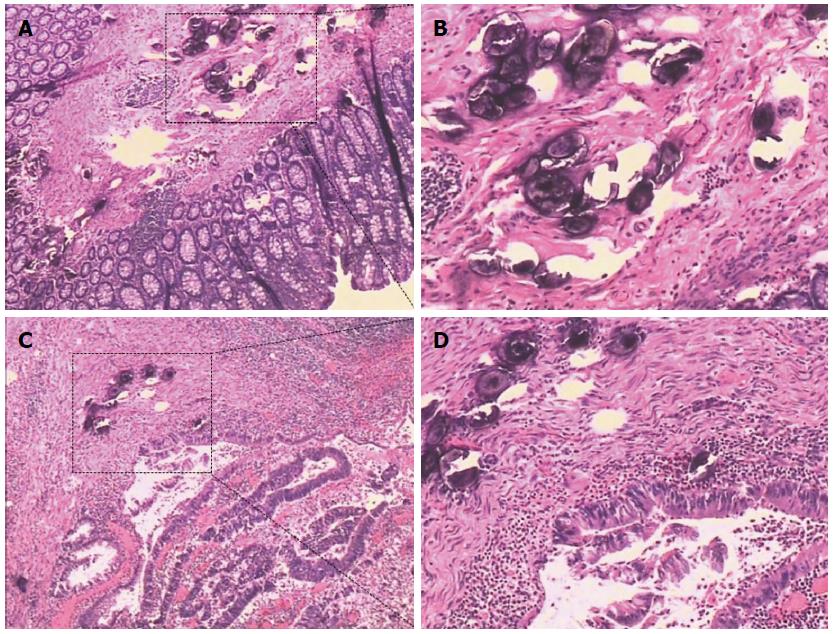
Irregular thickening of the intestinal wall was found in 25 patients (96%) during operations or postoperative sample assessments. Most of the patients were at Stage I (62%), and 23% and 15% were at Stage II and Stage III, respectively. In 18 patients (69%), schistosomal ova were only found in the submucosal layer; in 19% the ova had infiltrated muscularis propria, and serosal infiltrations were found in 12% of the patients. In 8% of the patients, schistosomal ova could be found infiltrating into the surrounding lymph nodes postoperatively. In 21 patients (81%), schistosomal ova could be found inside the tumor, while ova from the remaining 19% of patients were found in the adjacent tissues (Figure 4). Considering the pathological profiles, the largest percentage had well differentiated tumors and adenocarcinoma observed in postoperative pathological examination. The information on signet-ring cell carcinomas (8%) and mucinous adenocarcinomas (31%) were also included in the present study (Table 2).
| Male, n | Female, n | Total | |
| Schistosomal ova position | |||
| Submucosa infiltration | 12 | 6 | 18 (69.2) |
| Muscularis propria | 4 | 1 | 5 (19.2) |
| Serosal infiltration | 2 | 1 | 3 (11.5) |
| Infiltration in the sLNs | 1 | 1 | 2 |
| Intra-tumor tissue | 14 | 7 | 21 (80.8) |
| Para-tumor tissue | 4 | 1 | 5 (19.2) |
| Calcification ova (CT) | 15 | 7 | 22 (84.6) |
| Irregular thickening of the intestinal wall | 17 | 8 | 25 (96.2) |
| Rough serosal surface | 2 | 1 | 3 (11.5) |
Although schistosomiasis has been controlled in endemic regions[10] in the tropics and subtropics, previous S. japonicum infection might lead to complications, such as chronic intestinal schistosomiasis and hepatosplenic schistosomiasis, and this condition is significantly associated with both liver cancer and colorectal cancer[11]. Schistosome infection may have a negative effect on the prognosis of colorectal cancer[12]; it has been reported that the five-year survival rate was 45.6% out of 430 cases complicated with schistosomiasis, which was significantly lower than in those without schistosomiasis (50.9% out of 2717)[13]. In the present study, schistosomal rectosigmoid cancer seemed to be related to early tumor stage, possibly because schistosomiasis-related intestinal damages are mainly granuloma and fibrosis resulting from schistosomal ova deposition, especially in the large intestine[14-16]. Continuous epithelial proliferation adjacent to a chronic schistosomal ulcer and polyp formation, which lead to more obvious symptoms, might encourage the patients to seek a medical examination earlier. Wang et al[17] analyzed 30 patients with schistosomal rectal cancer and showed that schistosomiasis (P = 0.026) was statistically significantly correlated with overall survival (OS). Schistosomiasis was an independent prognostic factor for worse DFS and OS in multivariate analysis[17].
In cases of intestinal cancer associated with schistosomiasis, the location of the cancer was predominately the rectum[18], followed by the sigmoid colon, and then the other parts of the colon, while small intestinal cancer with a relatively lower distribution of S. japonicum eggs is quite rare[4,19]. Fan et al[20] analyzed 285 pathological specimens with colorectal cancer from surgical operations in an endemic area for schistosomiasis and found that cancer in the rectum and sigmoid colon accounted for 44% and 27% in the 220 cases of cancer combined with schistosomiasis, respectively. In those patients without schistosomiasis, the comparative figure was 23% and 18%, respectively, with a significant difference[20]. Peripheral blood tumor marker IL-2, TNF-2 and CEA might be elevated in those patients[21]. Yu et al[22] divided schistosomal egg induced polyps into three types: fibrous type, mixed type and epithelial proliferative type; CEA and PNA receptors were present in 18/20 (90%) and 6/18 of epithelial proliferative type, respectively. In the present study, 46% and 47% patients had elevated CEA or CA-125, whilst few patients had abnormal CA19-9. On the other hand, after statistical analysis, schistosomal rectosigmoid carcinoma was only associated with a higher CA-125 level.
Colonoscopy provides valuable information for the diagnosis of colonic schistosomiasis[23]. Liu et al[3] systematically described morphological types of schistosomal colorectal cancer (endophytic/ulcerative, exophytic/fungating, annular, giant polyp and IIc), and found that the ulcerative type were the most common cases. However, in this study, which focused on rectosigmoid cancers, fungating masses seemed form the majority of the cases. The endoscopic findings of schistosomal rectosigmoid cancer were non-specific.
Considering the diagnoses of carcinoma, only 27% of the patients were diagnosed with rectal carcinomas preoperatively, 19% of the biopsies showed hyperplastic polyps, and 8% and 23% revealed low or high grade intraepithelial neoplastic changes, respectively. Considering the diagnosis of colonic schistosomiasis: if schistosoma ova are not observed in biopsies, the near-normal crypts with excess mucus and diffuse or focal infiltration of eosinophilic granulocytes may be highly suggestive of colonic schistosomiasis[24]. Therefore, multisite biopsies are recommended to improve the accuracy of diagnosis. Ye et al[25] analyzed clinical and endoscopic manifestations for 96 patients, and found that epidemiological investigations and colonoscopic examinations combined with multi-block and multi-site biopsies may improve the rate of correct diagnosis of intestinal schistosomiasis.
Recent technological advances have significantly enhanced the role of imaging in the detection, characterization, and management of infectious diseases involving the large intestine. Lee et al[26] reported that CT demonstrated calcifications resembling tram tracks in the sigmoid colon and postulated that the tram-track appearance is noted only in the distal large intestine because this portion of the colon has a thicker, muscular layer than the proximal colon. Irregular thickening of the intestinal wall, soft tissue masses, multiple S. japonicum ova calcifications inside the tumor with obscured margins and multiple intestinal masses in some patients, are important CT features of CRC with schistosomiasis. Zhang et al[27] compared the CT presentation and pathological characteristics and found that the intestinal wall was irregularly thickened in 95% of the patients, with soft tissue masses in 5% patients. Linear, spotty and small patchy calcifications were seen in 104 (80%) patients, with 96 out of 130 patients having ill-defined margins[27]. In support of this view, in our study, CT scan and CT virtual colonography have a reasonable sensitivity and specificity for detecting these lesions. CT allowed the visualization of evenly thickened intestinal wall combined with linear and tram-track calcifications in all 26 patients.
Recent studies have also thrown some light on the molecular events associated with schistosomal colorectal cancer. Ruan et al[28] found that the expressions of vascular growth factors including PD-ECGF and VEGF are higher in the colorectal carcinoma patients with schistosomiasis than in those without. Zalata et al[29] found that signet ring cell carcinoma and mucinous adenocarcinoma both exhibited intense c-myc expression compared with non-mucinous carcinoma (P = 0.001). When adjusting for S. mansoni infection, 58% of schistosomal colorectal cancer cases were Bcl-2 positive compared with only 33% of non-schistosomal colorectal cancers (P = 0.046). They also suggested that the genotoxic agents produced endogenously through the course of Schistosomiasis mansoni infection may play a role in CRC- Schistosoma mansoni pathogenesis through the dysregulation of apoptosis by the alteration of the expression pattern of Bcl-2 protein[29].
A recent study showed that the prognosis of patients with schistosomal rectal cancer is worse than those with non-schistosomal rectal cancer; therefore, a diagnosis of schistosomiasis might be necessary[17]. In the present research, CA-125 levels and CT scans have a sufficient sensitivity to diagnose rectosigmoid carcinoma combined with schistosomiasis.
Colorectal cancer coexisting with schistosomiasis is a typical schistosomiasis-related intestinal damage, especially in the sigmoid colon and rectum.
Epidemiological data have suggested that a close relationship exists between colorectal cancer and schistosomiasis, especially when infected with Schistosoma japonicum. However, there have been little available data regarding the role of Schistosoma japonicum in rectosigmoid carcinoma.
The authors compared the clinicopathological features of patients with non-schistosomal rectosigmoid cancer or schistosomal rectosigmoid cancer, and analyzed the laboratory examinations, endoscopic findings and clinicopathological characteristics between those two groups. There were no significant differences in CEA values, CA19-9 values or findings from preoperative biopsies. However, patients with rectosigmoid schistosomiasis had significantly higher CA-125 values.
According to this study, rectosigmoid carcinoma combined with schistosomiasis might be associated with higher CA-125 values and early stages; CA-125 and computed tomography (CT) scans have a sufficient sensitivity for accurate diagnosis.
Endoscopy and CT scans contribute to the diagnosis of schistosomal rectosigmoid carcinoma, although they are nonspecific. A correct diagnosis of schistosomal rectosigmoid carcinoma can be established by endoscopy as well as CT scans in combination with its clinicopathological characteristics and laboratory tests (CA-125).
The manuscript written by Feng et al retrospectively evaluated the endoscopic findings and clinicopathological characteristics of schistosomal rectosigmoid cancer. The findings are important and provide novel information for the management of patients with rectosigmoid carcinoma, as well as schistosomiasis. However, there are some concerns that need to be addressed.
| 1. | Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 1793] [Article Influence: 149.4] [Reference Citation Analysis (7)] |
| 2. | Chen MG. Assessment of morbidity due to Schistosoma japonicum infection in China. Infect Dis Poverty. 2014;3:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Liu W, Zeng HZ, Wang QM, Yi H, Mou Y, Wu CC, Hu B, Tang CW. Schistosomiasis combined with colorectal carcinoma diagnosed based on endoscopic findings and clinicopathological characteristics: a report on 32 cases. Asian Pac J Cancer Prev. 2013;14:4839-4842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1471] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 5. | Konishi T, Watanabe T, Shibahara J, Nagawa H. Surveillance colonoscopy should be conducted in patients with colorectal Shistosomiasis even after successful treatment of the disease. Int J Immunopathol Pharmacol. 2006;19:245-246. [PubMed] |
| 6. | Mohamed AR, al Karawi M, Yasawy MI. Schistosomal colonic disease. Gut. 1990;31:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Botes SN, Ibirogba SB, McCallum AD, Kahn D. Schistosoma prevalence in appendicitis. World J Surg. 2015;39:1080-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Li WC, Pan ZG, Sun YH. Sigmoid colonic carcinoma associated with deposited ova of Schistosoma japonicum: a case report. World J Gastroenterol. 2006;12:6077-6079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Issa I, Osman M, Aftimos G. Schistosomiasis manifesting as a colon polyp: a case report. J Med Case Rep. 2014;8:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1559] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 11. | Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Chen MC, Chang PY, Chuang CY, Chen YJ, Wang FP, Tang YC, Chou SC. Colorectal cancer and schistosomiasis. Lancet. 1981;1:971-973. [PubMed] |
| 13. | Schistosomiasis and its prognostic significance in patients with colorectal cancer. National Cooperative Group on Pathology and Prognosis of Colorectal Cancer. Zhonghua Zhongliu Zazhi. 1986;8:149-151. [PubMed] |
| 14. | Madbouly KM, Senagore AJ, Mukerjee A, Hussien AM, Shehata MA, Navine P, Delaney CP, Fazio VW. Colorectal cancer in a population with endemic Schistosoma mansoni: is this an at-risk population? Int J Colorectal Dis. 2007;22:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Singh KP, Gerard HC, Hudson AP, Boros DL. Differential expression of collagen, MMP, TIMP and fibrogenic-cytokine genes in the granulomatous colon of Schistosoma mansoni-infected mice. Ann Trop Med Parasitol. 2006;100:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Boros DL. Polarization of the immune response to the single immunodominant epitope of p38, a major Schistosoma mansoni egg antigen, generates Th1- or Th2-type cytokines and granulomas. Infect Immun. 1999;67:4570-4577. [PubMed] |
| 17. | Wang M, Zhang YC, Yang XY, Wang ZQ. Prognostic analysis of schistosomal rectal cancer. Asian Pac J Cancer Prev. 2014;15:9271-9275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 19. | Hao WG, Wang XM, Yi WQ, Gao YP. [Analysis of lesions in 498 schistosomiasis patients]. Zhongguo Xuexichongbing Fangzhi Zazhi. 2013;25:428, 432. [PubMed] |
| 20. | Fan XL, Cheng HJ. Discussion on the relationship between schistosomiasis and colorectal cancer – histopathological analysis of 285 cases. Zhongguo Xuexichongbing Fangzhi Zazhi. 1992;3:62. |
| 21. | Hamed MA, Ahmed SA, Khaled HM. Efficiency of diagnostic biomarkers among colonic schistosomiasis Egyptian patients. Mem Inst Oswaldo Cruz. 2011;106:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Yu XR, Chen PH, Xu JY, Xiao S, Shan ZJ, Zhu SJ. Histological classification of schistosomal egg induced polyps of colon and their clinical significance. An analysis of 272 cases. Chin Med J (Engl). 1991;104:64-70. [PubMed] |
| 23. | Cao J, Liu WJ, Xu XY, Zou XP. Endoscopic findings and clinicopathologic characteristics of colonic schistosomiasis: a report of 46 cases. World J Gastroenterol. 2010;16:723-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Yosry A. Schistosomiasis and neoplasia. Contrib Microbiol. 2006;13:81-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ye C, Tan S, Jiang L, Li M, Sun P, Shen L, Luo H. Endoscopic characteristics and causes of misdiagnosis of intestinal schistosomiasis. Mol Med Rep. 2013;8:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Lee RC, Chiang JH, Chou YH, Rubesin SE, Wu HP, Jeng WC, Hsu CC, Tiu CM, Chang T. Intestinal schistosomiasis japonica: CT-pathologic correlation. Radiology. 1994;193:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Zhang W, Wang PJ, Shen X, Wang GL, Zhao XH, Seema SF, Zheng SQ, Li MH. CT presentations of colorectal cancer with chronic schistosomiasis: A comparative study with pathological findings. Eur J Radiol. 2012;81:e835-e843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Ruan SL, Wang B, Lu QM, Dong LR, Cao CX, Xu SL, Shen WY. [Expression of vascular growth factors in intestinal tissues in colorectal carcinoma patients with schistosomiasis japonica]. Zhongguo Xuexichongbing Fangzhi Zazhi. 2013;25:250-254. [PubMed] |
| 29. | Zalata KR, Nasif WA, Ming SC, Lotfy M, Nada NA, El-Hak NG, Leech SH. p53, Bcl-2 and C-Myc expressions in colorectal carcinoma associated with schistosomiasis in Egypt. Cell Oncol. 2005;27:245-253. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Correa P, Mura B, Wexner SD S- Editor: Qi Y L- Editor: Stewart G E- Editor: Liu XM