Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7155
Peer-review started: January 4, 2015
First decision: January 22, 2015
Revised: February 13, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: June 21, 2015
Processing time: 168 Days and 0.9 Hours
AIM: To evaluate the protective effect of bicyclol against bile duct ligation (BDL)-induced hepatic fibrosis in rats.
METHODS: Sprague-Dawley male rats underwent BDL and sham-operated animals were used as healthy controls. The BDL rats were divided into two groups which received sterilized PBS or bicyclol (100 mg/kg per day) orally for two consecutive weeks. Serum, urine and bile were collected for biochemical determinations. Liver tissues were collected for histological analysis and a whole genome oligonucleotide microarray assay. Reverse transcription-polymerase chain reaction and Western blotting were used to verify the expression of liver fibrosis-related genes.
RESULTS: Treatment with bicyclol significantly reduced liver fibrosis and bile duct proliferation after BDL. The levels of alanine aminotransferase (127.7 ± 72.3 vs 230.4 ± 69.6, P < 0.05) and aspartate aminotransferase (696.8 ± 232.6 vs 1032.6 ± 165.8, P < 0.05) were also decreased by treatment with bicyclol in comparison to PBS. The expression changes of 45 fibrogenic genes and several fibrogenesis-related pathways were reversed by bicyclol in the microarray assay. Bicyclol significantly reduced liver mRNA and/or protein expression levels of collagen 1a1, matrix metalloproteinase 2, tumor necrosis factor, tissue inhibitors of metalloproteinases 2, transforming growth factor-β1 and α-smooth muscle actin.
CONCLUSION: Bicyclol significantly attenuates BDL-induced liver fibrosis by reversing fibrogenic gene expression. These findings suggest that bicyclol might be an effective anti-fibrotic drug for the treatment of cholestatic liver disease.
Core tip: Cholestasis often fails to respond to medical therapy, resulting in liver necrosis, fibrosis, cirrhosis and subsequent liver failure. Bicyclol has significant liver protective effects but little is known about the effect of this drug on cholestatic liver fibrosis. Therefore, the present study was designed to investigate the protective effects of bicyclol in a rat model of liver fibrosis induced by bile duct ligation (BDL). Bicyclol can improve liver function after BDL and decrease bile duct proliferation, inflammation and fibrosis. Bicyclol might be an effective anti-fibrotic drug for the treatment of cholestatic liver disease.
- Citation: Zhen YZ, Li NR, He HW, Zhao SS, Zhang GL, Hao XF, Shao RG. Protective effect of bicyclol against bile duct ligation-induced hepatic fibrosis in rats. World J Gastroenterol 2015; 21(23): 7155-7164
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7155
Biliary obstruction is characterized by portal tract expansion, bile duct proliferation, hepatic stellate cell activation, extracellular matrix accumulation, liver fibrosis and subsequent cirrhosis. Cirrhosis, which is the end stage of progressive fibrosis, is a major health problem worldwide and is characterized by the accumulation of extracellular matrix proteins and the distortion of the hepatic architecture. Current evidence suggests that the process of hepatic fibrosis is driven by a complex network of cytokines[1-4]. In particular, the fibrogenic cytokine transforming growth factor-beta (TGF-β) is central to the development of cirrhosis due to its stimulating effect on matrix protein generation and its inhibitory effect on matrix protein removal. TGF-β plays a pivotal role in the development of hepatic fibrosis and cirrhosis[3,5-7]; therefore, the inhibition of TGF-β has been used in several therapeutic approaches to prevent hepatic fibrosis. However, no clinically effective treatment for liver fibrosis is available and an effective therapeutic approach that blocks the development of hepatic fibrosis is needed.
Although much has been learned in recent decades about the molecular basis of cholestasis and the pathophysiology of hepatic fibrosis, the mechanisms that underlie the development of cholestatic liver disease have not been well elucidated and new therapeutic approaches have been limited[8]. Few effective treatments for cholestasis are currently approved by the Food and Drug Administration; these treatments include ursodeoxycholic acid (UDCA) which achieves limited benefits during the early stages of primary biliary cirrhosis. Alternative therapies, including the combination of UDCA with immunomodulatory drugs, have been attempted but have not been successful in limited clinical trials[9]. Therefore, new therapeutic agents are needed for these chronic cholestatic diseases[10,11].
Bicyclol (4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxy-2-hydroxymethyl-2′-carbonyl biphenyl) is a synthetic drug that was developed by Chinese scientists and is widely used in the clinic to treat patients with chronic hepatitis B and C[12-14]. Pharmacologically, bicyclol exhibits anti-hepatitis virus activity in duck viral hepatitis and in the 2.2.15 cell line. Furthermore, bicyclol also exhibits a protective effect against liver injury induced by hepatotoxins in mice and rats[13,15-17]. Recent studies demonstrated that bicyclol markedly attenuates experimental liver fibrosis induced by chemical toxins, including carbon tetrachloride (CCl4) and dimethylnitrosamine, in mice and significantly protects against chemotherapeutic agent-induced liver damage in elderly patients with cancer[18-20]. These results suggest that bicyclol has a chemopreventive effect on liver injury and fibrogenesis. Therefore, the present study was designed to investigate the effect of bicyclol on cholestatic liver fibrosis induced by bile duct ligation (BDL) and the possible mechanisms that underlie this effect in rats.
Bicyclol was purchased from Beijing Union Pharmaceutical Factory (Beijing, China). Antibodies that target TGF-β1/2 were purchased from Epitomics (Burlingame, CA). Antibodies specific for α-smooth muscle actin (α-SMA) and GAPDH were purchased from Sigma-Aldrich (St. Louis, MO). The TaqMan real-time polymerase chain reaction (RT-PCR) master mix was purchased from Roche (Indianapolis, IN). ABI TaqMan primer/probes were purchased from Applied Biosystems (Foster City, CA).
Male adult Sprague-Dawley rats weighing 180-220 g were purchased from the Institute of Laboratory Animal Science (Chinese Academy of Medical Sciences, Beijing, China). The rats were maintained in a temperature-controlled room (22 ± 2 °C) with a 12 h light/dark cycle and a relative humidity of 40%-60%. The rats were given access to food and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Hebei United University and the animal protocol was designed to minimize the pain and discomfort of the animals. For experiments using BDL, the rats were randomly divided into three groups of six animals. Each day, six animals underwent BDL and three sham-operated animals were used as a healthy control. Twenty-four hours after surgery, the BDL animals were randomly assigned to receive a daily gavage consisting of PBS (i.e., the treatment control and solvent) or 100 mg/kg of bicyclol suspended in PBS for 14 d. The sham controls also received PBS by gavage. All BDL rats received 0.1 mL of vitamin K (100 μg/mL) every other day via subcutaneous injection. The body weight was measured daily. The animals were sacrificed in random order between 9:00 am and 11:00 am after an overnight fast. Samples of serum, bile (from bile duct cysts in BDL animals), urine (the urine of each rat was collected during an overnight fast using a metabolic cage) and liver were collected for further analyses, as described previously[21].
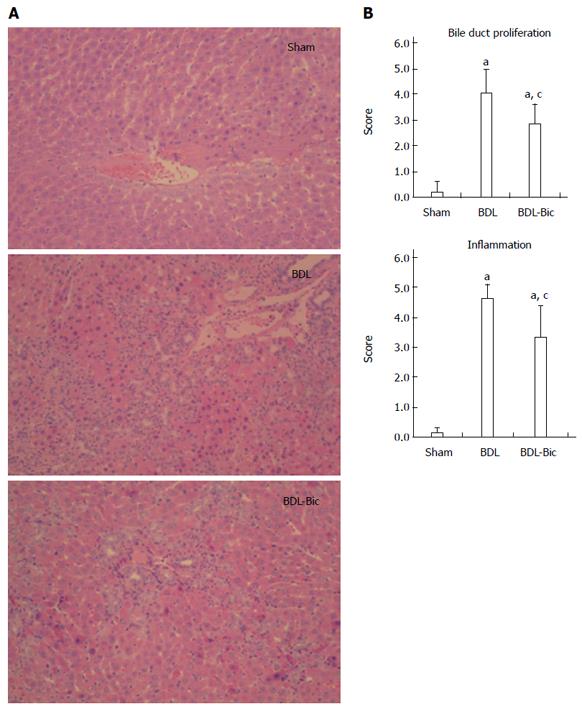
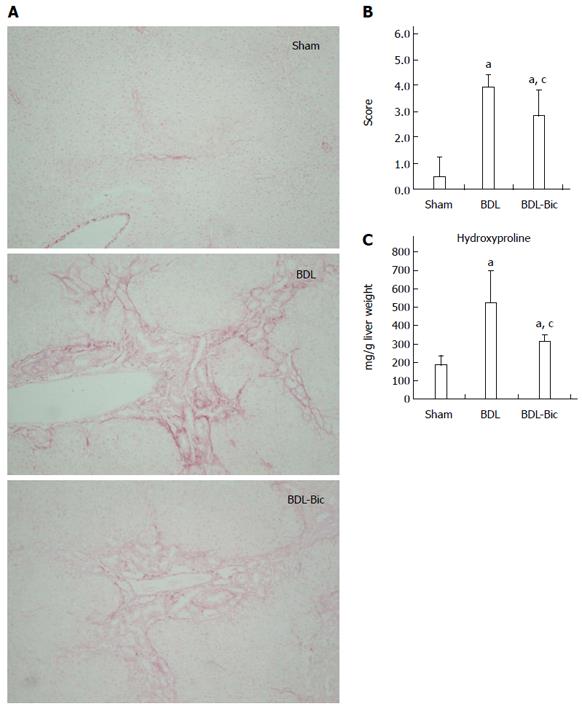
Serum alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol, total bile acid and total bilirubin of serum, bile and urine were analyzed using a Hitachi 7170 chemistry analyzer and kits from Zhongsheng Beikong Biotechnology (Beijing, China). Formalin-fixed liver tissue was embedded in paraffin and sections were stained with hematoxylin and eosin and Sirius red. Histological evaluation of the liver sections was performed by a single pathologist who assessed for bile duct proliferation, inflammation and fibrosis on a 1 to 4+ scale in a blinded manner. Hydroxyproline was evaluated using a kit from Nanjing Jiancheng Company (Nanjing, China) according to the manufacturer’s instructions.
Total RNA was isolated from the liver tissues using the TRIzol reagent (Invitrogen) and purified using the NucleoSpin RNA clean-up kit (Macherey-Nagel, Germany). An RNA sample for each group was obtained by mixing the same amount of total RNA from each animal in the group. The samples were then labeled with Cy3 and Cy5 during the reverse transcription process using Cy3/Cy5 labeling kits (Genesphere Inc., Hatfield, PA) according to the manufacturer’s instructions. The labeled DNA was hybridized with the microarrays (Phanlanx, Taiwan) overnight at 45 °C. After hybridization and subsequent washing, the arrays were analyzed using a LuxScan 10K/A dual channel laser scanner (CapitalBio, Beijing). The data were normalized using the Lowess method and only those genes that exhibited a consistent alteration tendency (both ≥ 1.5-fold) in both microarrays were selected as differentially expressed genes. Fibrogenesis-related genes were selected from the differentially expressed genes (Ratio ≥ 1.5) by searching in PubMed using the gene description/symbol and liver fibrosis as keywords.
Total RNA from each animal was extracted from the liver tissue using TRIzol (Invitrogen, Carlsbad, CA, United States) and purified using the NucleoSpin RNA clean-up kit (Macherey-Nagel, Duren, Germany). cDNA was generated using the AffinityScript multiple temperature cDNA synthesis kit (Agilent Technologies, Santa Clara, CA) and the relative expression of specific genes was determined using the TaqMan real-time PCR master mix (Roche) with TaqMan probe/primer mixes (ABI) in an ABI 7500 Fast Real-Time PCR System. The Gapdh gene was used as an endogenous control to normalize for differences in the amount of total RNA present in the samples. All of the animals were assayed. Calculations were then made and statistical analysis was performed between the groups.
The homogenized liver tissues were lysed in RIPA buffer (50 mmol/L Tris-HCl, pH 7.5, 1% NP-40, 0 mmol/L NaCl, 1 mg/mL of aprotinin, 1 mg/mL of leupeptin, 1 mmol/L Na3VO4 and 1 mmol/L NaF) for 30 min at 4 °C. Cell debris was removed by centrifugation at 12000 ×g for 20 min at 4 °C. Protein concentrations were determined using the BCA assay. Equal amounts of lysate were resolved via SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore, Bedford, MA). The membranes were blocked with 5% nonfat milk in TBS-T buffer at room temperature for 1 h and then incubated for 2 h or overnight with primary antibodies. After three 10 minute washes with 0.1% TBS-T buffer, the membranes were incubated for 1 h at room temperature with a species-specific horseradish-peroxidase-linked secondary antibody (Cell Signaling Technology). After three washes with 0.1% TBS-T buffer, the membranes were soaked in enzyme-linked chemiluminescence detection reagent (Millipore) and protein bands were visualized using the Fluorchem HD2 imaging system (Protein simple, Santa Clara, CA).
The data are presented as the mean ± SD. The significance of differences between experimental groups was assessed using the two-tailed Student’s t-test. The value of statistical significance was set at P < 0.05.
The results of the serum, urine and bile biochemistry are shown in Table 1. The activities of ALT and AST in the sham-operated rats remained within control values. Serum levels of ALT and AST were increased by approximately eight- and ten-fold, respectively, after bile duct ligation in comparison to the values of sham-operated rats. A 100 mg/kg dose of bicyclol in BDL rats significantly reduced these values.
| Sham-PBS(n = 6) | BDL-PBS(n = 6) | BDL-Bic100(n = 6) | |
| Serum ALT (U/L) | 29.3 ± 3.8 | 230.4 ± 69.6a | 127.7 ± 72.3a,c |
| Serum AST (U/L) | 98.3 ± 18.1 | 1032.6 ± 165.8a | 696.8 ± 232.6a,c |
| Serum total bilirubin (μmol/L) | 0.1 ± 0.2 | 161.5 ± 19.2a | 152.4 ± 41.8a |
| Serum total cholesterol (mmol/L) | 1.5 ± 0.5 | 3.0 ± 0.6a | 3.1 ± 0.7a |
| Serum LDL (mmol/L) | 0.2 ± 0.0 | 1.5 ± 0.3a | 1.8 ± 0.3a |
| Serum HDL (mmol/L) | 0.9 ± 0.2 | 0.3 ± 0.2a | 0.3 ± 0.1a |
| Serum total bile acid (μmol/L) | 8.0 ± 3.0 | 173.0 ± 38.0a | 172.6 ± 39.1a |
| Urine total bile acid (μmol/L) | 3.4 ± 1.5 | 247.2 ± 30.9a | 246.5 ± 59.6a |
| Urine total bilirubin (μmol/L) | 0.3 ± 0.7 | 100.4 ± 27.5a | 105.8 ± 58.0a |
| Bile total bile acid (μmol/L) | Not applicable | 3183.5 ± 908.6 | 3261.0 ± 488.8 |
| Bile total bilirubin (μmol/L) | Not applicable | 96.3 ± 35.9 | 126.3 ± 46.9 |
The concentration of serum total cholesterol and LDL, total bilirubin and total bile acid in the serum,bile and urine were significantly increased after 2 wk of BDL. In addition, the concentration of HDL in the serum was reduced. These changes are classical markers of cholestasis in BDL animals. Unexpectedly, pharmacological treatment with bicyclol failed to change the levels of these markers (Table 1).
After hematoxylin and eosin staining, the histological structure of the liver was observed to be normal in the sham group, as shown in Figure 1A. Portal inflammation, parenchymal necrosis and ductal proliferation, including bridging of the portal tracts, were observed in the BDL group. Portal inflammation and ductal proliferation were markedly reduced in the bicyclol-treated group compared to the BDL group. A blinded assessment also revealed significantly lower scores for bile duct proliferation and inflammation in the bicyclol-treated groups compared to the BDL group (Figure 1B).
After Sirius red staining, liver damage was visualized based on the hyperplasia of the lattice fibers and collagenous fibers in the portal area; this damage extended outwards in BDL group rats. These liver histopathological changes were improved in bicyclol-treated rats (Figure 2A). A blinded assessment also revealed significantly lower scores for the level of fibrosis in the bicyclol-treated groups compared to the BDL group. This finding was further confirmed using a hydroxyproline assay (Figure 2B and C).
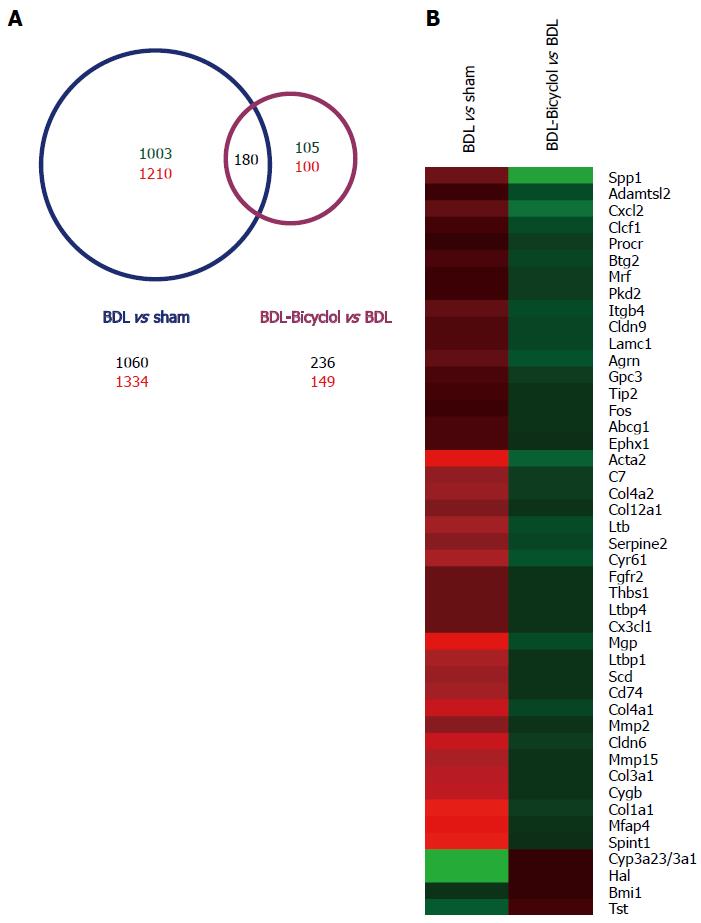
To analyze genome-wide alterations in gene expression, total RNA was extracted from sham, BDL and bicyclol-treated group rat livers for whole genome microarray experiments. For our analysis, a 1.5-fold alteration in the signal intensity was regarded as a significant change in mRNA expression. We found that 1060 genes were down-regulated and 1334 genes were up-regulated in BDL rat livers compared with sham rat livers. In addition, 236 genes were down-regulated and 149 genes were up-regulated in bicyclol-treated rat livers compared with BDL rat livers (Figure 3A). We found that a total of 180 genes were included in both sets of significantly changed genes. In addition to analyzing the effects of bicyclol treatment, we performed a gene functional enrichment analysis using the DAVID Bioinformatics Resources[22,23] to further characterize those 180 genes. The results demonstrated that these genes are related to ECM-receptor interactions, fatty acid metabolism, the PPAR signaling pathway, focal adhesion, the biosynthesis of unsaturated fatty acids, oxidation/reduction and the integrin signaling pathway, among other processes (Table 2). We also observed that among those 180 genes, 45 genes were reported to be associated with liver fibrosis, including Spp1, collagen 1a1 (Col1a1), matrix metalloproteinase 2 (Mmp2), α-SMOOTH MUSCLE ACTIN (α-Sma, Acta2) and other genes (Figure 3B).
| Pathway | Relate genes | P value |
| ECM-receptor interaction | Lamc1, Col4a2, Spp1, Col3a1, Col1a1, Lamb2, Col4a1, Agrn, Itgb4, Thbs1 | 0.0000003 |
| Fatty acid metabolism | Acadsb, Ehhadh, Cyp4a3/4a2, Acsl1, Cpt1a, Acaa1b | 0.00012 |
| PPAR signaling pathway | Ehhadh, Scd, Cyp4a3/4a2, Acsl1, Cpt1a, Acaa1b | 0.00017 |
| Focal adhesion | Lamc1, Col4a2, Spp1, Col3a1, Col1a1, Lamb2, Col4a1, Itgb4, Thbs1 | 0.00192 |
| Biosynthesis of unsaturated fatty acids | Scd1, Acot2, Scd, Acaa1b | 0.00267 |
| Oxidation reduction | Acadsb, Dhcr24, Gpx3, Scd, Rdh2, Akr1b1, Cyp4a3/4a2, Steap4, Cyp3a23/3a1, Loxl1, Ehhadh, Scd1, Aldh1a2, Hsd17b6 | 0.00575 |
| Integrin signaling pathway | Lamc1, Col4a2, Col3a1, Col1a1, Lamb2, Col4a1, Itgb4, Col12a1 | 0.00793 |
| Retinol metabolism | Cyp3a23/3a1, Ugt2a3, Cyp4a3/4a2, Aldh1a2 | 0.03196 |
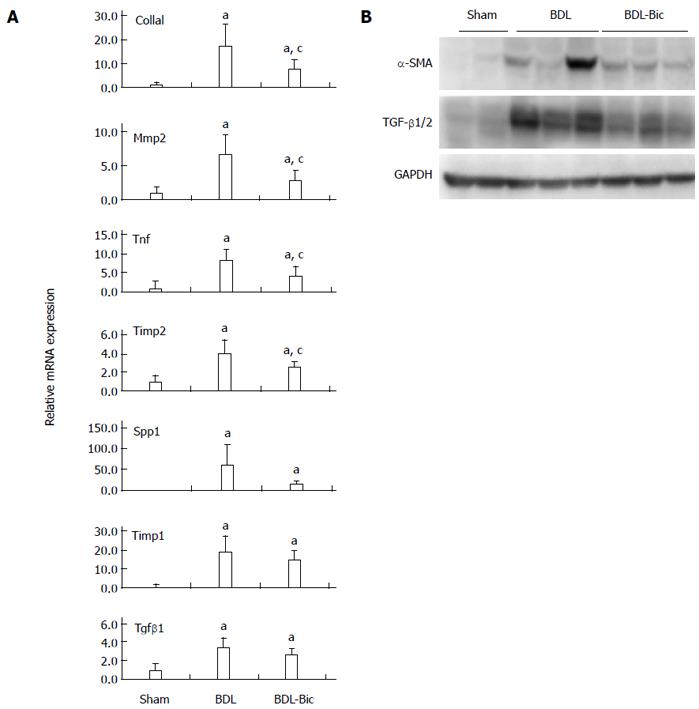
To verify the anti-fibrotic effects of bicyclol and the microarray data, some fibrogenic genes were assayed using TaqMan real-time PCR and/or Western blot. Our results demonstrated that bicyclol significantly reduces liver mRNA expression of Col1a1, Mmp2, tumor necrosis factor (Tnf) and tissue inhibitors of metalloproteinases 2 (Timp2). Bicyclol also reduced the mRNA levels of Spp1, Timp1 and Tgf-β1, but these differences failed to reach statistical significance (Figure 4A). Based on Western blots, the levels of TGF-β1/2 and α-SMA were increased in the BDL group and decreased in the bicyclol-treated groups (Figure 4B).
Cholestasis is caused by dysfunction of the hepatobiliary system which results from either a functional defect in bile formation or an impairment in bile secretion and flow, leading to the intracellular accumulation of bilirubin, toxic bile salts and cholesterol in the blood causing hepatocellular injury[24]. Cholestasis has been shown to stimulate hepatic stellate cells to express collagen, leading to the accumulation of extracellular matrix protein and progressive fibrogenesis. Chronic cholestasis eventually results in liver fibrosis, cirrhosis and subsequent liver failure[25].
In this report, we assessed the therapeutic effects of bicyclol in a BDL cholestatic rat model. Our results demonstrated that bicyclol significantly protects against BDL-induced liver injury and fibrosis, as evidenced by the reduction of elevated serum AST and ALT activities and the reduction of liver inflammation, fibrosis and bile duct proliferation.
The presence of cirrhosis indicates a perturbation of liver homeostasis and the intracellular release of cytokines and signaling molecules occurs during the development of cirrhosis. The mechanisms that underlie the development of cholestatic liver disease are not well understood. Current evidence suggests that the process of hepatic fibrosis is driven by a complex network of cytokines[26-30]. TGF-β1 was demonstrated to play an important role in the progression of liver fibrosis[31,32]. The release of TGF-β1 may be one of the first signals to activate quiescent hepatic stellate cells (HSCs), resulting in the transdifferentiation of these cells into proliferative, fibrogenic and contractile myofibroblasts. Activated HSCs, which express myogenic markers such as α-SMA, are considered to be central extracellular matrix (ECM)-producing cells within the injured liver. Concomitant with the increased activity of TGF-β that occurs during liver fibrogenesis, HSCs increase the production and deposition of collagen, leading to progressive scarring and the loss of organ function[33-37]. Thus, a reduction in TGF-β indicates a reduction in liver damage and the down-regulation of α-SMA indicates the inactivation of HSCs. The results of our whole genome oligonucleotide microarray revealed that some fibrosis-related pathways are regulated by bicyclol. These pathways include ECM-receptor interactions, the PPAR signaling pathway, focal adhesion, oxidation/reduction, the integrin signaling pathway and other pathways. Interestingly, fatty acid metabolism and the biosynthesis of unsaturated fatty acids were also regulated in this assay; this finding indicates that bicyclol might improve fatty acid metabolism in some types of liver disease, such as non-alcoholic fatty liver disease. The microarray data also demonstrated that numerous fibrogenic genes are reversed by bicyclol; some of these genes are potential anti-fibrotic targets, including Spp1[38,39], Fgfr2[40,41] and others.
RT-PCR and Western blot verified that bicyclol significantly down-regulates the mRNA and/or protein expression of liver fibrogenic genes, such as Col1a1, Mmp2, Tnf, Timp2 and the protein level of TGF-β1 and α-SMA.
Although our study demonstrated that bicyclol has a protective effect against bile duct ligation (BDL)-induced hepatic injury in rats, pharmacological treatment with bicyclol has no effect on total bile acid and total bilirubin in the serum, urine and bile. Microarray assays also demonstrated that bicyclol failed to regulate the bile acid synthesis pathway and transport protein expression; these findings were consistent with the data obtained from biochemical assays that indicated that bicyclol represses liver fibrosis through fibrogenesis-related pathways or targets but not through bile acid-related pathway or targets. This drug has the potential to repress liver fibrogenesis in the context of most types of chronic liver injury.
In summary, we demonstrated that bicyclol treatment improves liver condition in vivo in BDL rats. We also reported the whole genome mRNA expression profile of bicyclol-treated rat livers, which was verified using Taqman real-time PCR and Western blot. These findings suggest that bicyclol might be a potential therapy for liver fibrosis in patients with chronic cholestatic disorders. Further research is needed to clarify the molecular targets and related pathways of bicyclol. Such studies might identify potential novel therapeutic targets for liver fibrosis.
Liver fibrosis is a histological hallmark of liver injury characterized by abnormal deposition of extracellular matrix with subsequent destruction of the normal liver architecture, which has become an important target for pre-cancer prevention and treatment of liver diseases. There are a variety of pathogens that can trigger liver fibrogenesis, including cholestasis, hepatic viral infection, nonalcoholic steatohepatitis and drug toxicity. Among these pathogens, cholestasis is the major cause of liver fibrosis in children and can be induced by bile duct ligation (BDL) in animal models. Current studies have shown that a synthetic drug bicyclol has effective anti-hepatitis virus activity and improves liver injury and fibrogenesis induced by hepatotoxins and chemical toxins. However, no previous studies have evaluated whether bicyclol has any effects on cholestatic liver fibrosis. In this report, we assessed the therapeutic effect of bicyclol after BDL and verified this effect.
Considerable progress has been made in the research of cholestatic liver diseases. Pharmacological therapy for cholestasis is limited and ursodeoxycholic acid is the only Food and Drug Administration approved medicine for disease-modifying therapy with evidence of efficacy. So, exploring and screening effective and reliable drugs and therapies is urgent.
No studies have previously evaluated the influence of bicyclol on cholestatic liver injury in BDL rats and the molecular targets or related pathways. This study is the first to investigate and confirm the protective effect of bicyclol after BDL. These results indicate that bicyclol is a potential anti-fibrotic drug. Furthermore, this study demonstrated the bicyclol attenuates BDL-induced liver injury by reversing fibrogenic gene expression but not bile acid synthesis and transport. This provides a guide for further exploring molecular targets and/or mechanisms that underlie this effect.
The study results suggest that bicyclol significantly protected against BDL-induced liver injury and fibrosis, as evidenced by the reduction of elevated serum aspartate transaminase and alanine transaminase activities and reducing liver inflammation, necrosis and bile duct proliferation. The chemopreventive effect of bicyclol is by repressing fibrogenesis-related markers but it failed to regulate bile acid metabolism. It implies that bicyclol might be a potential anti-fibrotic drug for treatment of cholestatic liver disease.
BDL is an established animal model of cholestasis, with complete biliary obstruction and accumulation of multiple primary bile acids, bilirubin and circulating endotoxins, parallel to those that occur in humans with cholestasis. Chronic cholestasis can result in bile duct proliferation, liver fibrosis, cirrhosis and eventually liver failure. Therefore, BDL was used to screen and evaluate drugs for cholestatic liver diseases. Bicyclol is a synthetic drug and is widely used in the clinic to treat patients with chronic hepatitis B and C due to its anti-hepatitis virus activity. Besides, bicyclol exhibits a chemopreventive effect on liver injury and fibrogenesis induced by hepatotoxins and chemical toxins.
The authors evaluated the protective effect of bicyclol on bile duct ligation-induced hepatic fibrosis in rats. The authors report that 14 d post-BDL, rats treated with 100 mg/kg bicyclol had significantly less liver fibrosis and bile duct proliferation. Liver function was partially restored and liver fibrosis was reversed by down-regulation of fibrogenic genes assessed by real-time polymerase chain reaction and microarray assay. This report is well written, the experiments were well done and they corroborate the effect of bicyclol in a different animal model. Minor language polishing is needed.
| 1. | Nieto JC, Sánchez E, Román E, Vidal S, Oliva L, Guarner-Argente C, Poca M, Torras X, Juárez C, Guarner C. Cytokine production in patients with cirrhosis and TLR4 polymorphisms. World J Gastroenterol. 2014;20:17516-17524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Mitra A, Satelli A, Yan J, Xueqing X, Gagea M, Hunter CA, Mishra L, Li S. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology. 2014;60:2027-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Kim B, Park YK, Koo SI, Lee JY. Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochim Biophys Acta. 2015;1850:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 572] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 5. | Lee JH, Jang EJ, Seo HL, Ku SK, Lee JR, Shin SS, Park SD, Kim SC, Kim YW. Sauchinone attenuates liver fibrosis and hepatic stellate cell activation through TGF-β/Smad signaling pathway. Chem Biol Interact. 2014;224C:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Huang W, Li L, Tian X, Yan J, Yang X, Wang X, Liao G, Qiu G. Astragalus and Paeoniae radix rubra extract inhibits liver fibrosis by modulating the transforming growth factor-β/Smad pathway in rats. Mol Med Rep. 2015;11:805-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Hohenester S, Oude-Elferink RP, Beuers U. Primary biliary cirrhosis. Semin Immunopathol. 2009;31:283-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Silveira MG, Lindor KD. Treatment of primary biliary cirrhosis: therapy with choleretic and immunosuppressive agents. Clin Liver Dis. 2008;12:425-443; x-xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 853] [Article Influence: 53.3] [Reference Citation Analysis (2)] |
| 11. | Zein CO, Lindor KD. Latest and emerging therapies for primary biliary cirrhosis and primary sclerosing cholangitis. Curr Gastroenterol Rep. 2010;12:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 12. | Tang Y, Hu W, Li Y, Zhang CZ. [Synthesis of metabolites of bicyclol]. Yaoxue Xuebao. 2007;42:1054-1057. [PubMed] |
| 13. | Xie W, Shi GF, Zhang HF, Zhao GM, Yu ZJ, Lang ZW, Zhao H, Yan J, Cheng J. A randomized, multi-central, controlled study of patients with hepatitis B e antigen-positive chronic hepatitis B treated by adefovir dipivoxil or adefovir dipivoxil plus bicyclol. Hepatol Int. 2012;6:441-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Liu GT. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med Chem. 2009;5:29-43. [PubMed] |
| 15. | Li M, Liu GT. Inhibition of Fas/FasL mRNA expression and TNF-alpha release in concanavalin A-induced liver injury in mice by bicyclol. World J Gastroenterol. 2004;10:1775-1779. [PubMed] |
| 16. | Zhao D, Liu G. [Protective effect of bicyclol on concanavalin A-induced liver nuclear DNA injury in mice]. Zhonghua Yixue Zazhi. 2001;81:844-848. [PubMed] |
| 17. | Yu HY, Wang BL, Zhao J, Yao XM, Gu Y, Li Y. Protective effect of bicyclol on tetracycline-induced fatty liver in mice. Toxicology. 2009;261:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 18. | Hu QW, Liu GT. Effects of bicyclol on dimethylnitrosamine-induced liver fibrosis in mice and its mechanism of action. Life Sci. 2006;79:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Gu Y, Zhao J, Yao XM, Li Y. Effects of bicyclol on immunological liver fibrosis in rats. J Asian Nat Prod Res. 2010;12:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Li XY, Zhou JF, Chen SC, Guan M, Wang YY, Zhao L, Ying HY, Zhou YP. Role of bicyclol in preventing chemotherapeutic agent-induced liver injury in patients over 60 years of age with cancer. J Int Med Res. 2014;42:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | He H, Mennone A, Boyer JL, Cai SY. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology. 2011;53:548-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169-W175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1781] [Cited by in RCA: 1719] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 23. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24735] [Cited by in RCA: 28589] [Article Influence: 1681.7] [Reference Citation Analysis (0)] |
| 24. | Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Jüngst C, Berg T, Cheng J, Green RM, Jia J, Mason AL, Lammert F. Intrahepatic cholestasis in common chronic liver diseases. Eur J Clin Invest. 2013;43:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Pérez-Vargas JE, Zarco N, Shibayama M, Segovia J, Tsutsumi V, Muriel P. Hesperidin prevents liver fibrosis in rats by decreasing the expression of nuclear factor-κB, transforming growth factor-β and connective tissue growth factor. Pharmacology. 2014;94:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Li L, Wu T, Huang J, Ma K, Xu L, Wang H, Fan X, Tao R, Ai G, Ning Q. Expression of heat shock protein 47, transforming growth factor-beta 1, and connective tissue growth factor in liver tissue of patients with Schistosoma japonicum-induced hepatic fibrosis. Parasitology. 2015;142:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153-159. [PubMed] |
| 29. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 30. | Chen N, Geng Q, Zheng J, He S, Huo X, Sun X. Suppression of the TGF-β/Smad signaling pathway and inhibition of hepatic stellate cell proliferation play a role in the hepatoprotective effects of curcumin against alcohol-induced hepatic fibrosis. Int J Mol Med. 2014;34:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1870] [Article Influence: 85.0] [Reference Citation Analysis (1)] |
| 32. | Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 625] [Article Influence: 41.7] [Reference Citation Analysis (2)] |
| 33. | Duval F, Moreno-Cuevas JE, González-Garza MT, Rodríguez-Montalvo C, Cruz-Vega DE. Liver fibrosis and protection mechanisms action of medicinal plants targeting apoptosis of hepatocytes and hepatic stellate cells. Adv Pharmacol Sci. 2014;2014:373295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Fang L, Huang C, Meng X, Wu B, Ma T, Liu X, Zhu Q, Zhan S, Li J. TGF-β1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-β1/Smad pathway. Toxicol Appl Pharmacol. 2014;280:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clermont PJ, Diehl AM. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 750] [Article Influence: 50.0] [Reference Citation Analysis (1)] |
| 37. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4209] [Article Influence: 200.4] [Reference Citation Analysis (10)] |
| 38. | Whitington PF, Malladi P, Melin-Aldana H, Azzam R, Mack CL, Sahai A. Expression of osteopontin correlates with portal biliary proliferation and fibrosis in biliary atresia. Pediatr Res. 2005;57:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC, Rosenbaum J, Desmoulière A. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Böhm F, Speicher T, Hellerbrand C, Dickson C, Partanen JM, Ornitz DM, Werner S. FGF receptors 1 and 2 control chemically induced injury and compound detoxification in regenerating livers of mice. Gastroenterology. 2010;139:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 41. | Nakamura I, Zakharia K, Banini BA, Mikhail DS, Kim TH, Yang JD, Moser CD, Shaleh HM, Thornburgh SR, Walters I. Brivanib attenuates hepatic fibrosis in vivo and stellate cell activation in vitro by inhibition of FGF, VEGF and PDGF signaling. PLoS One. 2014;9:e92273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Atta H, Gorrell MD, Panduro A S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Liu XM