Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6974
Peer-review started: September 21, 2014
First decision: October 19, 2014
Revised: December 3, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: June 14, 2015
Processing time: 270 Days and 18.6 Hours
AIM: To evaluate the diagnostic potential of Lugol’s chromoendoscopy-guided confocal laser endomicroscopy (CLE) in detecting superficial esophageal squamous cell neoplasia (ESCN).
METHODS: Between December 2008 and September 2010, a total of 52 patients were enrolled at the Chinese PLA General Hospital in Beijing, China. First, Lugol’s chromoendoscopy-guided CLE was performed in these patients and the CLE in vivo histological diagnosis was recorded. Then, chromoendoscopy-guided biopsy was performed in the same patients by another endoscopist who was blinded to the CLE findings. Based on the biopsy and CLE diagnosis, en bloc endoscopic resection was performed. The CLE in vivo diagnosis and the histological diagnosis of biopsy of ESCN were compared, using a histological examination of the endoscopic resection specimens as the standard reference.
RESULTS: A total of 152 chromoendoscopy-guided biopsies were obtained from 56 lesions. In the 56 lesions of 52 patients, a total of 679 CLE images were obtained vs 152 corresponding biopsies. The sensitivity, specificity, negative predictive value and positive predictive value of chromoendoscopy-guided CLE compared with biopsy were 95.7% vs 82% (P < 0.05), 90% vs 70% (P < 0.05), 81.8% vs 46.7% (P < 0.05), and 97.8% vs 92.7% (P > 0.05), respectively. There was a significant improvement in sensitivity, specificity, negative predictive value, and accuracy when comparing chromoendoscopy-guided CLE with biopsy.
CONCLUSION: Lugol’s chromoendoscopy-guided CLE is a real-time, non-invasive endoscopic diagnostic technology; the accuracy of the detection of superficial ESCN is equivalent to or may be superior to biopsy histology.
Core tip: The aim of the present study was to determine the diagnostic potential of confocal laser endomicroscopy (CLE) combined with Lugol’s iodine chromoendoscopy in detecting superficial esophageal squamous cell neoplasia (ESCN). Lugol’s chromoendoscopy-guided CLE was performed in 52 enrolled patients. In the same patients, chromoendoscopy-guided biopsy was performed by another endoscopist. A comparison of the detection rates between the CLE finding and biopsy was performed. The sensitivity, specificity, negative predictive value and positive predictive value of chromoendoscopy-guided CLE were 95.7%, 90%, 81.8% and 97.8%, respectively. There was a statistically significant difference in the detection of dysplasia between chromoendoscopy-guided CLE and biopsy. Lugol’s chromoendoscopy-guided CLE is a real-time, non-invasive endoscopic diagnostic technology; the accuracy of the detection of superficial ESCN is equivalent to or may be superior to biopsy histology.
- Citation: Huang J, Yang YS, Lu ZS, Wang SF, Yang J, Yuan J. Detection of superficial esophageal squamous cell neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. World J Gastroenterol 2015; 21(22): 6974-6981
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6974
Esophageal carcinoma is one of the most aggressive tumors and has a poor prognosis; it is the eighth leading cause of cancer-related death worldwide[1]. The morbidity and mortality rates associated with esophageal carcinoma are the highest in China. Squamous cell carcinoma and adenocarcinoma are the most common types of esophageal carcinoma. The former has always been the dominant histological type of esophageal cancer in China. Since most patients are diagnosed when the cancer has grown quite large or has spread to lymph nodes or other structures, the prognosis for esophageal squamous cell carcinoma (ESCC) is poor. Superficial esophageal squamous cell neoplasia (ESCN) is usually asymptomatic and curable if detected early, so early detection is the key to reducing the high mortality of ESCC.
Lugol’s iodine chromoendoscopy (LIC) is an easy and inexpensive method that improves the detection of dysplastic lesions of the esophagus. This simple technique is highly sensitive for identifying squamous lesions but has a low specificity and requires biopsy pathology to confirm the diagnosis[2,3].
Confocal laser endomicroscopy (CLE) is a new approach. This technique allows not only the observation of living cells and tissue but also of the vascular networks of the mucosal layer in the gastrointestinal tract during ongoing endoscopy[4]. With 1000-fold magnification ability, this technique enables the visualization of cells of the esophageal squamous epithelium and intraepithelial papillary capillary loops (IPCLs).
En bloc endoscopic resection (ER) is desirable for accurate histopathological assessment of tissue specimens obtained using endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). ER is well-recognized as the standard treatment for superficial ESCC in situ.
The purpose of this study was to determine the diagnostic potential of CLE combined with LIC in detecting superficial ESCN, using histological examination of en bloc ER specimens as the standard.
The inclusion criteria were patients aged 18-80 years whose endoscopic appearance of the esophageal lesion was type 0-II according to the Paris classification[5]. The exclusion criteria were patients who did not provide informed consent and were allergic to fluorescein, and those who had other co-existing cancers.
The study was performed as a single-center study at the Chinese PLA General Hospital in Beijing, China. The protocol was approved by the medical ethical committee of the Chinese PLA General Hospital and the clinical study was registered as a clinical trial (Registry No. NCT01156064 and No. NCT01378507).
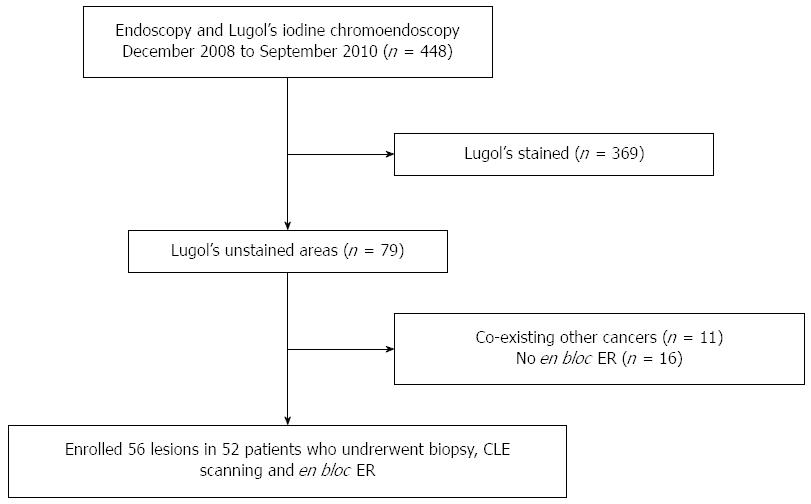
Between December 2008 and September 2010, 448 patients underwent upper gastrointestinal (GI) endoscopy and Lugol’s iodine test during endoscopy at the Department of Gastroenterology and Hepatology in our hospital. Areas unstained for Lugol’s iodine in the esophageal mucosa were revealed in 79 patients. CLE scanning for Lugol-voiding areas was carried out in 52 patients (Figure 1). A single endoscopist was engaged in the procedures of CLE combined with Lugol’s staining. The CLE endoscopist had gained experience with the CLE system for at least three months before the initiation of the study (Huang J). The CLE images were collected in real-time during the procedure, and the CLE in vivo histological diagnosis was recorded shortly after the procedure. Biopsy combined with Lugol’s staining chromoendoscopy (mainframe: CLV-260SL, endoscope: GIF-H260, Tokyo, Japan) was performed by another experienced endoscopist (Lu ZS) who was blinded to the CLE diagnosis. En bloc ER was performed in 52 patients.
The CLE system used was an integrated confocal laser endomicroscope (Pentax Co. Ltd., Mainframe: ISC-1000, endoscopy: EC-3878K, Tokyo, Japan) which combines a confocal laser microscope into the distal tip of a conventional video endoscope, thus enabling confocal microscopy in addition to standard video endoscopy. The scanning rate of the confocal images was 0.8 frames/s (1024 × 1024 pixels) or 1.6 frame/s (1024 × 512 pixels); the optical slice thickness of each scan was 4 μm, the resolution was 0.7 μm, and the maximum depth for observation was 250 μm. During the endoscopic examination, 5 mL of 10% fluorescein sodium was administered via intravenous injection, and the confocal laser scanning system was used to observe the blood vessels, cellular and subcellular structures of Lugol stained areas. CLE images were collected using a foot pedal. All lesions were diagnosed correctly by endomicroscopy in accordance with the criteria previously described[6]. The CLE in vivo histological diagnosis was identified as non-neoplasm or neoplasm.
Once the diagnosis of ESCN was confirmed by either CLE or biopsy, en bloc ER was performed by a single endoscopist (Lu ZS) who had gained experience with more than 50 ESD and 100 EMR of esophageal lesions before performing ESD on any patient in this study; the endoscopist controlled the single-channel endoscope (GIFQ260J; Olympus Optical Co, Ltd, Tokyo, Japan).
The transparent distal translucent cap EMR (EMR-C) method[7] was used to resect lesions < 1.5 cm. ESD was used to treat neoplastic lesions > 1.5 cm. The typical ESD procedure involved marking, incision, and submucosal dissection with simultaneous hemostasis. After making several marking dots outside the lesion, a saline solution containing epinephrine (0.01 mg/mL) and indigo carmine was injected into the submucosal layer. A circumferential incision was made in the mucosa using a needle-knife (KD-1L; Olympus Optical Co., Ltd., Tokyo, Japan) or an IT-knife (KD-610L; Olympus Optical Co., Ltd., Tokyo, Japan). The submucosal layer was dissected directly, mainly with the IT-knife, until complete removal was achieved. Endoscopic hemostasis was performed either with the knife or hemostatic forceps (FD-410LR; Olympus Optical Co., Ltd., Tokyo, Japan) whenever bleeding was noted. After dissection, preventive endoscopic hemostasis was performed for any oozing or exposed vessel. Only en bloc ER was included in order to completely capture the histopathologic examination.
Biopsies were obtained by standard biopsy forceps from the Lugol-voiding areas. The resected (EMR or ESD) specimen was stretched out and pinned to a soft board with the oral and anal orientations clearly marked, and then measured. The specimen was fixed in a 10% formaldehyde solution, continuously sectioned at 2 mm from the proximal end to the distal end, paraffin embedded, and stained with hematoxylin and eosin (HE). Before sectioning, one side of the specimen was marked with ink. Then, all slides were checked under the microscope from the side with the ink mark to the other side, to observe the histological type.
The biopsy and resected specimens were evaluated by an experienced GI pathologist (Wei LX). The histological diagnosis was identified as inflammation, low-grade intraepithelial neoplasia (LGIN), high-grade intraepithelial neoplasia (HGIN) or ESCC.
Experimental data were recorded and saved in Microsoft Excel 2003. All statistical analyses were performed using the SPSS 13.0 software for Windows (SPSS Inc., Chicago, IL, United States). For descriptive statistics, mean ± SD was used in the case of a normal distribution of variables. A Pearson χ2 test was applied to compare the sensitivity and specificity of chromoendoscopy-guided CLE and biopsy in the diagnosis of ESCN. A significant difference was assumed for P < 0.05.
A total of 56 lesions in 52 patients were enrolled for the study, including 16 females and 36 males (mean age, 60.5 years; range, 43-78 years). Five lesions were located in the cervical and upper thoracic esophagus, 40 in the middle thoracic esophagus, and 11 in the lower thoracic esophagus and abdominal esophagus. The mean size of the lesions was 2.3 cm (range, 1.0-6.0 cm).
Inflammation and LGIN by histologic diagnosis and non-neoplasm by CLE in vivo diagnosis classified the low-risk group, while HGIN or ESCC by histologic diagnosis and neoplasm by CLE comprised the high-risk group.
Of the 56 demarcated iodine-unstained areas that were studied, 42 lesions were endoscopically diagnosed as neoplasms before endoscopic biopsy and CLE. Among these 42 lesions, 41 were en bloc resection specimens histologically confirmed to be neoplasms; one lesion was confirmed to be inflammation. Among the 14 lesions diagnosed as non-neoplasms, nine were histologically confirmed to be non-neoplasms and five were confirmed to be neoplasms. The sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) of chromoendoscopy were 89.1%, 90%, 64.3% and 97.6%, respectively.
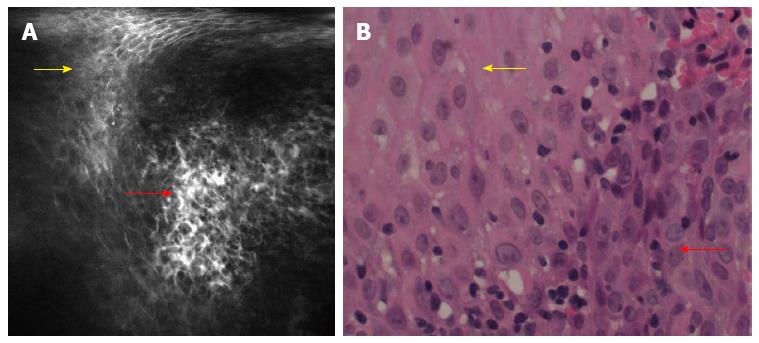
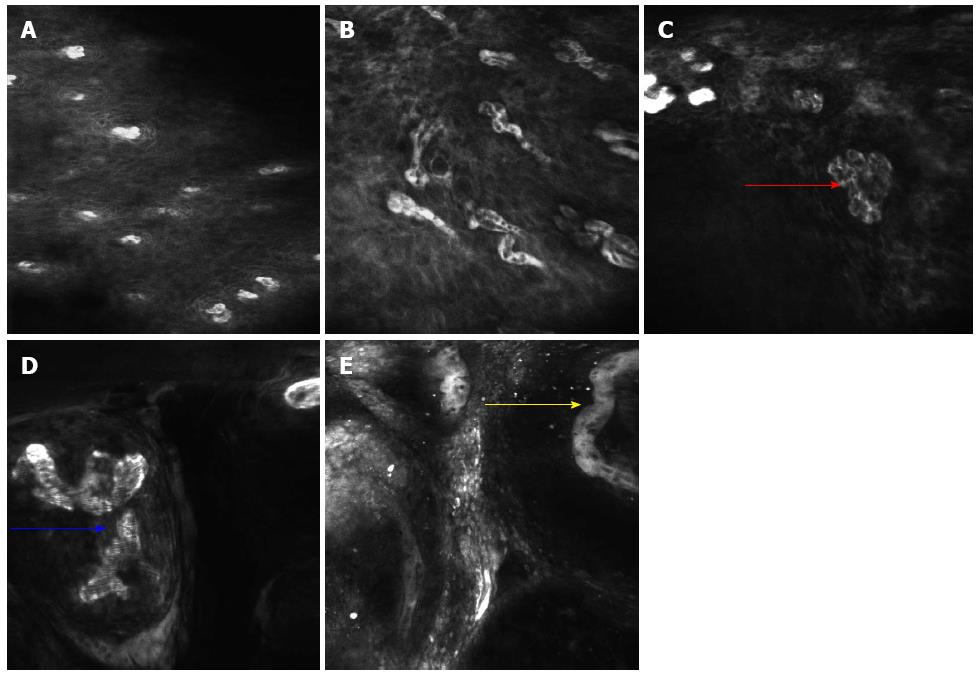
A total of 679 CLE images were obtained from the 56 lesions. Regular squamous epithelium was characterized by regular IPCLs and homogenous epithelial cells with regular architecture and clear borders[6,8]. Neoplastic lesions in the esophagus were characterized by irregular IPCLs and heterogeneous epithelial cells with irregular architecture, varying sizes and invisible borders (Figure 2). Various types of irregular IPCLs including dilatation, twist, caliber change and various shape changes were observed in the CLE images (Figure 3). The mean duration of the CLE procedure was 20 ± 13 min, including staining with Lugol’s solution in a total of 56 Lugol-voiding areas.
Histological examination of en bloc ER specimens revealed that 46 of the 56 lesions had HGIN or ESCC in situ. Inflammation and LGIN of biopsy histologic diagnosis were classified as the non-neoplasms, while HGIN or ESCC in situ indicated neoplasms.
A total of 152 chromoendoscopy-guided biopsies were obtained from the 56 lesions. The biopsy histopathologic assessment revealed the presence of neoplasms in 41/56 lesions, which was confirmed by histopathologic analyses of en bloc ER specimens in 38/41 lesions.
CLE in vivo histological diagnosis revealed non-neoplasms in 11/56 and neoplasms in 45/56 (80.3%), which was confirmed by histopathologic analyses of en bloc ER specimens in 9/11 (81.8%) and 44/45 (97.8%) of lesions. The sensitivity, specificity, NPV and PPV of chromoendoscopy-guided CLE were 95.7%, 90.0%, 81.8% and 97.8%, respectively. The results show a significant improvement in sensitivity, specificity, NPV, FPR, FNR, and accuracy compared with chromoendoscopy-guided CLE and biopsy.
The CLE procedure was generally well-tolerated by all patients. No fluorescein injection-induced side effects were observed, except for mild urine and skin discoloration. In particular, no allergic reactions occurred, because the sensitivity test for fluorescein was undertaken before CLE.
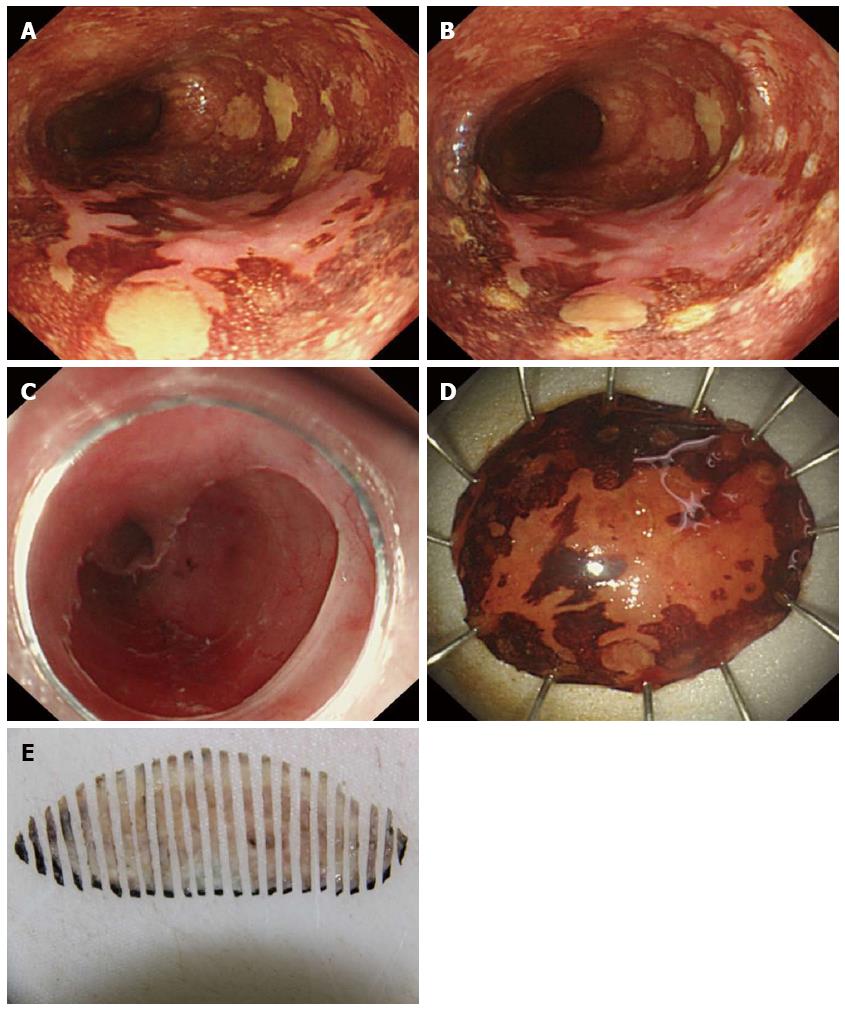
The study included 15 lesions treated by EMR-C and 41 lesions treated by ESD (Figure 4). The duration of the procedure was 27 min (range, 17-39 min) for EMR-C and 113 min (range, 47-175 min) for ESD. Minor bleeding was encountered in all ER procedures, but successful hemostasis was always achieved using thermocoagulation. No patient experienced massive hemorrhage requiring a blood transfusion or postprocedural emergent endoscopy. No delayed hemorrhage occurred. Exposure of the muscular layer during ESD occurred in four cases, but no overt esophageal perforation or the presence of pneumomediastinum was observed by computed tomography scanning.
The aim of the present study was to determine the diagnostic potential of CLE combined with LIC in detecting superficial ESCN. In order to completely capture the histological analysis, histological examination of the en bloc ER specimens was used as the standard reference. Our result supports the hypothesis that CLE is non-inferior to biopsy in detecting ESCN.
Lugol’s solution is an absorptive stain containing iodine, potassium iodide and distilled water. The solution has an affinity for glycogen in non-keratinized squamous epithelium, and therefore is often used in the esophagus to detect squamous dysplasia and squamous cell carcinoma[9]. The absence of staining results from the depletion of glycogen in squamous cells; this occurs in dysplasia, squamous cell carcinoma, Barrett’s epithelium, and inflammation[10]. Although various techniques for endoscopic diagnosis have been developed, iodine staining is still the most useful screening method for early esophageal carcinoma[2,11]. Lugol’s staining also improves the visualization of the lateral margins of lesions, which can result in a significant increase in the size of lesions compared with the size estimates obtained by standard endoscopy[12]. However, iodine is an irritant that may cause a choking sensation or chest discomfort during or after the procedure, and some patients are hypersensitive to iodine. HGIN and ESCC barely react with iodine due to the small number of glycogen-containing cells and are therefore seen as completely unstained areas with a reddish color after the brown color of iodine solution has faded. On the other hand, LGIN and areas of inflammation react slightly with iodine and are therefore seen as unstained areas with a yellowish-white color. It is difficult to distinguish HGIN from LGIN without biopsy histological diagnosis even if the pink-color sign is used[13-16]. Chromoendoscopy with iodine staining has a low specificity and requires many biopsy specimens. Choosing an adequate biopsy point in a scattered-type esophagus[17], which is characterized by the existence of multiple Lugol-voiding lesions[18], is quite difficult because many of the iodine-unstained lesions are inflammatory areas of the mucosa or LGINs.
CLE enables the endoscopist to perform an in vivo histologic examination of the gastrointestinal mucosa and distinguish between neoplastic and non-neoplastic tissues during ongoing endoscopy[19-22]. Currently, two confocal imaging systems are available: integrated CLE and probe-based CLE. Integrated CLE was used in our research because the endoscope’s working channel can still be used for Lugol staining while confocal images are generated simultaneously with the endoscopic images. CLE images showed a significantly higher proportion of squamous epithelial cells with an irregular arrangement and alteration in IPCLs in ESCC. Squamous cell neoplasia showed dark cells of variable size, no clearly visible borders and an irregular architecture. Various alterations in IPCLs such as increased diameter, tortuous vessels and long branching have been observed[8,23]. In this study, CLE scanning used in Lugol-unstained areas could easily find typical lesions, thus reducing the procedure time and the false positive rate and false negative rate.
Pech et al[6] compared CLE and biopsy histology; the accuracy was 95%, and the sensitivity and specificity were 100% and 87%, respectively. Unfortunately, a high false positive rate (30%) and false negative rate (17.3%) of biopsy pathology were noted in our research because it was difficult to find typical biopsy lesions in the Lugol-unstained areas. Tissue acquired by biopsy forceps was limited and may be blind; biopsies only reflect limited characteristics of pathological lesions. Endoscopic biopsy also causes mucosal bleeding and consequently makes it difficult to find other target lesions. As a result, it was not possible to observe all pathologic changes, and therefore difficult to perform an objective pathological evaluation on the lesions[23].
In this study, CLE was compared with en bloc ER specimen histology. Only en bloc ER was used in this study because one of the benefits of en bloc resection is the more accurate histological assessment compared to biopsy. All en bloc resected specimens were continuously sliced into 2 mm sections from the proximal end to the distal end, such that en bloc resection made an entire histopathologic evaluation possible. CLE was able to scan the entire lesion with high accuracy, which not only avoided complications such as bleeding and tissue damage from repeat multiple endoscopy and biopsies, but also reduced the time of histologic examination of the biopsy. Therefore, the diagnostic value of CLE for superficial ESCN is confirmed, but the depth of CLE scanning is limited. Currently, the ability to diagnose esophageal carcinoma is limited to the superficial type; application of these methods to other types of esophageal carcinomas remains to be studied.
ER has become an established standard treatment for patients with superficial ESCN in recent years. In the present study, ESD was used in large lesions (> 1.5 cm), while EMR-C was used in small lesions. We defined the strict criterion of a size of 1.5 cm to ensure en bloc resection of the lesions. In our study, ER was performed for either CLE or biopsy-proven ESCN. Five lesions were identified as neoplasms by CLE while LIN/non-neoplasia by biopsy. ER was finally performed in these patients after informed consent was obtained, not only because the patients (including smokers and alcohol users) were highly suspected of having esophageal squamous cell cancer, but because the endoscopic appearance of these lesions was type 0-II according to the Paris classification and Lugol-voiding was obvious. Histological examination of the ESD specimens confirmed the CLE in vivo histological diagnosis in these lesions.
Our study had several limitations. First, it was a single-center study. Second, the number of the enrolled cases was limited. Although EMR-C was widely used, the application of ESD was limited due to the technical difficulty and the high complication rate in China. The CPLA General Hospital was one of the first hospitals to use ESD and CLE in China. Although the number of cases is limited, it is still acceptable due to our strict inclusion criteria. Third, the depth of lesion invasion was not predictable by CLE due to the limited laser penetration depth of integrated CLE.
In conclusion, this study demonstrates that ESCN can be diagnosed reliably by Lugol’s chromoendoscopy-guided CLE. The accuracy, sensitivity, specificity, PPV, and NPV were high in our series. CLE combined with LIC is a real-time, non-invasive endoscopic diagnostic technology. Directly progressing to ER without further biopsy procedures may became the standard after further research into CLE in the future.
Superficial esophageal squamous cell neoplasia (ESCN) is usually asymptomatic and curable if detected early. Lugol’s iodine chromoendoscopy (LIC) is highly sensitive for identifying squamous lesions, but has a low specificity. Confocal laser endomicroscopy (CLE) can show the cells of the esophageal squamous epithelium and intraepithelial papillary capillary loops in vivo, thus contributing to an accurate diagnosis of ESCN when combined with LIC.
CLE has been used to investigate inflammatory disease, gastrointestinal cancer and precancer including Barrett’s esophagus, gastric intestinal metaplasia gastric neoplasms and early cancer, colon polyps, colonic neoplasia and cancer, and ulcerative colitis.
The early detection of esophageal squamous cell carcinoma is the key to reducing the high mortality. This study used CLE combined with LIC in detecting superficial ESCN, first using histological examination of en bloc endoscopic resection specimens as the standard pathology.
This study has demonstrated that ESCN can be diagnosed reliably by using Lugol’s chromoendoscopy-guided CLE. Clinical endoscopists could use CLE combined with LIC to quickly and accurately identify ESCN.
CLE is novel digestive endoscope which enables the endoscopist to perform in vivo histologic examination of the gastrointestinal mucosa, and distinguish between neoplastic and non-neoplastic tissues during ongoing endoscopy.
The authors have demonstrated an effective method for the detection and diagnosis of ESDN by Lugol’s chromoendoscopy-guided CLE, which has a higher sensitivity and specificity than conventional endoscopy.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1257] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 2. | Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Yokoyama A, Ohmori T, Makuuchi H, Maruyama K, Okuyama K, Takahashi H, Yokoyama T, Yoshino K, Hayashida M, Ishii H. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928-934. [PubMed] |
| 4. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (2)] |
| 6. | Pech O, Rabenstein T, Manner H, Petrone MC, Pohl J, Vieth M, Stolte M, Ell C. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol. 2008;6:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 374] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Deinert K, Kiesslich R, Vieth M, Neurath MF, Neuhaus H. In-vivo microvascular imaging of early squamous-cell cancer of the esophagus by confocal laser endomicroscopy. Endoscopy. 2007;39:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Freitag CP, Barros SG, Kruel CD, Putten AC, Dietz J, Gruber AC, Diehl AS, Meurer L, Breyer HP, Wolff F. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 10. | Weinstein WM. Vital staining of esophageal and gastric mucosa: not vital but may be helpful. Gastrointest Endosc. 1992;38:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Fagundes RB, de Barros SG, Pütten AC, Mello ES, Wagner M, Bassi LA, Bombassaro MA, Gobbi D, Souto EB. Occult dysplasia is disclosed by Lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the esophagus. Endoscopy. 1999;31:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231. [PubMed] |
| 13. | Mori M, Adachi Y, Matsushima T, Matsuda H, Kuwano H, Sugimachi K. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol. 1993;88:701-705. [PubMed] |
| 14. | Kitamura K, Kuwano H, Yasuda M, Sonoda K, Sumiyoshi K, Tsutsui S, Kitamura M, Sugimachi K. What is the earliest malignant lesion in the esophagus? Cancer. 1996;77:1614-1619. [PubMed] |
| 15. | Shimizu Y, Omori T, Yokoyama A, Yoshida T, Hirota J, Ono Y, Yamamoto J, Kato M, Asaka M. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008;23:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 16. | Ishihara R, Yamada T, Iishi H, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Takeuchi Y, Higashino K. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc. 2009;69:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc. 2001;54:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 18. | Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 20. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T, Chu CL, Zhang TG, Li YQ. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut. 2011;60:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruïne A, Masclee A. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2010;8:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zuo XL, Li CQ, Zhang JN, Guo YT, Zhang TG. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Benz C, Hardy T, Vela S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM