Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6924
Peer-review started: December 21, 2015
First decision: January 22, 2015
Revised: February 23, 2015
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: June 14, 2015
Processing time: 180 Days and 8 Hours
AIM: To evaluate the correlation between the immunoexpression of angiogenic markers [CD31, CD105 and vascular endothelial growth factor (VEGF)], proliferative index (Ki67), and prognosis of patients with gastrointestinal stromal tumors (GIST).
METHODS: This is a retrospective study of 54 GIST cases. Medical records were searched to obtain the GIST patients’ demographic and clinical data, and paraffin-embedded blocks of tumor samples were retrieved from the hospital archives to conduct a new immunohistochemical evaluation. The tumor samples of GIST patients were subject to immunohistochemical evaluation for endoglin (CD105), CD31, VEGF, and Ki67 expression. The CD105 and CD31 intratumoral microvascular density (IMVD) was measured using automated analysis. We determined the correlation between the immunoexpression of CD105, CD31, VEGF, Ki67 and prognosis. In addition, we conducted a cutoff analysis using the receiver-operating characteristic curve. VEGF positivity was classified as either null/weak or strong. Ki67 was evaluated using a cutoff of 5% positive cells. The prognosis was classified as good (patient alive without recurrence) or poor (patient with recurrence/death).
RESULTS: The distribution of tumor sites among the 54 analyzed samples was as follows: 27 (50%) in the stomach, 20 (37.1%) in the small intestine, 6 (11.1%) in the colon, and 1 (1.8%) in the esophagus. The size of the tumors ranged from 2 to 33 cm (median: 8 cm); in 12 cases (22.2%), the tumor was below 5 cm at the largest diameter, but in 42 cases (77.7%), the tumor was larger than 5 cm. The means of CD105 and CD31 were significantly higher in the group with poor prognosis (P < 0.001). The cut-off values of CD105 (> 1.2%) and CD31 (> 2.5%) in the receiver-operating characteristic curve were related to a poorer prognosis. Cases with a better prognosis showed significantly null/weak staining for VEGF (P < 0.001). Ki-67 expression of ≥ 5% was strongly correlated with a worse prognosis (P < 0.001). In the multivariate analysis, CD105 was the variable that most strongly correlated with prognosis.
CONCLUSION: The IMVD cutoff values for the angiogenic markers CD105 and CD31, may be prognostic factors for GIST, in addition to VEGF and Ki67.
Core tip: Prognosis of gastrointestinal stromal tumors (GIST) is a longstanding challenge. Association of angioimmunomarkers with poor prognosis has recently been demonstrated, but few studies have evaluated the relevance of vascular endothelial growth factor (VEGF) and CD31, and none has analyzed the role of CD105 expression in prognosis. Our results suggest that angiogenic markers (intratumoral microvascular density cut-off of CD105 and CD31 besides VEGF) and Ki67 (tumor cell proliferation marker), may be prognostic factors for GIST, besides and Ki67 (tumor cell proliferation). However, further studies are necessary before considering such angiogenic molecules as possible therapeutic targets.
- Citation: Basilio-de-Oliveira RP, Nunes Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol 2015; 21(22): 6924-6930
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6924.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6924
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract[1]. Although a great deal is already understood about the biology, diagnosis, and treatment of this tumor type, predicting prognosis in some cases remains a challenge[2]. The primary goal of a prognostic determination to discern patients with localized, resectable disease who require only clinical follow-up from those in need of adjuvant therapy[3]. Currently, there are five different classifications[4-8] used to stratify tumors into groups associated with a greater or lesser risk of tumor recurrence and/or distant metastasis[9]. However, these classifications still leave room for doubt, as there are some groups of rare tumors with an insufficient number of cases for effective analysis, and others with highly variable recurrence/tumor progression rates, e.g., from 24% to 73%, leaving a high percentage of tumors with an indefinite prognosis[10,11].
In addition to morphological criteria, some immunohistochemical markers have been used as prognostic markers of GIST. Among these are the proliferating cell nuclear antigen Ki-67, which is widely used with a cut-off point of 5% as an indicator of poor prognosis. More recently, the angiogenic markers, especially CD31 and vascular endothelial growth factor (VEGF)[12], which is considered the main mediator of tumor angiogenesis have also been used as prognostic[13]. Some studies[14-18] have shown an association between high levels of tissue VEGF and poorer prognosis of patients with GIST.
In addition to VEGF, another molecule that is involved in the process of tumor angiogenesis is the transmembrane glycoprotein endoglin (CD105). Endoglin may or may not be proangiogenic, depending on whether it is bound to the activin receptor-like kinase[19], and is primarily expressed in activated endothelial cells[20,21]. Compared with other pan-endothelial markers (e.g., CD34 and CD31), endoglin shows a characteristic property as a “neovessel” marker, i.e., in a state of proliferation. Therefore, CD105 is considered a more specific immunohistochemical marker for tumor neovasculature[22].
Various studies have demonstrated the importance of CD105, using intratumoral microvessel density (IMVD), as a prognostic factor correlated with overall and disease-free survival, tumor recurrence, and the presence of metastasis in different tumor types[23,24]. However, to our knowledge, only one study[25] has addressed the association of the immunohistochemical marker CD105 in GIST with morphological factors, and no correlation with prognosis was found. Other angiogenic markers have been studied in relation to cancer prognosis, including CD34, CD31, and factor VIII[26-28]. However, the results are contradictory, with some studies[26,29,30], including those on GIST[17], that have identified an association, and others that have not[31]. In addition, volumetric growth and the development of metastases in cases of GIST appear to be related to the development of a new vascular network[17]. Another fact that corroborates the importance of vascularization in the context of GIST is the mechanism of action of the second-generation drug sunitinib, which is based on the blockade of VEGF activity along with tyrosine kinase receptor blockade that has been used with success in some GIST patients[32]. Another key factor used in defining prognosis in cases of GIST is cell cycle markers, especially Ki67, which is an indicator of proliferating cells[33].
In an attempt to better understand the behavior of GIST, we performed immunohistochemical assays to analyze the expression of angiogenic markers (CD105, CD31 and VEGF) as well as the cell proliferation index (Ki-67) and determined their correlation with the clinical progression of patients. The results of this study should provide valuable information with respect to the effectiveness of these markers as prognostic factors in the context of GIST.
We conducted a retrospective study of all cases of GIST evaluated in the Pathology Laboratory of two university hospitals in Rio de Janeiro, Brazil (Gaffrée and Guinle University Hospital and Clementino Fraga Filho University Hospital). After obtaining approval from the Ethics Committee (protocol number 079/05), medical records were searched to obtain the patients’ demographic and clinical data, and paraffin-embedded blocks with tumor samples were retrieved from the hospital archives for a new immunohistochemical evaluation, as described in detail below. The archives were searched for cases of GIST with positivity for the CD117 antibody, with totally resected tumors. Patients with disseminated GIST or with other types of cancer were excluded from the study.
From the clinical records, gender, age, tumor site and size, and clinical progression were recorded. The follow-up period of patients was calculated from the date of surgery until the last follow-up visit. The prognosis was classified as good (disease-free survival) or poor (the patient died due to GIST, or survived but had metastases during follow-up). There were no deaths due to other causes in this case series.
The CD105, CD31, VEGF, and Ki67 expression levels were determined by immunohistochemical analysis, according to the methods and criteria described below.
Formalin-fixed, paraffin-embedded tissues, sectioned into 5-μm-thick slices, were mounted on poly-L-lysine-coated slides (Sigma, St. Louis, MO, United States; code P8920). The sections were deparaffinized by xylene and dehydrated by a graded series of ethanol. The Novolink polymer (Novocastra, Newcastle, United Kingdom) was used. The chromogen was 3,3’-diaminobenzidine tetrahydrochloride, followed by Mayer’s hematoxylin counterstain were applied to the slides. Negative controls lacking primary antibody application were run simultaneously. The following antibodies with respective dilutions were used: anti-Ki-67 (M7240, 1:250; Dako Dk A/S), anti-CD31 (JC70A, 1:50; Dako Dk A/S), anti-VEGF C-1 (sc-7269, 1:6000; Santa Cruz Biotechnology, Heidelberg, Ger), and anti-CD105 (clone 4G11, 1:60; Leica Biosystems Newcastle Ltd, United Kingdom).
Two computer tools were used for the CD105 and CD31 immunohistochemical IMVD analysis: Qcapture and ImageLab. The former is an image-capturing system in an Olympus digital 3.3-megapixel camera attached to an Olympus BX-40 microscope. The three vascular fields showing the highest intensity antibody signals, such as vessels or groups of endothelial cells, were searched at a magnification of × 100 and captured for each case with a × 200 magnifying lens. The images were then analyzed using the “color function” and area/density measurement function in ImageLab software[34]. The percentages of marked areas (hotspots) were calculated in a fixed area of 216 μm on each image. The average of the three areas selected for analysis was recorded into an Excel worksheet.
The tumors were classified into two categories based on VEGF expression: null/weakly positive or moderate/markedly positive. The classification was based on the staining intensity of the vascular structures.
Positive cells for Ki-67 were counted in a field of 1000 cells[33]. The tumors were classified into those with less than 5% positive cells and those with 5% or more positive cells.
CD31 and CD105 positivity and Ki-67 expression were analyzed in relation to clinical status[4,10]. A receiver-operating characteristic (ROC) curve was constructed to determine a cut-off for poor prognosis.
In the univariate analysis, the χ2, Wilks G2, Fisher and Student’s t tests were used, with a significance threshold value of less than 0.05. In the multivariate analysis, for factors that showed statistical significance in the univariate analysis, we used the Jaccard index, which compares the similarity and diversity of samples. The highest rated factor was the one determined to be most closely linked to prognosis. The statistical methods of this study were reviewed by Mauricio Gama, Associate Professor of Research Department of Clementino Fraga Filho University Hospital.
Among the 54 cases of GIST that were studied, the patients’ mean age was 57.34 ± 13.71 years (range: 24-83 years), and 30 (59.5%) patients were women. The distribution of the tumor sites among patients was as follows: 27 (50%) in the stomach, 20 (37.1%) in the small intestine, 6 (11.1%) in the colon and 1 (1.8%) in the esophagus. The size of the tumors ranged from 2 to 33 cm (median: 8 cm); in 12 cases (22.2%), the tumor was below 5 cm at the largest diameter, but in most cases (42; 77.7%), the tumor was larger than 5 cm.
Of the 54 patients, 33 (61.2%) were alive and disease-free (i.e., good prognosis) and 21 (38.8%) patients were alive with disease or had died due to cancer. The follow-up period ranged from 1 to 242 mo, with a median time of 35 mo.
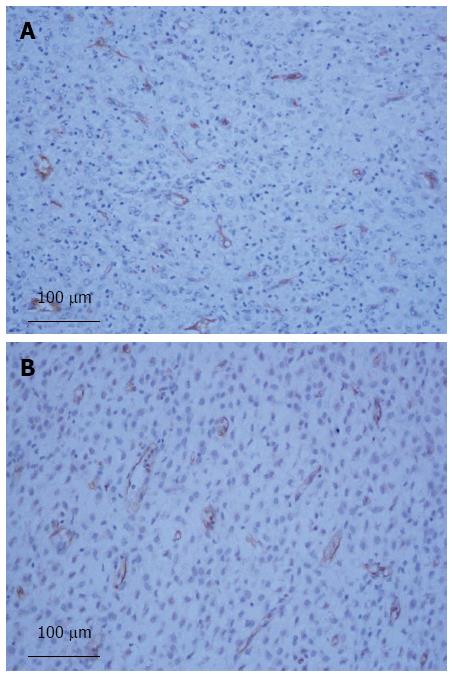
The mean positivity values of CD105 and CD31 ranged from 0.37% to 4.21% and 0.39% to 7.83%, with median values of 1.14% and 1.96%, respectively (Figure 1).
In relation to VEGF, 21 tumors (38.9%) showed null/weakly positive staining, while 33 (61.1%) showed moderate/strong staining.
The anti-Ki-67 antibody was expressed in 31 (57.4%) tumors with a cell proliferation index of less than 5%, and the remaining 23 tumors (42.6%) had an index greater than or equal to 5%.
The average value for the CD105 evaluation was significantly higher in the group of patients with poor prognosis than in the group of patients who were alive without recurrence (1.98% vs 1.04%, P < 0.001, Student’s t-test). Based on the ROC curve for CD105, a cutoff point of 1.21% was determined. This cutoff point was corroborated by the area under the curve value of 0.88, which indicates good power of discrimination.
The average value obtained with CD31 was significantly higher in the poor prognosis group than in the group of patients who survived without recurrence (3.61% vs 1.94%, P < 0.001, Student’s t-test). Based on the ROC curve for CD31, a cutoff point of 2.50% was determined. This cutoff point was corroborated by the area under the curve value of 0.92, which indicates strong discriminatory power.
A relatively better patient prognosis was associated with null/weak VEGF expression in the tumors; of the 21 cases with null/weak VEGF expression, only 3 had an unfavorable prognosis (P = 0.002). A rate of ≥ 5% Ki67 expression was strongly associated with reduced overall survival (P < 0.001) (Table 1). In the multivariate analysis, the factors that showed statistical significance in the univariate analysis (CD105, CD31, VEGF and Ki-67) were submitted to analysis of similarity (Jaccard index), which revealed that CD105 had the strongest association with prognosis-(Jaccard index value: 0.69278), followed by CD31 (0.66471), Ki67 (0.54286) and VEGF (0.50000).
Determining the prognosis of GIST is essential, given that 60% of tumors are larger than 5 cm, which is associated with a relatively poor prognosis[5,35,36], 50% of patients are considered high-risk[37], and 20%-55% of patients will experience tumor recurrence[38-40].
In our case series, we demonstrated an association between immunohistochemical markers related to angiogenesis and prognosis. CD105 (endoglin) showed an association with prognosis through the IMVD measurement. The plotted ROC curve indicated a cut-off point of 1.2%, which was established as a dividing factor in our series for good and poor prognosis, as tumors with an IMVD above this value were related to a relatively worse prognosis. No previous study has directly evaluated the role of CD105 expression in prognosis in the context of GIST. The only previously reported study[25] on the association between GIST and CD105 demonstrated a link between the strong intensity of immunohistochemical staining with some morphological criteria that is associated with a worse prognosis, such as a mitotic index above 5 mitoses per 50 high-power fields and a high degree of risk.
The average IMVD value of CD31 was higher in tumors from patients with a worse prognosis than in those with a good prognosis, demonstrating a clear relationship between this marker and prognosis, as has been previously reported[17,18,41]. Although these previous studies all established a correlation between the IMVD of CD31 and prognosis, there is marked variation among the cut-off values reported for malignancy, which might be due to the different methods used for evaluation and analysis[17,18]. There are also some differences between the methods adopted in our study and others. For example, the count of vascular structures was conducted in a semi-automated manner using a computer program, which significantly reduces bias in counting. In addition, the cut-off point value of 2.50% found in our series was validated through analysis of a ROC curve.
VEGF expression was null or weakly positive in 21 tumors, and only 3 of these cases showed poor prognosis, while the 33 patients with strong VEGF positivity in tumors (approximately 60%) experienced recurrence or died. This association between VEGF and GIST prognosis has also been found in other studies[15-18]. In addition, high VEGF expression has been associated with a poorer prognosis, independent of the tumor genotype, and with a low therapeutic response to imatinib mesylate[42]. In other tumors, such as those of the lung and breast[26,27], high IMVD is associated with a reduction in survival and a shorter time to tumor recurrence[22,29].
We also found a strong association between a marker for nuclear antigen cell proliferation (Ki67) and prognosis in our series, similar to previous studies[43,44]. Therefore, the present results demonstrate the high prognostic potential of Ki-67 for GIST. Of the 31 patients with an index of less than 5%, 29 were alive without recurrence and only 2 had progressed to a poor prognosis. In contrast, of the 23 tumors with an index greater than 5%, 19 progressed to metastasis/death. There are other advantages of using Ki-67 as a prognostic marker: unlike the mitotic index, which shows an association with the topography of the tumor[43], it can be used independently of the location of the tumor. It also constitutes a potential discriminator in localized and/or disseminated disease[43]. Therefore, despite its inability to determine risk levels in all classifications proposed, we believe that Ki-67 is an important parameter in the prognosis of GIST and that it should not be neglected as a prognostic marker.
Our results suggest that angiogenic markers, including the CD105 and CD31 IMVD cut-off values, VEGF and the tumor cell proliferation marker Ki67, may be useful prognostic factors in GIST. However, further studies are necessary before considering such angiogenic molecules as possible therapeutic targets.
The authors wish to thank Professor Mauricio Pinto Gama for guidance and help in the statistical analysis of data collected in this study.
Angiogenesis contributes to the growth and spread of gastrointestinal stromal tumors (GIST). Endoglin, vascular endothelial growth factor (VEGF) and CD31 are directly involved in this process. However to date the use of these molecules as prognostic factors remains controversial.
Previous studies have shown that high levels of VEGF and CD31 are associated with a poor prognosis. However no studies have addressed the prognostic potential of endoglin. In the present study, independent prognostic implications of endoglin were investigated.
Tumors with intratumoral microvessel density above the cut-off found in the respective receiver-operating characteristic curves were directly associated with a poor prognosis. In the univariate and multivariate analyses, vascular markers, especially endoglin were associated with prognosis.
The results of this study suggest that high levels of molecules related to angiogenesis (endoglin, VEGF and CD31) are an independent indicator of poor prognostic in GIST.
Endoglin is a type I membrane glycoprotein located on cell surfaces and is part of the TGFbeta receptor complex. It has a crucial role in angiogenesis and is an important protein for tumor growth, survival and metastasis
This is a retrospective study in which the authors analyzed the main molecules of angiogenesis (CD31, VEGF and endoglin) and related them to disease prognosis. It is noteworthy that endoglin has not been previously studied in the context of GIST. The results are interesting and suggest that tumors that demonstrate a high microvessel vascular intratumoral had a worse prognosis.
| 1. | Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48:83-96. [PubMed] |
| 3. | Boichuk S, Parry JA, Makielski KR, Litovchick L, Baron JL, Zewe JP, Wozniak A, Mehalek KR, Korzeniewski N, Seneviratne DS. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Cancer Res. 2013;73:5120-5129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] |
| 5. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 6. | Joensuu H. Predicting recurrence-free survival after surgery for GIST. Lancet Oncol. 2009;10:1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Huang H, Liu YX, Zhan ZL, Liang H, Wang P, Ren XB. Different sites and prognoses of gastrointestinal stromal tumors of the stomach: report of 187 cases. World J Surg. 2010;34:1523-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, Ooi BS, Chung YF, Wong WK. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008;15:2153-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Wong NA. Gastrointestinal stromal tumours--an update for histopathologists. Histopathology. 2011;59:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [PubMed] |
| 11. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 12. | Takahashi R, Tanaka S, Hiyama T, Ito M, Kitadai Y, Sumii M, Haruma K, Chayama K. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797-802. [PubMed] |
| 13. | Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469-478. [PubMed] |
| 14. | Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1774] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 15. | Miao R, Liu N, Wang Y, Li L, Yu X, Jiang Y, Li J. Coexpression of cyclooxygenase-2 and vascular endothelial growth factor in gastrointestinal stromal tumor: possible relations to pathological parameters and clinical behavior. Hepatogastroenterology. 2008;55:2012-2015. [PubMed] |
| 16. | Wang TB, Qiu WS, Wei B, Deng MH, Wei HB, Dong WG. Serum vascular endothelial growth factor and angiogenesis are related to the prognosis of patients with gastrointestinal stromal tumors. Ir J Med Sci. 2009;178:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Imamura M, Yamamoto H, Nakamura N, Oda Y, Yao T, Kakeji Y, Baba H, Maehara Y, Tsuneyoshi M. Prognostic significance of angiogenesis in gastrointestinal stromal tumor. Mod Pathol. 2007;20:529-537. [PubMed] |
| 18. | Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266-274. [PubMed] |
| 19. | Koleva RI, Conley BA, Romero D, Riley KS, Marto JA, Lux A, Vary CP. Endoglin structure and function: Determinants of endoglin phosphorylation by transforming growth factor-beta receptors. J Biol Chem. 2006;281:25110-25123. [PubMed] |
| 20. | Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci. 2003;116:2677-2685. [PubMed] |
| 22. | Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984-992. [PubMed] |
| 23. | Svatek RS, Karam JA, Roehrborn CG, Karakiewicz PI, Slawin KM, Shariat SF. Preoperative plasma endoglin levels predict biochemical progression after radical prostatectomy. Clin Cancer Res. 2008;14:3362-3366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Gromova P, Rubin BP, Thys A, Cullus P, Erneux C, Vanderwinden JM. ENDOGLIN/CD105 is expressed in KIT positive cells in the gut and in gastrointestinal stromal tumours. J Cell Mol Med. 2012;16:306-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941-2955. [PubMed] |
| 27. | Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, Vincenzi B, Tonini G. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591-597. [PubMed] |
| 28. | Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46:481-489. [PubMed] |
| 29. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [PubMed] |
| 30. | Weidner N. The importance of tumor angiogenesis: the evidence continues to grow. Am J Clin Pathol. 2004;122:675-677. [PubMed] |
| 31. | Medri L, Nanni O, Volpi A, Scarpi E, Dubini A, Riccobon A, Becciolini A, Bianchi S, Amadori D. Tumor microvessel density and prognosis in node-negative breast cancer. Int J Cancer. 2000;89:74-80. [PubMed] |
| 32. | Chen YY, Yeh CN, Cheng CT, Chen TW, Rau KM, Jan YY, Chen MF. Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance. World J Gastroenterol. 2011;17:2113-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828-837. [PubMed] |
| 34. | Hadlich MS, Oliveira GM, Feijóo RA, Azevedo CF, Tura BR, Ziemer PG, Blanco PJ, Pina G, Meira M, Souza e Silva NA. Free and open-source software application for the evaluation of coronary computed tomography angiography images. Arq Bras Cardiol. 2012;99:944-951. [PubMed] |
| 35. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [PubMed] |
| 36. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [PubMed] |
| 37. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 636] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 38. | Ahmed I, Welch NT, Parsons SL. Gastrointestinal stromal tumours (GIST) - 17 years experience from Mid Trent Region (United Kingdom). Eur J Surg Oncol. 2008;34:445-449. [PubMed] |
| 39. | Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, Schleck CD, Hodge DO, Donohue JH. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52-59. [PubMed] |
| 40. | Oliveira RPB, Pannain VL, Portari Filho PE, Salomão AR, Iglesias AC, Oliveira CAB. Gastrointesinal stromal tumor: analysis of factors related to the prognostic. Rev Col Bras Cir. 2007;34:374-380. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Zhao Y, Wang Q, Deng X, Zhao Y. Altered angiogenesis gene expression in gastrointestinal stromal tumors: potential use in diagnosis, outcome prediction, and treatment. Neoplasma. 2012;59:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | McAuliffe JC, Lazar AJ, Yang D, Steinert DM, Qiao W, Thall PF, Raymond AK, Benjamin RS, Trent JC. Association of intratumoral vascular endothelial growth factor expression and clinical outcome for patients with gastrointestinal stromal tumors treated with imatinib mesylate. Clin Cancer Res. 2007;13:6727-6734. [PubMed] |
| 43. | Belev B, Brčić I, Prejac J, Golubić ZA, Vrbanec D, Božikov J, Alerić I, Boban M, Razumović JJ. Role of Ki-67 as a prognostic factor in gastrointestinal stromal tumors. World J Gastroenterol. 2013;19:523-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 44. | Avanzolini G, Barbini P, Cappello A, Cevenini G. Real-time tracking of parameters of lung mechanics: emphasis on algorithm tuning. J Biomed Eng. 1990;12:489-495. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Giordano A, Nanavati AJ S- Editor: Qi Y L- Editor: A E- Editor: Ma S