Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6310
Peer-review started: January 2, 2015
First decision: January 22, 2015
Revised: February 14, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: May 28, 2015
Processing time: 149 Days and 22.7 Hours
AIM: To evaluate the efficacy and safety of modified sequential therapy and to compare modified sequential therapy with standard quadruple therapy for Helicobacter pylori (H. pylori) eradication.
METHODS: In total, 200 consecutive patients who were diagnosed with H. pylori-infected chronic gastritis by electronic endoscopy and rapid urease testing from December 2012 to October 2013 were enrolled in this study. The patients had not previously received H. pylori eradication treatment, and were randomized into two groups. The patients in Group A (n = 101) were treated with ilaprazole + bismuth potassium citrate + amoxicillin and clavulanate potassium + levofloxacin, and the patients in Group B (n = 99) were administered a modified sequential therapy composed of ilaprazole at 5 mg bid and amoxicillin and clavulanate potassium at 914 mg for the first five days followed by ilaprazole at 5 mg bid, furazolidone at 100 mg bid and levofloxacin at 500 mg qid for the next five days. Four to six weeks after the end of treatment, a 14C-urea breath test was performed for all the subjects to confirm the eradication of H. pylori. The intention-to-treat and per-protocol eradication rates were determined.
RESULTS: A total of 190 of the 200 patients completed the study. All 200 patients were included in the intention-to-treat analysis, whereas 190 patients were included in the per-protocol analysis. In the intention-to-treat analysis, the rates of H. pylori eradication in Groups A and B were 85.15% (86/101) and 81.82% (81/99), respectively. In the per-protocol analysis, the H. pylori eradication rates in Groups A and B were 88.66% (86/97) and 87.09% (81/93), respectively. No significant difference was observed (χ2 = 0.109, P = 0.741) in the eradication rate between Groups A and B. The rates of adverse effects observed in the groups were similar at 6.19% (6/97) for Group A and 7.53% (7/93) for Group B (P > 0.05). No mortality or major morbidities were observed in any of the patients. Symptomatic improvements in the presentation of stomachache, acid regurgitation, and burning sensation were not significantly different between the two groups.
CONCLUSION: Ilaprazole-based 10-d standard quadruple therapy does not offer an incremental benefit over modified sequential therapy for the treatment of H. pylori infection, as both treatment regimens appear to be effective, safe, and well-tolerated as initial treatment options.
Core tip: As the prevalence of antibiotic-resistant Helicobacter pylori (H. pylori) has increased in recent years, the eradication rate of H. pylori has simultaneously declined each year. The aim of this randomized controlled clinical trial was to better characterize the safety and efficacy of a modified sequential therapy regimen for the initial treatment of H. pylori and to compare this treatment regimen with a 10-d standard quadruple treatment regimen for the eradication of H. pylori.
-
Citation: Liao XM, Nong GH, Chen MZ, Huang XP, Cong YY, Huang YY, Wu BH, Wei JQ. Modified sequential therapy
vs quadruple therapy as initial therapy in patients withHelicobacter infection. World J Gastroenterol 2015; 21(20): 6310-6316 - URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6310
Helicobacter pylori (H. pylori) infection is widespread in humans. An epidemiological study has indicated that the prevalence of H. pylori infection in China remains high, reaching 40%-60% in adults[1]. H. pylori is involved in chronic gastritis, non-ulcerative dyspepsia, peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphomas. Additionally, H. pylori is related to unexplained iron deficiency anemia, chronic idiopathic thrombocytopenic purpura, Alzheimer’s disease, colorectal adenomas and colon cancer and is possibly related to atherosclerosis, diabetes, hypertension, obesity and other diseases[2]. The eradication of H. pylori facilitates the control of H. pylori-related diseases. However, as the prevalence of antibiotic-resistant H. pylori has increased in recent years, the eradication rate of H. pylori has declined yearly. In 2012, the Chinese Consensus Report showed that the resistance rates to metronidazole, clarithromycin, and levofloxacin were 60%-70%, 20%-38%, and 30%-38%, respectively; however, the resistance rates to amoxicillin, furazolidone and tetracycline remained low (1%-5%). According to a 2013 antibiotic resistance study in Guangdong province in China, the resistance rates to metronidazole, furazolidone, amoxicillin, clarithromycin and levofloxacin were 88.4%, 61.1%, 47.4%, 42.1% and 21.1%, respectively[3]. In 2014, a review noted that the lack of therapeutic compliance and the incidence of side effects might lead to the development of antibiotic resistance. Resistance to metronidazole has reached approximately 40% in developed countries and exceeds 90% in developing countries. The resistance to clarithromycin has been increasing, reaching more than 20% in southern Europe[4]. The Fourth Chinese National Consensus Report[1] showed that the eradication rate of the standard triple therapy [proton pump inhibitor (PPI) + clarithromycin + amoxicillin or PPI + clarithromycin + metronidazole] is lower or far lower than 80%. Increasing the duration of the standard triple therapy from 7 to 10 or 14 d could increase the eradication rate by 5%. To improve the H. pylori eradication rate, several regimens of H. pylori eradication therapy have been recommended internationally, including sequential therapy (5 d of PPI + amoxicillin followed by 5 d of PPI + clarithromycin + metronidazole, for a total of 10 d), concomitant therapy (PPI + clarithromycin + amoxicillin + metronidazole taken simultaneously) and levofloxacin triple therapy (PPI + levofloxacin + amoxicillin). There has been no controlled study comparing the efficacy of the PPI + amoxicillin + fluoroquinolone regimen with and without the addition of bismuth; however, the use of PPI + amoxicillin + fluoroquinolone + bismuth quadruple therapy as a rescue therapy was shown to be safe and effective in several studies[1].
In this randomized controlled trial, we selected 200 patients with H. pylori-positive chronic gastritis who had never received H. pylori eradication treatment. These patients were treated for 10 days with ilaprazole + bismuth potassium citrate + amoxicillin and clavulanate potassium + levofloxacin or ilaprazole + amoxicillin and clavulanate potassium + levofloxacin + furazolidone. The aim of the study was to better characterize the safety and efficacy of the modified sequential therapy regimen for the initial treatment of H. pylori and compare it with the standard quadruple treatment for H. pylori eradication.
This was a prospective study. The protocol was approved by the Ethical Investigation Committee of our institution, and informed consent was obtained from all the patients after a full informative session. All patients were managed by a single gastroenterologist, and their details were recorded.
The inclusion criteria were as follows: 200 consecutive patients from December 2012 to October 2013 who visited our hospital clinic for upper abdominal pain, heartburn, acid reflux and other gastrointestinal symptoms, aged 18-65 years old, male or female, and H. pylori-positive with chronic gastritis confirmed by electronic endoscopy and a rapid urease test.
The exclusion criteria were as follows: (1) pregnant or breast-feeding women; (2) merged ulcers and ulcer complications; (3) cancer patients; (4) previous upper gastrointestinal surgery; (5) therapy with PPIs, H2 receptor antagonists, bismuth, or antimicrobial drugs 2 wk before treatment; (6) a history of previous H. pylori eradication therapy; (7) significant organ dysfunction (hepatic, cardiorespiratory, renal diseases, neoplastic diseases, or coagulopathy); (8) allergy to any of the drugs used in the study or similar drugs; and (9) other cases of interference studies.
A total of 200 patients participated in the study and were randomized into 2 groups. The patients in Group A were treated with standard quadruple therapy (n = 101) consisting of AIBL (amoxicillin and clavulanate potassium at 914 mg bid, ilaprazole at 5 mg bid, bismuth potassium citrate at 220 mg bid, and levofloxacin at 500 mg qid for 10 d). The patients in Group B (n = 99) were administered a modified sequential therapy composed of ilaprazole at 5 mg bid, amoxicillin and clavulanate potassium at 914 mg bid, for the first 5 d followed by ilaprazole at 5 mg bid, furazolidone at 100 mg bid and levofloxacin at 500 mg qid, for the next five days. The proton pump inhibitor and bismuth potassium citrate were administered 30 min before meals, whereas the antibiotics were administered after meals. These drugs were prescribed to the patient one time, and a specific gastroenterologist contacted the patients by telephone to ask them to take the prescribed medication and to inquire whether adverse drug reactions occurred at a fixed time daily. The patients were advised of the possibility of experiencing nausea, taste disturbance, diarrhea, vomiting, dizziness and headaches during the treatment period. The patients were asked to return at the end of eradication therapy to assess the side effects and compliance with the therapy. The incidence of adverse effects was evaluated by a specific questionnaire. Eradication was defined as a negative result on the 14C-urea breath test which was performed 4-6 wk after the end of the course of treatment. None of the patients used antibacterial drugs, PPIs, H2 receptor antagonists, or bismuth after the treatment until a review was conducted regarding the 14C-urea breath test after H. pylori infection.
A technician who was blinded to the assigned protocol performed all of the 14C-urea breath tests.
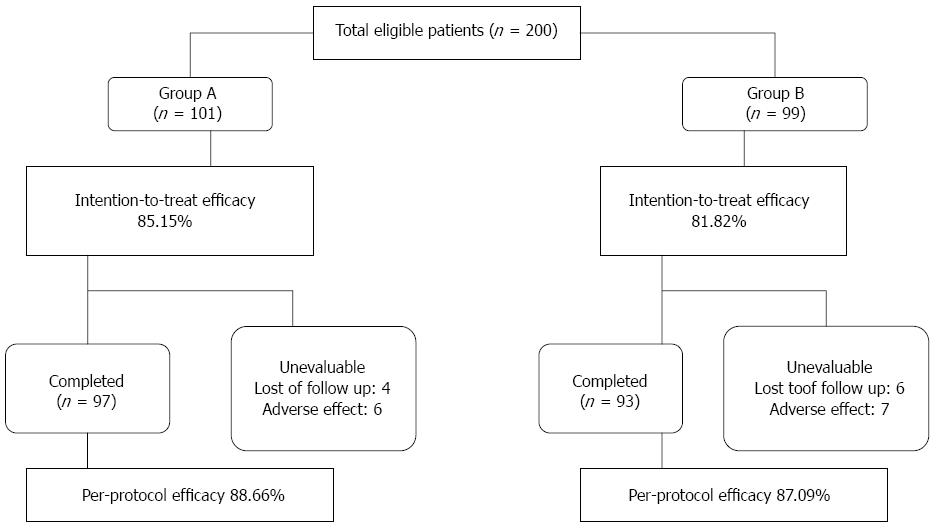
The eradication rates in the intention-to-treat (ITT) and per-protocol (PP) analyses were calculated (Figure 1). The patients who took at least one dose of drugs were included in the ITT analysis, whereas the patients who completed the entire therapy period and completed the follow-up were considered in the PP analysis. The data were analyzed using SPSS 13.0 software. The χ2 test was used for a comparison between the groups, and values of P < 0.05 were considered significant.
A total of 200 patients were included in the ITT analysis, whereas 190 patients were considered in the PP analysis. A total of 97 patients were included in Group A, and 55.67% and 44.33% of these patients were male and female, respectively. The mean age ± SD of the patients in Group A was 40.91 ± 12.10 years. A total of 93 patients were included in group B; 51.61% were male, and 48.39% were female. The mean age ± SD of the Group B patients was 42.88 ± 11.59 years. Of the Group A patients, 23 smoked and 32 used alcohol; in Group B, 25 patients smoked and 30 patients used alcohol. No significant differences between the two groups in terms of drinking, smoking, gender or age were detected (P > 0.05). According to the ITT analysis, H. pylori eradication was achieved in 86 of the 101 patients in the standard quadruple treatment group (Group A) and in 81 of the 99 patients in the modified sequential treatment group (Group B). The ITT eradication rates of the standard quadruple therapy and sequential therapy were 85.15% and 81.82%, respectively (P = 0.741). The PP eradication rates of the standard quadruple therapy and sequential therapy were 88.66% and 87.09%, respectively (P = 0.741). The reported side effects included diarrhea (three patients in total), nausea (one patient in total), dizziness or headache (four patients in total), insomnia (three patients in total), nausea (five patients in total), vomiting (one patient in total), taste disturbance (six patients in total), menstrual period extension (two patients in total); some patients experienced multiple side effects. However, the extent of the mild adverse reactions could be tolerated and disappeared after treatment. Only 10 patients showed poor compliance (four patients in group A and six patients in group B), and 4 patients (two patients in group A and two patients in group B) were lost to follow-up because of job transfers. The rates of adverse effects for the two groups were similar at 6.19% (6/97) for Group A and 7.53% (7/93) for Group B (P > 0.05). No mortality or major morbidity was recorded in the study (Table 1).
| Side effects | 13 (6.8) |
| Nausea | 5 (2.6) |
| Taste disturbance | 6 (3.2) |
| Vomiting | 1 (0.5) |
| Diarrhea | 3 (1.6) |
| Dizziness, headache | 4 (2.1) |
| Insomnia | 3 (1.8) |
| Menstrual period extension | 2 (1.1) |
Over the past decade, eradication programs regarding H. pylori-related diseases have been based on standard triple therapy worldwide. However, the eradication rate of the standard triple therapy (PPI + clarithromycin + amoxicillin or PPI + clarithromycin + metronidazole) is lower or far lower than 80% with the increase in drug-resistant H. pylori. Increasing the duration of standard triple therapy from 7 to 10 or 14 d could increase the eradication rate by 5%[1]. The cure rates of H. pylori infection are influenced by several factors such as antibiotic susceptibility, insufficient inhibition of acid secretion [e.g., the cytochrome P450 2C19 (CYP2C19) genotype, the PPI dose, and the PPI treatment schedule], bacterial genotypes that reduce virulence (e.g., cagA-negative strains and the vacA s2 genotype), the environment (e.g., smoking), and protocol compliance[5,6]. Reports indicated that the effectiveness of eradication can be influenced by the genetic type of H. pylori, better effects of eradicative treatment can be expected if one is infected with the strains of smaller virulence, and cure rates seem to be higher for patients with cagA+/vacA s1 H pylori strains[7,8]. A previous randomized open trial[9] showed that smoking significantly decreased the cure rate of H. pylori infection, while another study suggested that smoking and drinking habits when analyzed jointly are more useful for predicting the outcome of H. pylori eradication than when analyzed separately[10]. Because of the decreased eradication rate, the search for more effective treatment programs or the use of new alternative drugs for H. pylori eradication therapy has become imperative. Recently[1,11], H. pylori treatment with bismuth-containing quadruple therapy or sequential therapy was recommended as the first-line treatment. A review article[12] showed that quadruple therapy should be considered the first-line treatment in areas of high clarithromycin resistance. A 10-d sequential therapy as a novel therapy shows an impressive eradication rate greater than 90%. The rationale for sequential therapy includes the following: (1) amoxicillin would decrease the bacterial load and the risk of the selection of a clarithromycin-resistant mutant strain; and (2) amoxicillin might disrupt the efflux pump, preventing clarithromycin resistance. Choi et al[13] performed a meta-analysis (8 Italian studies) that showed a trend in preferring sequential therapy to triple therapy. Others have suggested that there is insufficient data to recommend sequential therapy as an alternative first-line therapy for H. pylori therapy in Asia[14].
This trial was conducted to establish simple and short-term regimens with effective and nontoxic agents for an applicable initial therapy for H. pylori eradication in China. Our study showed that 10-d standard quadruple ilaprazole and modified sequential therapy were satisfactory and safe, and they appear to be well tolerated for initial therapy. In this trial, we used a new PPI, ilaprazole [the compound designated as IY-81149,2 {[(4-methoxy-3-methyl)-2-pyridinyl]-ethylsulfinyl}-5-(1H-pyrrol-1-lyl) 1H-benzimidazole], which belongs to a class of substituted benzimidazole molecules that are chemically related to omeprazole and lansoprazole. The mechanism of action for the suppression of gastric acid secretion is almost identical in ilaprazole and omeprazole. For both drugs, the protonated substituted benzimidazoles suppress gastric acid secretion through the inhibition of H+/K+-ATPase at the secretory surfaces of the gastric parietal cells[15]. Pre-clinical studies and both national and international phase I and II clinical trials showed that ilaprazole is a strong, stable, long-lasting inhibitor of gastric acid secretion. A multicenter, randomized, double-blinded, positive-controlled clinical trial which was conducted at 20 hospitals in China concluded that ilaprazole was not affected by CYP2C19 polymorphisms[16]. An additional article reported that ilaprazole provided a higher suppression of gastric acid secretion in a dose-dependent manner, a longer half-life, higher bacteriostasis, and a safety profile similar to that of omeprazole[16]. In agreement with an open randomized crossover study[17] and a previous review[18], which indicated that the metabolism of ilaprazole was not related to CYP2C19 and showed that ilaprazole at 5 mg resulted in an effect comparable to 20 mg of omeprazole, 10 mg and 20 mg of ilaprazole provided a significantly greater and prolonged suppression of gastric acid. The resistance of H. pylori to antimicrobial drugs is an important reason for the low eradication rate[19,20]. Amoxicillin and clavulanic acid are two antibiotics that are frequently used in the treatment of H. pylori. Amoxicillin is a semi-synthetic β-lactam antibiotic with high selectivity and low toxicity, whereas clavulanic acid is a β-lactamase inhibitor that blocks the activity of the β-lactamase produced by bacteria. Clavulanic acid can reduce bacterial resistance and enhance the antibacterial effect of amoxicillin when used in combination with amoxicillin and other β-lactam antibiotics[21]. Although H. pylori does not produce β-lactamase, others[21] have shown that an amoxicillin and clavulanate potassium-containing eradication regimen is safe and effective; therefore, we selected amoxicillin and clavulanate potassium for our study. In this study, we included amoxicillin, levofloxacin and furazolidone in the treatment regimens according to a 2013 antibiotic resistance study in Guangdong province in China which showed that the resistance rates against metronidazole and clarithromycin were 88.4% and 42.1%, respectively, which were relatively lower than the resistance rates for amoxicillin and clavulanate potassium, levofloxacin and furazolidone[3]; the difference in resistance rate between Guangdong province and the entire country might have been related to the specific geography, ethnicity, economic level, drug habits and time span in Guangdong province. A previous randomized controlled trial[22] indicated that bismuth salts had a synergistic effect on antibiotics by destroying bacteria in the manner of an antiseptic. A meta-analysis[23] concluded that bismuth for the treatment of H. pylori is safe and well-tolerated, the only adverse event occurring significantly more commonly was dark stools. Recently, a study[24] including one hundred and forty-two H. pylori-positive patients in Turkey showed that the 14-d modified sequential treatment, including bismuth, achieved a significantly high eradication rate in patients with H. pylori infection, with satisfactory patient compliance and minor side effects. Therefore, we added this agent to the initial therapy regimen.
In this clinical study, 200 consecutive patients with H. pylori-positive chronic gastritis who had never received H. pylori eradication treatment were randomized into two groups and administered ilaprazole + bismuth + amoxicillin and clavulanate potassium and levofloxacin in a 10-d standard quadruple treatment or amoxicillin and clavulanate potassium + levofloxacin and furazolidone for 10 d in a modified sequential program. The results showed that the ITT eradication rates with the standard quadruple therapy and modified sequential therapy were 85.15% and 81.82%, respectively (P = 0.741). The PP eradication rates with the standard quadruple therapy and modified sequential therapy were 88.66% and 87.09%, respectively (P = 0.741). The H. pylori eradication rates in both groups were significantly higher than those in patients in this region who received H. pylori eradication treatment containing ilaprazole or esomeprazole + amoxicillin and clavulanate potassium and furazolidone in a 7-d standard triple therapy or amoxicillin and clavulanate potassium + clarithromycin and furazolidone in a 10-d sequential therapy[25]. The eradication rates for both of our study groups were also higher than the eradication rates reported in a national multicenter study evaluating bismuth-containing ilaprazole + amoxicillin and clarithromycin in a 7-d quadruple therapy[26]. These findings suggest that the experimental therapy used in the present study is reasonable regarding the antimicrobial resistance in this region and that antimicrobial drugs and prolonged treatment would improve H. pylori eradication by ilaprazole in combination with bismuth quadruple and sequential programs. The rates of side effects in both groups were similar (6.19% vs 7.53%, P > 0.05), which indicated that ilaprazole in the 10-d standard quadruple and modified sequential regimen had a better safety profile in the treatment of H. pylori-positive patients with chronic gastritis and offered a clinical basis for H. pylori-positive treatment programs in the region. Regarding the sequential therapy, our results are similar to a previous randomized, double-blinded, comparative clinical trial in China which reported that the eradication rate with sequential therapy (20 mg of omeprazole bid and 1000 mg of amoxicillin for 5 d followed by 20 mg of omeprazole, 500 mg of metronidazole, and 500 mg of clarithromycin for an additional 5 d) was 88.89%[27]. In the same year, 2012, a randomized study in Japan reported that the eradication rate of non-bismuth quadruple therapy (lansoprazole at 30 mg, amoxicillin at 750 mg, clarithromycin at 200 mg and metronidazole at 250 mg, twice daily for 7 d) was 94.9% and 98.3%, respectively, by ITT analysis and PP analysis[28]. Recently, a Korean article reported that 7-d and 14-d quadruple therapy with PPI, tripotassium dicitrato bismuthate, tetracycline, and metronidazole showed eradication rates of 66.4% and 71.1%, respectively, by an ITT analysis and 76.5% and 83.8%, respectively, by PP analysis[29].
This study has two limitations. The eradication rate with these two regimens did not achieve the desirable eradication rate of 90%[30]. Selecting the modified sequential therapy as an initial treatment, which requires three antibiotic drugs, has the possibility of increasing the adverse reactions of the drugs and reducing the availability of antibiotic drugs when therapeutic failure occurs.
In conclusion, our study suggests that for H. pylori patients in the Guangdong province, China, ilaprazole-based 10-d standard quadruple therapy does not offer an incremental benefit over modified sequential therapy, as both regimens appear to be effective, safe, and well-tolerated as initial treatment options. Additional studies comparing the treatment dose and duration are needed to further evaluate these two regimens as initial therapy protocols in our population.
As the prevalence of antibiotic-resistant Helicobacter pylori (H. pylori) has increased in recent years, the eradication rate of H. pylori has simultaneously declined annually. To improve the rate of H. pylori eradication in patients, several regimens of H. pylori eradication therapy have been recommended internationally, including sequential therapy [5 d of proton pump inhibitor (PPI) + amoxicillin followed by 5 d of PPI + clarithromycin + metronidazole for a total of 10 d], concomitant therapy (PPI + clarithromycin + amoxicillin + metronidazole taken simultaneously), and levofloxacin triple therapy (PPI + levofloxacin + amoxicillin).
Controlled studies have not been conducted comparing the relative efficacies of a PPI + amoxicillin + fluoroquinolone regimen with and without the addition of bismuth. However, the use of PPI + amoxicillin + fluoroquinolone + bismuth quadruple therapy as a rescue therapy has been shown to be safe and effective in several studies.
Firstly, because no controlled studies have been conducted comparing the efficacy of a PPI + amoxicillin + fluoroquinolone regimen with and without the addition of bismuth, the authors evaluated the efficacy and safety of a new sequential therapy regimen and compared this regimen with the standard quadruple treatment regimen for H. pylori eradication. Secondly, the authors chose amoxicillin, levofloxacin, and furazolidone for these regimens based on an antibiotic resistance study conducted in patients from the Guangdong province in China. Finally, the authors chose two four-drug regimens as initial therapies despite the fact that standard triple therapy is considered standard of care in many countries. Furthermore, both of the regimens tested were well-tolerated as initial therapies.
The results of this study suggest that ilaprazole-based 10-d standard quadruple therapy does not offer an incremental benefit over modified sequential therapy for the treatment of H. pylori patients, as both regimens are effective, safe, and well-tolerated as initial treatment options.
H. pylori infection is widespread in humans. H. pylori is involved in chronic gastritis, non-ulcerative dyspepsia, peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphomas. Additionally, H. pylori is related to idiopathic iron deficiency anemia, chronic idiopathic thrombocytopenic purpura, Alzheimer’s disease, colorectal adenomas and colon cancer, and may be related to atherosclerosis, diabetes, hypertension, and obesity among other diseases. The eradication of H. pylori could ameliorate many of these H. pylori-related diseases.
This is a well-designed, performed and written clinical trial study to compare the efficacy and safety of a modified sequential therapy with the standard quadruple treatment for H. pylori eradication in 200 consecutive patients who were diagnosed with H. pylori-infected chronic gastritis in China were the eradication rate of H. pylori has yearly declined.
| 1. | Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Dong FY, Huang YQ, Bao ZJ. Regimens and Influencing Factors of Helicobacter pylori Eradication. Weichang Bingxue. 2013;18:565-568. [DOI] [Full Text] |
| 3. | Wei JQ, Nong GH, Chen MZ, Cong YY, Wu BH, Huang XP. In vitro drug resistance of Helicobacter pylori strain isolated from patients to commonly used antibacterial drugs in Zhuhai area. Zhongguo Linchuang Yaoxue Zazhi. 2013;22:234-237. |
| 4. | Lopes D, Nunes C, Martins MC, Sarmento B, Reis S. Eradication of Helicobacter pylori: Past, present and future. J Control Release. 2014;189:169-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Sugimoto M, Furuta T. Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J Gastroenterol. 2014;20:6400-6411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 6. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (7)] |
| 7. | Parzecka M, Szaflarska-Popławska A, Mierzwa G, Gorzkiewicz M, Łuczak S, Grzybowski T. [Genetic type of Helicobacter pylori and the efficacy of eradication therapy]. Pol Merkur Lekarski. 2009;26:105-109. [PubMed] |
| 8. | van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WG, de Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut. 2000;46:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Miyoshi M, Mizuno M, Ishiki K, Nagahara Y, Maga T, Torigoe T, Nasu J, Okada H, Yokota K, Oguma K. A randomized open trial for comparison of proton pump inhibitors, omeprazole versus rabeprazole, in dual therapy for Helicobacter pylori infection in relation to CYP2C19 genetic polymorphism. J Gastroenterol Hepatol. 2001;16:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Namiot DB, Leszczyńska K, Namiot Z, Kurylonek AJ, Kemona A. Smoking and drinking habits are important predictors of Helicobacter pylori eradication. Adv Med Sci. 2008;53:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cai W, Zhou L, Ren W, Deng L, Yu M. Variables influencing outcome of Helicobacter pylori eradication therapy in South China. Helicobacter. 2009;14:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kuo CH, Kuo FC, Hu HM, Liu CJ, Wang SS, Chen YH, Hsieh MC, Hou MF, Wu DC. The Optimal First-Line Therapy of Helicobacter pylori Infection in Year 2012. Gastroenterol Res Pract. 2012;2012:168361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, Song MJ, Park SK, Park JH, Kim HJ. [Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea]. Korean J Gastroenterol. 2008;51:280-284. [PubMed] |
| 14. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 15. | Wang L, Zhou L, Lin S, Hu H, Xia J. A new PPI, ilaprazole compared with omeprazole in the treatment of duodenal ulcer: a randomized double-blind multicenter trial. J Clin Gastroenterol. 2011;45:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Ilaprazole research group. Effect of Ilaprazole on duodenal ulcer and the influence of CYP2C19 polymorphisms: a multicenter clinical trial. Zhonghua Xiaohua Neijing Zazhi. 2009;26:475-479. [DOI] [Full Text] |
| 17. | DU YQ, Guo WY, Zou DW, Zhan XB, Li Z, Hu JH, Gong YF, He J, Lu JP, Li ZS. Acid inhibition effect of ilaprazole on Helicobacter pylori-negative healthy volunteers: an open randomized cross-over study. J Dig Dis. 2012;13:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | de Bortoli N, Martinucci I, Giacchino M, Blandizzi C, Marchi S, Savarino V, Savarino E. The pharmacokinetics of ilaprazole for gastro-esophageal reflux treatment. Expert Opin Drug Metab Toxicol. 2013;9:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20:9912-9921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 20. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Zhang CJ, Lu HM. Effect of trilogy treatment of rabeprazole, amoxicillin and clavulanate potassium and tinidazole on eradication Helicobacter pylori infection. Linchuang Yixue. 2008;28:17-18. [DOI] [Full Text] |
| 22. | Minakari M, Davarpanah Jazi AH, Shavakhi A, Moghareabed N, Fatahi F. A randomized controlled trial: efficacy and safety of azithromycin, ofloxacin, bismuth, and omeprazole compared with amoxicillin, clarithromycin, bismuth, and omeprazole as second-line therapy in patients with Helicobacter pylori infection. Helicobacter. 2010;15:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14:7361-7370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Uygun A, Ozel AM, Sivri B, Polat Z, Genç H, Sakin YS, Çelebi G, Uygur-Bayramiçli O, Erçin CN, Kadayifçi A. Efficacy of a modified sequential therapy including bismuth subcitrate as first-line therapy to eradicate Helicobacter pylori in a Turkish population. Helicobacter. 2012;17:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Wei JQ, Chen MZ, Wu BH, Huang XP. Clinical efficacy of Helicobacter pylori eradication in 7-day triple regimen and 10-day sequential therapy with ilaprazole. Zhongguo Linchuang Yaoxue Zazhi. 2012;21:280-283. |
| 26. | Gao W, Cheng H, Hu FL, Lü NH, Xie Y, Sheng JQ, Xu JM, Zhang LX, Zhang L, Chen Y. [Ilaprazole based bismuth-containing quadruple regimen for the first-line treatment of Helicobacter pylori infection: a multicenter, randomized, controlled clinical study]. Zhonghua Yi Xue Zazhi. 2012;92:2108-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol. 2010;16:4357-4362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Yanai A, Sakamoto K, Akanuma M, Ogura K, Maeda S. Non-bismuth quadruple therapy for first-line Helicobacter pylori eradication: A randomized study in Japan. World J Gastrointest Pharmacol Ther. 2012;3:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Lee ST, Lee DH, Lim JH, Kim N, Park YS, Shin CM, Jo HJ, Song IS. Efficacy of 7-Day and 14-Day Bismuth-Containing Quadruple Therapy and 7-Day and 14-Day Moxifloxacin-Based Triple Therapy as Second-Line Eradication for Helicobacter pylori Infection. Gut Liver. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Vorobjova T S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN