Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6271
Peer-review started: November 26, 2014
First decision: January 22, 2015
Revised: February 10, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: May 28, 2015
Processing time: 186 Days and 1.3 Hours
AIM: To assess the expression and prognostic value of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) in gastric cancer, and its correlation with vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR).
METHODS: Tumor and adjacent tissues were obtained from 123 patients who underwent radical surgery for gastric cancer at Renmin Hospital of Wuhan University from 2008-2009. The expression of NOX2, VEGF, EGFR and CD68 in tumor tissues was detected by immunohistochemistry. The expression of NOX2 in gastric cancer and adjacent tissues was detected by Western blot analysis. Spearman’s correlation was performed to elucidate the relationship of NOX2 with VEGF and EGFR. The Kaplan-Meier method was used to calculate survival time, and the log-rank test was used to evaluate differences in survival. Cox‘s proportional hazards regression model was applied in a stepwise manner to analyze the independent prognostic factors.
RESULTS: NOX2 exhibited positive expression in 47.2% (58/123) of the gastric cancer tissues. Western blot analysis revealed that NOX2 was up-regulated in tumor tissues compared to the adjacent tissue [39.0% (48/123)]. Immunohistochemistry staining revealed that CD68, which is a specific marker of macrophages, and NOX expression presented a similar localization and staining intensity. The expression of NOX2 was positively correlated with that of VEGF and EGFR. Comparison of the 5-year survival rates of the NOX2 positive and NOX2 negative groups showed that the NOX2 positive group presented a poor prognosis.
CONCLUSION: NOX2 positively correlates with the levels of VEGF and EGFR. NOX2 may be used as a new biomarker and a potential therapeutic target for gastric cancer.
Core tip: In this article, the authors used immunohistochemistry to analyze the expression of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) in formalin-fixed paraffin-embedded tissues from 123 gastric cancer patients. Western blot analysis was employed to examine NOX2 expression in tumor and adjacent tissues. NOX2 was up-regulated in tumor tissues. NOX2 was positively correlated with vascular endothelial growth factor and epidermal growth factor receptor. The results also showed that patients who were NOX2 positive clearly presented worse outcomes than NOX2-negative patients. The expression of NOX2 could be used as a prognostic biomarker for gastric cancer.
- Citation: Wang P, Shi Q, Deng WH, Yu J, Zuo T, Mei FC, Wang WX. Relationship between expression of NADPH oxidase 2 and invasion and prognosis of human gastric cancer. World J Gastroenterol 2015; 21(20): 6271-6279
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6271
Reactive oxygen species (ROS) are considered toxic by-products of various types of cellular aerobic respirations. The role of ROS in vivo is to modulate various cell functions, such as cell migration, proliferation and phagocytosis[1]. Recent studies have shown that ROS are implicated in many human diseases, including cancer, but the precise mechanisms for ROS to regulate cancer remain elusive. ROS and oxidative stress have been linked to cancer initiation and progression through their propensity to induce DNA mutations or DNA damage, genome instability and cell proliferation[2], therefore, the chromosomal imbalance theory and the DNA, RNA, or protein damage theory are thought to be the key words. Thus, ROS are believed to play an important role in the activation of oncogene products[3,4]. Enzymes are the major sources of ROS generation[5], and among all the resources of ROS the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family is the most important[6]. The NADPH oxidase (NOX) homologues include NOX1 to NOX5, DUOX1 and DUOX2. These enzymes can transfer electrons from NADPH to molecular oxygen, and in this process, form ROS. Additionally, the NOX family can promote angiogenesis and has been confirmed to be associated with the metastatic potential of many solid tumor[7]. Within the NOX family, the NOX2 isoform can maintain the cytoskeletal structure of the cell and prevents cell apoptosis[8], in addition to potentially influencing tumor proliferation.
Gastric cancer is the fourth most common type of malignant tumor worldwide and the second most common cause of cancer-related death[9]. For advanced gastric cancer patients, radical gastrostomy and lymph node dissection with chemotherapy are the common therapeutic methods[10]. However, the prognosis of gastric cancer patients is still poor. The 5-year survival rate for advanced gastric cancer is approximately 5%-20%, and the median overall survival is less than 1 year[11,12]. The reason for the poor outcome of gastric cancer remains elusive, though the eutrophic condition of gastric tumors has recently become a research hot spot. Tumor angiogenesis is a key point in the progression of solid tumors including gastric cancer. Angiogenesis is a vital step in the metastasis of tumors[13]. Vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, can activate vascular endothelial growth factor receptor 2 (VEGFR2), a receptor tyrosine kinase (PTK) in endothelial cells to promote proliferation and migration. Thus, VEGF represents a key molecule in the angiogenesis of tumors[7]. The angiogenic effect of VEGF can stimulate ROS such as superoxides[14]. The major source of ROS is the NOX family of enzymes, and NOX2 is related to tumor-associated macrophages which are the key modulators of tumor angiogenesis[15]. Thus, NOX2 may stimulate tumor angiogenesis through VEGF.
Epidermal growth factor receptor (EGFR) belongs to the receptor tyrosine kinase family which plays an important role in cellular processes, such as cell proliferation, apoptosis, angiogenesis and metastasis[16]. In gastric cancer a high level of EGFR expression is always associated with an increased risk of invasion or metastasis, and EGFR positive gastric cancer patients exhibit a worse prognosis[17]. NOX2 also plays an important role in cellular processes and can stimulate angiogenesis. NOX2 has been found to be highly expressed in prostate cancer cells and tissues, and a high level of NOX2 is correlated with the progression of prostate cancer[18]. However, whether NOX2 is expressed in gastric cancer and its correlation with the expression of VEGF and EGFR in gastric cancer remain unexplored.
In this study, we examined the expression of NOX2 and its role in the processes of gastric cancer. Then, we evaluated the prognostic value of NOX2 in gastric cancer.
A total of 123 patients who underwent a radical operation for gastric cancer at Renmin Hospital of Wuhan University from July 2008 to July 2009 were included in this study. None of these patients received preoperative and intraoperative chemotherapy or radiotherapy. The gastric cancer tissues and adjacent tissues were divided into two groups, one was used to produce the paraffin-embedded tissue sections, and the other was placed in liquid nitrogen for Western blot analysis. Clinical-pathological data were collected from all of the patients to analyze the relationship between the expression of NOX2 and the clinical-pathological data.
No patients died during their hospital stay. All patients were followed until July 2014 or death. The methods employed for follow-up were out-patient review and a telephone follow-up study.
Paraffin-embedded tissues were obtained from Renmin Hospital of Wuhan University and were sectioned into 3 µm thick tissue sections. Immunohistochemical analyses of NOX2, CD68, VEGF and EGFR were performed using a standard strepavidin-peroxidase (SP) method[19]. Minor steps were changed as follows: sections were deparaffinized and dehydrated using a graded series of ethanol solutions. The slides were immersed in 10 mmol/L citrate buffer (pH 6.0) and boiled for 15 min in the microwave oven for the antigen retrieval. Then the slides were allowed to cool at room temperature. Hydrogen peroxidase (0.3%) was used to halt the endogenous peroxidase activity for 15 min at the room temperature. Non-specific binding was blocked by the goat serum (5%) for 10 min. The primary antibody was a rabbit antibody to NOX2 (1:100, abcam, United Kingdom), mouse antibody to CD68 (1:100, abcam, United Kingdom), rabbit antibody to VEGF (1:75, ZSGB-BIO, China), or rabbit antibody to EGFR (1:100, ZSGB-BIO, China). Sections were incubated with the primary antibody overnight at 4 °C, then incubated with a second antibody. The staining results was visualized using the 3,5-diaminobenzidine. In each immunohistochemistry staining analysis, PBS instead of the primary antibody was used as the negative control.
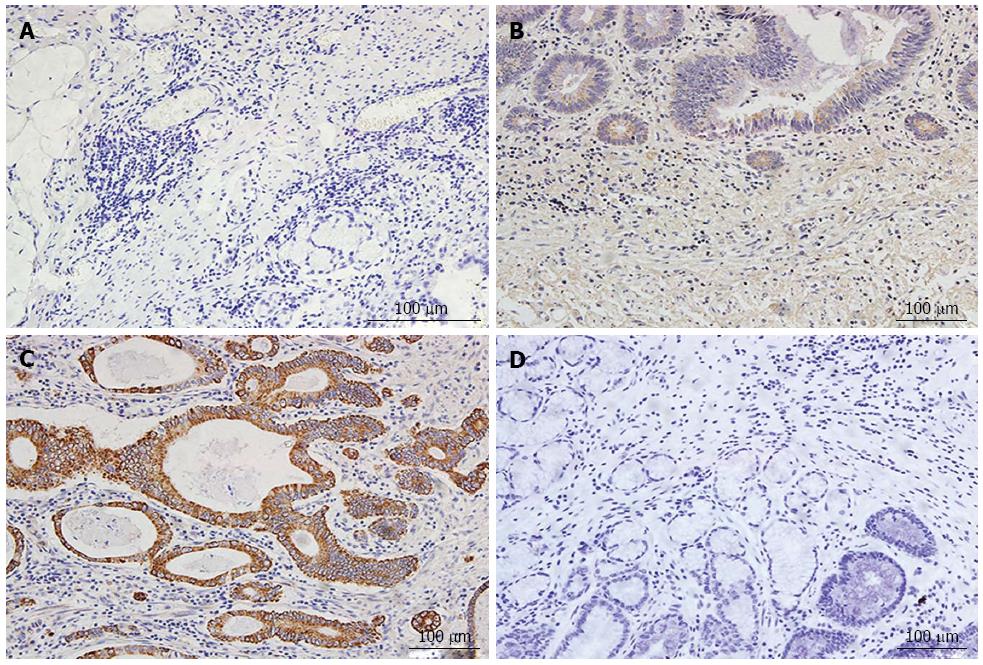
Two independent observers who were experienced in examining immunohistochemistry results evaluated the results. Both were blinded to the clinical data of the patients. An OLYMPUS BX53 upright microscope was used to obtain photographs of the immunohistochemistry staining results. Positive expression of NOX2 was observed as yellow or brown staining in the cytoplasm. Image-pro Plus 6.0 was used to judge the area of staining and to obtain the density and optical density values for the Immunohistochemsitry (IHC) sections. According to the observed staining intensity, the IHC results can be defined as 0 points, no staining; 1 point, pale yellow staining; 2 points, yellow staining; 3 points, brown staining. The percentage of positive tumor cells was determined in at least 5 areas under × 200 magnification and then averaged. The mean percentage was subsequently divided into four categories: 0, < 5%; 1, 5%-25%; 2, 26%-50%; and 3, 51%-100%. Finally, we combined the results for the staining intensity and the percentage of positive tumor cells as follows: 0-2 was defined as negative expression, and 3-6 as positive expression[20].
The fresh gastric cancer tissue and the adjacent tissue were homogenized in ice-cold lysis buffer in the presence of protease inhibitor cocktail. Concentrations of protein in the samples were determined using the Bradford method with bovine serum albumin as a standard. In brief, an equal amount of protein samples were separated on 10% sodium dodecyl sulfatepolyacrylamide gels and then transferred to a PVDF membrane. The membrane was blocked with 5% skim milk in the TBST buffer (TBS containing 0.1% Tween-20) at room temperature for 2 h and then incubated with the rabbit polyclonal anti-NOX2 antibody (1:1000, Abcam, United Kingdom) at 4 °C overnight. After extensive rinsing with TBST, the blots were incubated with IRDye 800CW goat anti-rabbit secondary antibody (926-32211 LI-COR, United States 1:10000) at room temperature for 1.5 h, and the expression of NOX2 was detected with the Odyssey imaging system.
The data were analyzed with SPSS 19.0 statistical software. The χ2 test and Fisher’s exact test were used to assess the association of NOX2 expression with the clinical-pathological characters. The Kaplan-Meier method was employed to calculate the survival curve, and the log-rank test was employed to evaluate the differences in survival. Cox’s regression model was applied in a stepwise manner to analyze the independent prognostic factors. The correlations of NOX2 with VEGF and EGFR were evaluated via Spearman’s rank correlation. A P-value less than 0.05 was considered to indicate statistical significance.
NOX2 was overexpressed in the gastric cancer tissues compared with the adjacent tissue. NOX2 expression was observed in the gastric cancer cytoplasm. Among the gastric cancer tissues, 47.2% (58/123) were positive for NOX2 expression. The rate of NOX2 expression in the adjacent tissue was 44.7% (55/123), and the staining density in the adjacent tissue was weaker than in the GC tissues (Figure 1).
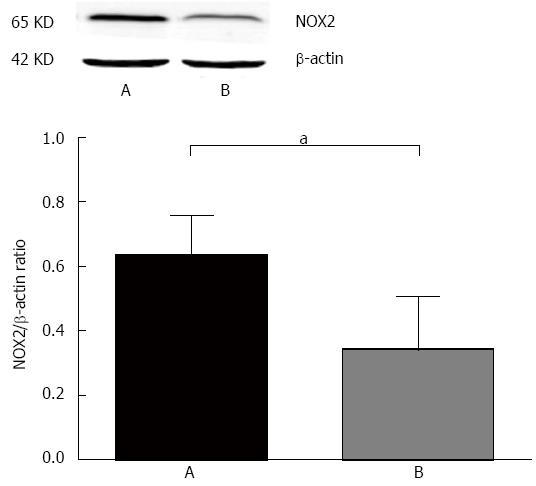
The rate of NOX2 up-regulation in gastric cancer was 39.0% (48/123), and the expression of NOX2 in gastric cancer tissues was higher than in the adjacent cancer tissues (Figure 2).
The histological types of the gastric cancer tissues from all the 123 patients were adenocarcinomas. The adenocarcinomas were divided into highly differentiated (22 patients), moderately differentiated (34 patients), poorly differentiated (62 patients) and undifferentiated (5 patients). The pathological TNM staging of gastric cancer revealed stages I + II in 35 patients and stages III + IV in 70 patients. In the patients showing a poor histological grade and tumor stage, NOX2 expression was higher than in those with a good grade, and NOX2 expression showed no significant correlation with age, sex or tumor size. Information about the relationship between NOX2 expression and clinical-pathological character is shown in Table 1.
| Clinical feature | Total number (n = 123) | NOX2 positivity negativity | χ2-value | P-value |
| Gender | ||||
| Male | 69 | 30 (43.5) | 0.852 | 0.229 |
| 39 (56.5) | ||||
| Female | 54 | 28 (51.9) | ||
| 26 (48.1) | ||||
| Age (yr) | ||||
| < 59 | 55 | 27 (49.1) | 0.150 | 0.419 |
| 28 (50.9) | ||||
| ≥ 59 | 68 | 31 (45.6) | ||
| 37 (54.4) | ||||
| Position | ||||
| Cardia and fundus | 30 | 8 (26.6) | 6.683 | 0.008 |
| 22 (73.3) | ||||
| Body and antrum | 93 | 50 (53.8) | ||
| 43 (46.2) | ||||
| Differentiation type | ||||
| High | 22 | 6 (27.3) | 5.062 | 0.042 |
| 16 (72.7) | ||||
| Moderate | 34 | 16 (47.1) | ||
| 18 (52.9) | ||||
| Poor | 62 | 34 (54.8) | ||
| 28 (45.2) | ||||
| Undifferentiation | 5 | 2 (40) | ||
| 3 (60) | ||||
| Lymph node metastasis | ||||
| Yes | 53 | 34 (64.2) | 10.796 | 0.001 |
| 19 (35.8) | ||||
| No | 70 | 24 (34.3) | ||
| 46 (65.7) | ||||
| Vascular invasion | ||||
| Yes | 60 | 35 (58.3) | 5.875 | 0.012 |
| 25 (41.7) | ||||
| No | 63 | 23 (36.5) | ||
| 40 (63.5) | ||||
| Clinical stage | ||||
| Early stage | 32 | 9(28.1) | 6.285 | 0.010 |
| 23(71.9) | ||||
| Advanced stage | 91 | 49(53.8) | ||
| 42(46.2) | ||||
| TNM | ||||
| I + II | 35 | 10(28.6) | 6.779 | 0.008 |
| 25(71.4) | ||||
| III + IV | 88 | 48(68.6) | ||
| 40(31.4) | ||||
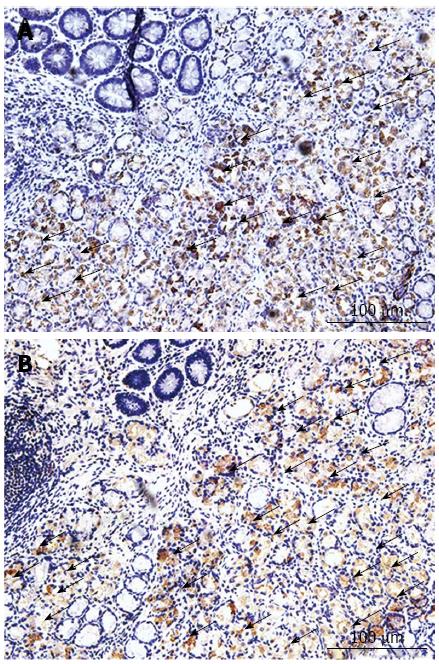
The IHC staining results presented in Figure 3 show that CD68 and the NOX expression presented similar locations and staining intensities.
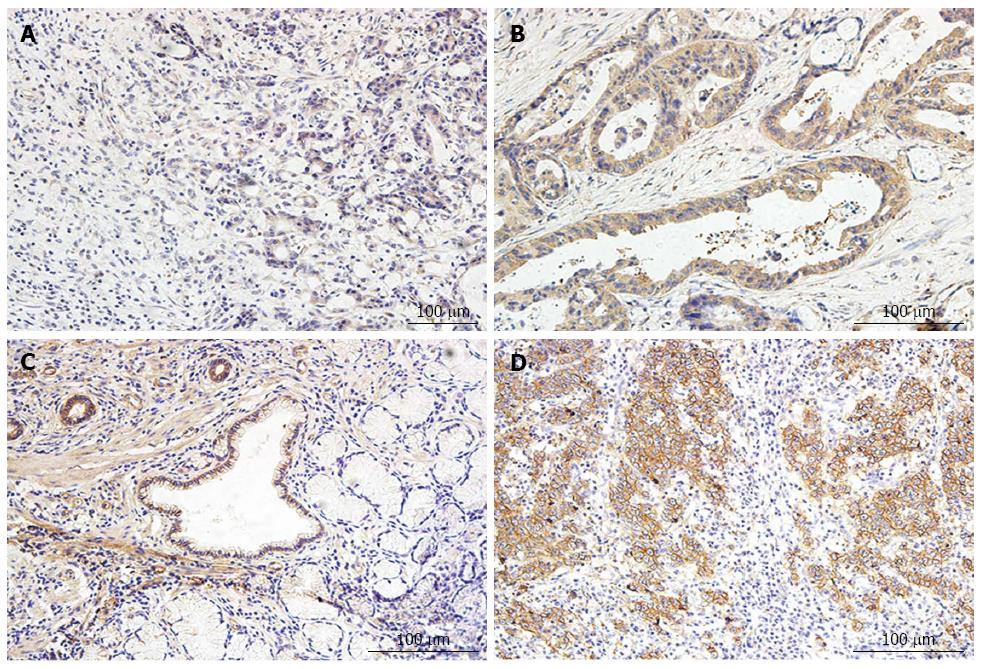
VEGF and EGFR expression was observed in gastric cancer. Positive expression of VEGF and EGFR was detected in the cytoplasm of the tumor tissues. The expression of VEGF and EGFR in the cancers was correlated with the clinical-pathological features. In patients with a poor tumor stage and histological grade the immunohistochemistry staining score was higher than in those presenting a good grade and stage. In Figure 4 strong positive expression of VEGF and weak expression of VEGF are shown.
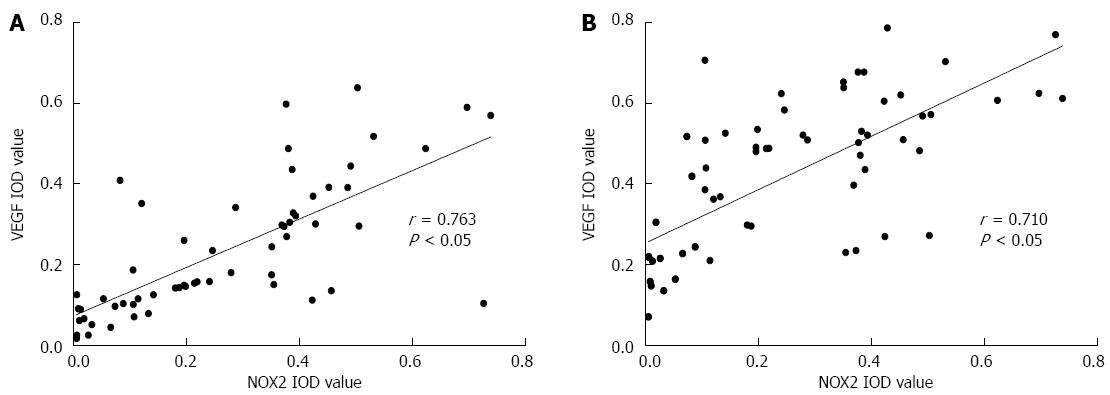
The mean IOD values of NOX2, VEGF and EGFR in the gastric cancer tissues were measured. Based on determination of the spearman rank correlation coefficient, as shown in Figure 5, we found that the expression of NOX2 was positively correlated with VEGF (r = 0.763, P < 0.05) and EGFR (r = 0.710, P < 0.05).
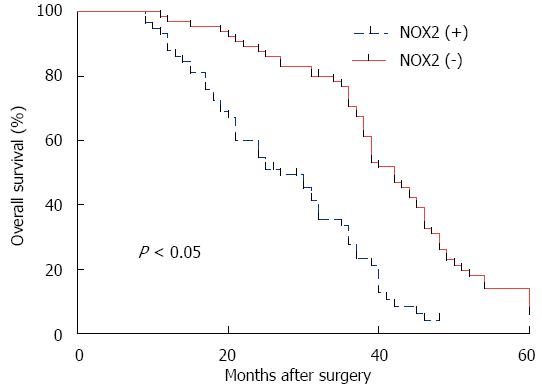
No patients died during their hospital stay. All patients were followed until July 1, 2014 or death. The follow-up investigation of the 5-year survival study showed that the patients who were NOX2 positive clearly presented a worse outcome than the NOX2 negative patients (Figure 6). Thus NOX2 may be a novel biomarker of gastric cancer for estimating its prognosis. Based on the univariate analysis of the clinical-pathological features related to prognosis in the 123 gastric cancer patients presented in Table 2, overall survival was significantly correlated with clinical stage, lymph node metastasis, vascular invasion, TNM stage, and NOX2 positivity. Furthermore, the association between NOX2 positivity and survival was still significant after controlling for other prognostic markers in the multivariate analysis, while in the outcome of the multivariate analysis, it was shown that the differentiation type, lymph node metastasis, vascular invasion and TNM stage were independent prognostic factors as well (Table 3).
| Variable | Coef | SE | Wald | RR value (95%CI) | P-value |
| Clinical stage | 0.531 | 0.221 | 5.7970 | 1.701 (1.104-2.620) | 0.016 |
| Differentiation type | 0.363 | 0.193 | 3.5250 | 1.438 (0.984-2.101) | 0.060 |
| Lymph node metastasis | 0.971 | 0.213 | 20.857 | 0.379 (0.250-0.575) | 0.000 |
| Vascular invasion | 0.926 | 0.198 | 21.854 | 0.396 (0.269-0.584) | 0.000 |
| TNM | 0.638 | 0.214 | 8.9030 | 1.892 (1.245-2.876) | 0.003 |
| NOX2 | 1.080 | 0.212 | 26.004 | 0.339 (0.224-0.514) | 0.000 |
| Variable | Coef | SE | Wald | RR value (95%CI) | P-value |
| Clinical stage | 0.670 | 0.236 | 8.0800 | 1.955 (1.230-3.103) | 0.004 |
| Differentiation type | 0.176 | 0.201 | 0.7710 | 1.193 (0.805-1.769) | 0.380 |
| Lymph node metastasis | 0.760 | 0.232 | 10.725 | 0.468 (0.297-0.737) | 0.001 |
| Vascular invasion | 1.061 | 0.220 | 23.330 | 0.346 (0.225-0.532) | 0.000 |
| TNM | 0.519 | 0.229 | 5.1360 | 0.637 (0.406-0.999) | 0.049 |
| NOX2 | 0.824 | 0.236 | 12.153 | 0.439 (0.276-0.697) | 0.000 |
Oxidative stress has been demonstrated to play a key role in many clinical phenomena, such as the inflammatory response and the ageing process[21]. Recent studies have shown that the expression of oxidative stress is higher in many malignancies, including breast cancer, colon cancer and head neck neoplasms[22]. Oxidative stress is the major cause of enhanced cell migration, and it can induce the expression of oncogenes and suppress the activity of anti-survival molecules[23]. The level of oxidative stress is associated with the intracellular ROS level, and NADPH oxidase is a major source of the ROS. NOX2 was the first identified member of the NADPH oxidase family[24], and its role in the progression of malignancies may be correlated with the promotion of angiogenesis[7]. In tumor progression, NADPH oxidases can play the role of a mediator, and may damage DNA within cells and activate oncogenic transformation[25]. In recent studies, it has been shown that ROS can also induce apoptosis of tumor cells[26]. This repeated apoptosis of tumor cells may promote the ability to resist apoptosis in tumor cells, which could introduce mutations in these cells. Therefore, in the present study, we examined the expression of NOX2 in gastric cancer and its correlation with clinical-pathological characters. We included CD68 in analyses because macrophages may one of the source of NOX2. IHC staining for CD68 and NOX2 in the same tissue sections showed that CD68 and NOX2 share the same positive sites. Therefore, we deduced that macrophages may be one of the sources of NOX2. We determined VEGF and EGFR levels in different clinical-pathological gastric tumor tissues. Then, we studied the correlation of NOX2 with VEGF and EGFR through Spearman’s rank correlation. The Spearman’s rank correlation coefficients for NOX2 with VEGF and EGFR were 0.763 and 0.710, respectively, and NOX2 was positively correlated with the levels of VEGF and EGFR in gastric cancer. Thus, sections with a high level of NOX2 always show high expression of VEGF and EGFR in gastric cancer. NOX2 may play a key role in the progression of gastric cancer.
This study reports the expression of NOX2, which is the central component of NADPH oxidase in gastric cancer for the first time. Based on the results of IHC staining, it can be observed that the staining of NOX2 occurs at similar sites to CD68 (Figure 2). This finding indicates that NOX2 is closely associated with inflammatory cells such as macrophages. Additionally, macrophages could be a critical determinant of angiogenesis and tumor progression[15]. Thus, it follows that NOX2 may also be associated with angiogenesis and tumor progression, and a recent study implied that NOX2 does, in fact, stimulate angiogenesis in mouse models[27]. Angiogenesis is widely accepted as an important indicator of tumor metastasis because without a blood supply the tumor itself may degenerate. Thus, regulation of angiogenesis through certain signaling pathways may promote the treatment of tumors[28]. Angiogenesis represents a key point in the tumor blood supply. Therefore, if we can control angiogenesis, we can control the blood supply to the tumor. ROS are the major cause of revascularization, and NOX2 can stimulate the human body to produce ROS. Increasing evidence has shown that ROS are implicated in tumor metastasis[29]. ROS can cause the release of VEGF[30], which is important for the angiogenesis of the tumor, and ROS can also stimulate VEGF expression in vascular smooth muscle cells and endothelial cells[31]. EGFR, which is a major treatment bio-target for gastric cancer, is an important biomarker for the prognosis of gastric cancer. A high expression level of EGFR may predict poor survival in gastric cancer. Inhibition of EGFR may suppress the growth, angiogenesis, and metastasis of gastric cancer cells. In this study, we chose to use EGFR as a biomarker for gastric cancer, and we subsequently applied Spearman correlation to analyze the relationship between NOX2 and EGFR levels in gastric cancer. The result of Spearman correlation showed that NOX2 was positively correlated with the level of EGFR. Therefore, as the major source of ROS, NOX2 may also play an important role in the progression of tumor and cardiovascular diseases.
Gastric cancer is a common digestive system malignancy, and although the diagnosis of and therapeutic strategies for gastric cancer have improved, it remains one of the most dangerous human malignancies[32]. Gastric cancer is the second most common cause of cancer-related death[9]. The classification of advanced gastric cancer is important for the prognosis of patients. The classification determines the growth and metastasis of the tumor cells and, thus, affects the prognosis of gastric cancer. Blood vessel and lymphatic invasion are also important phenomena regarding the prognosis of gastric cancer[33], and among the factors related to blood vessel invasion, VEGF is a core promoter. VEGF represents a key point in the angiogenesis, which is the foundation for the growth and metastasis of tumors, and anti-VEGF therapy has been applied in clinical research[34]. In the present study, we examined VEGF expression in 123 patients with different clinical-pathological features. Our data showed that positive expression of VEGF occurred in 73.4% of the total gastric cancer cases, and the immunohistochemical scores for patients presenting a poor clinical-pathology were higher than for those showing a good clinical-pathology. The correlation between the expression of NOX2 and VEGF was positive, with a correlation coefficient of 0.330. This result indicated that NOX2 could be used as a new prognostic bio-marker of gastric cancer, and anti-oxidative stress may be a new way to control the initiation and progression of gastric cancer.
In summary, the present study demonstrated that the expression level of NOX2 was increased in gastric cancer tissue compared with para-carcinoma tissue, and the obtained IHC scores were higher in the poor clinical-pathology group compared with the good clinical-pathology group. Based on these findings, NOX2 may be used as a new bio-marker of gastric cancer. As a key promoter of angiogenesis and tumorigenesis, NOX2 may promote pro-tumor processes. However, given the current paucity from the field of this research in gastric cancer, further studies must apply a logical and unifying approach to identify cell-specific pathways influenced by NOX2 and the underlying molecular mechanism in the progression and prognosis of gastric cancer.
Gastric cancer (GC) is one of the most common types of malignant tumors in the digestive system. Despite the progress in the diagnosis and treatment of GC, the outcome of GC is still poor. Exploring a novel biomarker of GC will aid in establishing early diagnoses, improving treatment regimens and determining the prognosis for GC patients.
Reactive oxygen species (ROS) represent a hot spot in the field of tumor research. The functions of ROS and oxidative stress in vivo are complicated, but in tumor studies, they have been demonstrated to be important participants in the initiation and progression of solid tumors. NADPH oxidase 2 (NOX2), as a main source of ROS, has been reported to be associated with the metastatic potential of many solid tumors and shows a close correlation with the angiogenesis of tumors. However, the expression of NOX2 in GC and its correlation with vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) have not been studied previously. In this study, the authors observed up-regulated expression of NOX2 in GC, and the expression of GC was positively correlated with the levels of VEGF and EGFR.
The results of this research showed that NOX2 expression was positively correlated with the levels of VEGF and EGFR. Patients with higher expression of NOX2 presented a worse outcome. NOX2 may be used as a novel indicator of a poor prognosis in patients with GC.
By studying the association of NOX2 with VEGF and EGFR and the prognosis of GC, this work may stimulate other researchers to pay special attention to the new biomarker NOX2. NOX2 could be included in the assessment of the prognosis of GC patients.
NOX2 belongs to the NADPH oxidase family. It was the first identified member of the NADPH oxidase family, and its role in the progression of malignancy may be correlated with the promotion of angiogenesis. VEGF represents a key point in angiogenesis, which is the foundation for the growth and metastasis of tumors. EGFR, which is a major treatment bio-target for many cancers, is an important biomarker for the prognosis of tumors. A high expression level of EGFR may predict poor survival in GC.
Through the immunohistochemical and western blot analyses of NOX2 in 123 GC tissues and corresponding adjacent tissues, the authors found that NOX2 was up-regulated in the GC tissues compared with the adjacent tissues. The levels of NOX2 were positively correlated with VEGF and EGFR levels. The relationship of NOX2 expression with the clinicopathological features and prognosis of GC patients showed that NOX2 may present potential value in the prognosis of GC.
| 1. | Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLoS One. 2011;6:e14665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240-245. [PubMed] |
| 3. | Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett’s esophagus, and the latter’s progression toward esophageal cancer? Am J Gastroenterol. 2002;97:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3-11. [PubMed] |
| 5. | Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal. 2011;14:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Guichard C, Pedruzzi E, Fay M, Ben Mkaddem S, Coant N, Daniel F, Ogier-Denis E. [The Nox/Duox family of ROS-generating NADPH oxidases]. Med Sci (Paris). 2006;22:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 8. | Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 9. | Hallinan JT, Venkatesh SK, Peter L, Makmur A, Yong WP, So JB. CT volumetry for gastric carcinoma: association with TNM stage. Eur Radiol. 2014;24:3105-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yonemura Y, Endo Y, Obata T, Sasaki T. Recent advances in the treatment of peritoneal dissemination of gastrointestinal cancers by nucleoside antimetabolites. Cancer Sci. 2007;98:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, Montgomery E, Heitmiller RE, Choti MA, Lillemoe KD. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 12. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 2655] [Article Influence: 132.8] [Reference Citation Analysis (1)] |
| 13. | Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;291:H1563-H1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal. 2012;16:1229-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Cui XF, Zhou WM, Yang Y, Zhou J, Li XL, Lin L, Zhang HJ. Epidermal growth factor upregulates serotonin transporter and its association with visceral hypersensitivity in irritable bowel syndrome. World J Gastroenterol. 2014;20:13521-13529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hong L, Han Y, Brain L. The role of epidermal growth factor receptor in prognosis and treatment of gastric cancer. Expert Rev Gastroenterol Hepatol. 2014;8:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Jones KJ, Chetram MA, Bethea DA, Bryant LK, Odero-Marah V, Hinton CV. Cysteine (C)-X-C Receptor 4 Regulates NADPH Oxidase-2 During Oxidative Stress in Prostate Cancer Cells. Cancer Microenviron. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 19. | Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S. Nox-2 is a modulator of fibrogenesis in kidney allografts. Am J Transplant. 2009;9:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6483] [Cited by in RCA: 6440] [Article Influence: 268.3] [Reference Citation Analysis (0)] |
| 22. | Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Chetram MA, Hinton CV. ROS-mediated regulation of CXCR4 in cancer. Front Biol (Beijing). 2013;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Minakami R, Sumimotoa H. Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (nox) family. Int J Hematol. 2006;84:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7-21. [PubMed] |
| 26. | Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415-418. [PubMed] |
| 27. | Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160-1167. [PubMed] |
| 28. | Takahashi H, Ojima H, Shimizu H, Furuse J, Furukawa H, Shibata T. Axitinib (AG-013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn J Clin Oncol. 2014;44:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Chen J, Liu B, Yuan J, Yang J, Zhang J, An Y, Tie L, Pan Y, Li X. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol Oncol. 2012;6:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Das S, Singh G, Baker AB. Overcoming disease-induced growth factor resistance in therapeutic angiogenesis using recombinant co-receptors delivered by a liposomal system. Biomaterials. 2014;35:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25:891-897. [PubMed] |
| 32. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 33. | Gresta LT, Rodrigues-Júnior IA, de Castro LP, Cassali GD, Cabral MM. Assessment of vascular invasion in gastric cancer: a comparative study. World J Gastroenterol. 2013;19:3761-3769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Di Domenico M, Ricciardi C, Fusco A, Pierantoni GM. Anti-VEGF therapy in breast and lung mouse models of cancers. J Biomed Biotechnol. 2011;2011:947928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Duan GC, Suo J S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN