Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6229
Peer-review started: September 26, 2014
First decision: October 29, 2014
Revised: November 17, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: May 28, 2015
Processing time: 250 Days and 18.8 Hours
AIM: To investigate whether children with congenital common bile duct dilatation (CBDD) differ from children with obstructive CBDD in cholangiographic characteristics.
METHODS: In this retrospective cohort study, the baseline data and the results of imaging analyses were reviewed among children who had endoscopic retrograde cholangiopancreatography (ERCP) due to CBDD. ERCP was performed on all pediatric patients by experienced pediatric endoscopists. The maximal transverse diameter of the common bile duct (CBD) was measured on ERCP. To assess whether age-adjusted CBDD could be used for differential diagnosis, a CBDD severity index (SI) was calculated by dividing the measured CBD diameter by the age-corrected maximal diameter of a normal CBD.
RESULTS: A retrospective medical chart review revealed that 85 consecutive children under 16 years of age with hepatobiliary disease and CBDD were referred to Seoul Asan Medical Center. Fifty-five (64.7%) children had congenital CBDD and 30 (35.3%) had obstructive CBDD. The two groups did not differ significantly in terms of clinical characteristics except for sex. The congenital and obstructive CBDD groups did not differ significantly in terms of mean CBD diameter (19.3 ± 9.6 mm vs 12.2 ± 4.1 mm, P > 0.05). However, congenital CBDD cases had a significantly higher mean SI than obstructive CBDD cases (3.62 ± 1.64 vs 1.98 ± 0.71, P = 0.01). In multivariate analysis, an SI value ≥ 2.32 and comorbidity with anomalous union of pancreaticobiliary duct (APBDU) in ERCP independently predicted congenital CBDD.
CONCLUSION: Measuring the CBD may aid the differential diagnosis of both CBDD and APBDU in children.
Core tip: A severity index calculated by measuring the diameter of the common bile duct (CBD) adjusted for age was a better method to discriminate between congenital common bile duct dilatation (CBDD) and secondarily obstructive CBDD in children compared with simply measuring the diameter of the CBD.
- Citation: Oh SH, Chang SH, Kim HJ, Cho JM, Hwang JH, Namgoong JM, Kim DY, Cho YA, Yoon CH, Kim KM. Cholangiographic characteristics of common bile duct dilatation in children. World J Gastroenterol 2015; 21(20): 6229-6235
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6229.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6229
The causes of common bile duct dilatation (CBDD) may differ according to age and geography. In adults, CBDD is generally caused by intrinsic luminal obstruction and thus investigative methods focus mainly on the causes of obstruction, such as biliary tract stones and pancreaticobiliary malignancies[1,2]. In children, congenital CBDD (i.e., choledochal cyst) must be considered when investigating the causes of CBDD[1,2]. However, in Western countries, pediatric choledochal cyst is rare, accounting for only 1.2%-8.6% of pediatric patients who undergo endoscopic retrograde cholangiopancreatography (ERCP) for pancreatic and biliary disease; by contrast, pancreatitis and choledolithiasis are diagnosed much more commonly in this pediatric population[3-5]. However, in East Asia, the most frequent diagnosis in children who are investigated by ERCP for pancreatic and biliary disease is choledochal cyst[6-8]. Moreover, the frequency of choledochal cyst differs depending on the patient’s country of origin: one-third of the patients who are reported to have this condition in the world are from Japan[6-9].
The clinical manifestations of choledochal cyst also differ depending on patient age[9]. In Japan, choledolithiasis has been reported in 18%-70% of adults with choledochal cyst. By contrast, only 9% of pediatric patients with choledochal cyst were reported to have choledolithiasis[9,10], although recent findings suggest that this prevalence may be higher than was previously believed (17%-29%)[9-12]. Such relatively high choledolithiasis co-morbidity may initially complicate the differential diagnosis of CBDD from choledolithiasis alone. Choledolithiasis can lead to CBDD that can be initially misdiagnosed as choledochal cyst in both children and adults[13-15]. Thus, choledochal cyst should be diagnosed on the basis of both the clinical features and the results of various diagnostic modalities[6,9,16].
In adults with CBDD, the diameter of the common bile duct (CBD) is considered to be of no clinical significance[17-19]. This is because the studies in adults aimed to differentially diagnose stones from mitotic lesions in the biliary tree. To our knowledge, studies of pediatric CBDD that examine whether bile duct size can help to differentiate between obstructive and congenital causes of CBDD have not yet been performed. Therefore, the aim of this study was to determine whether children with congenital CBDD differ from children with other CBDD causes in terms of cholangiographic characteristics.
A retrospective medical chart review revealed that 85 consecutive children under 16 years of age with hepatobiliary disease and CBDD were referred to Seoul Asan Medical Center, a tertiary referral center in Seoul, South Korea between January, 2000 and January, 2012[6,16]. The baseline data and the results of imaging analyses were documented. All children were screened by trans-abdominal ultrasonography (TUS) and more than one imaging modality, such as computed tomography and magnetic resonance cholangiopancreatography (MRCP). They underwent a total of 123 ERCP procedures. The study protocol was approved by the institutional review board of Asan Medical Center, Seoul.
ERCP was performed on all pediatric patients by experienced pediatric endoscopists[6]. The maximal transverse diameter of the CBD between the insertion of the cystic duct and the head of the pancreas was measured along its longitudinal axis via a cholangiogram[20,21]. Measurements were not taken within 5 mm of the origin of the CBD. The reference cut-off value for the normal maximum diameter of the CBD relative to age was obtained from the intravenous cholangiographic data of Witcombe et al[20]. To assess whether age-adjusted CBDD could be used for differential diagnosis, a CBDD severity index (SI) was calculated by dividing the measured CBD diameter by the age-corrected maximal diameter of a normal CBD. To avoid variation due to other causes, patients with a previous history of cholecystectomy, obstructive cholestasis, and premedication such as with opioids were excluded. Moreover, measurements were made on unmagnified cholangiograms by using electronic calipers. Neonatal cases were also excluded due to technical difficulties in ERCP. Although contrast dye was gently flushed into the CBD, the dilatation caused by direct dye injection into the duct was ignored. All ERCP findings were reviewed by radiologists and the diagnosis of choledochal cyst was confirmed by surgical excision and intraoperative cholangiography. To reduce intraobserver and interobserver variability, measurement of the diameter was performed three times and average values of the diameter were used. These were validated by gastroenterologists and radiologists at the same institution. This process led to patients being classified into those with congenital CBDD and those with obstructive CBDD due to secondary causes. The morphological descriptions of the CBD were based on Todani’s classification system[22,23].
For univariate analysis, continuous variables were assessed by using independent sample t-tests and categorical variables were assessed by using χ2 tests. For multivariate analysis, a logistic regression model was used to generate odds ratios (ORs), the corresponding 95% confidence intervals (95%CIs), and the P values. The optimal cut-off of CBD that allowed congenital CBDD to be differentiated from obstructive CBDD was determined by using a receiver operating characteristic (ROC) curve. All statistical calculations were performed by using SPSS software (SPSS for Windows, version 14.0; SPSS Inc., Chicago, IL). A P value less than 0.05 was considered to indicate statistical significance.
In total, 33 boys (38.8%) and 52 girls (61.2%) were diagnosed with CBDD according to our study criteria. The mean patient age was 6.3 ± 3.6 years. The indications for ERCP are summarized in Table 1. Fifty-five (64.7%) children had congenital CBDD and 30 (35.3%) had CBDD due to other secondary causes. The clinical and cholangiographic characteristics of the 85 patients are summarized in Table 2. The most common presenting clinical manifestations in both groups at the time of diagnosis were abdominal pain and jaundice. Some patients in both groups also presented with pancreatitis. This was regarded as a complication in the patients with choledochal cyst but as the underlying disease in the patients with obstructive CBDD. The two groups did not differ significantly in terms of clinical characteristics except for sex: the patients with congenital CBDD were significantly more likely to be female than the patients with obstructive CBDD (80% vs 26.7%, P = 0.032).
| Causes | n = 85 | Descriptions |
| Congenital CBDD | n = 55 (64.7%) | |
| Choledochal cyst | n = 55 | Type I (n = 26) |
| Type IVa (n = 29) | ||
| Obstructive CBDD | n = 30 (35.3%) | |
| Choledolithiasis | n = 23 | Idiopathic (n = 10) |
| Chronic pancreatitis (n = 6) | ||
| Leukemia (n = 2) | ||
| G6PD deficiency (n = 2) | ||
| Spherocytosis (n = 2) | ||
| Trauma (n = 1) | ||
| Miscellaneous | n = 7 | Chronic pancreatitis (n = 3) |
| Lymphoma/pancreatic cancer (n = 3) | ||
| Trauma (n = 1) |
| Characteristics total (n = 85) | Congenital CBDD (n = 55) | Obstructive CBDD (n = 30) | Univariate P value | Multivariate P value |
| Clinical | ||||
| Age, mean ± SD (mo) | 63.8 ± 36.4 | 82.4 ± 46.0 | NS | |
| Sex, M:F | 11:44 | 22:8 | 0.042 | |
| Abdominal pain | 50 (91.0) | 24 (80.0) | NS | |
| Jaundice | 23 (41.8) | 13 (43.3) | NS | |
| Abdominal mass | 2 (3.6) | 0 (0) | NS | |
| Vomiting | 11 (20.0) | 4 (13.3) | NS | |
| Cholangitis | 5 (9.0) | 1 (3.3) | NS | |
| Pancreatitis | 23 (43.6) | 9 (30.0) | NS | |
| Cholangiographic | ||||
| CBD diameter | 19.3 ± 9.6 | 12.2 ± 4.1 | NS | |
| Severity index ≥ 2.32 | 34 (61.8) | 1 (3.3) | 0.012 | 0.024 |
| Cystic features | 14 (21.8) | 2 (6.7) | NS | |
| Cylindrical-fusiform feature | 43 (78.2) | 28 (93.3) | NS | |
| APBDU | 39 (70.9) | 0 (0) | 0.005 | 0.001 |
| Choledolithiasis | 34 (61.8) | 23 (76.6) | NS | |
| Cholelithiasis | 3 (5.5) | 7 (23.3) | NS | |
| Pancreatic duct dilatation | 5 (9) | 2 (6.7) | NS |
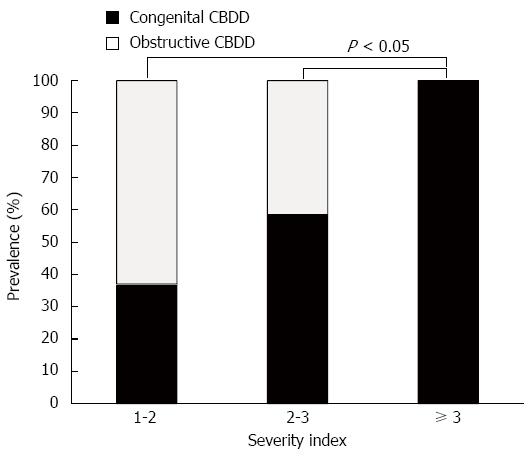
The congenital and obstructive CBDD groups did not differ significantly in terms of mean CBD diameter (19.3 ± 9.6 mm vs 12.2 ± 4.1 mm). However, the congenital group had a significantly higher mean CBDD SI (3.62 ± 1.64) than the obstructive CBDD group (1.98 ± 0.71). In addition, as the SI increased, so did the prevalence of choledochal cyst among children with CBDD (Figure 1). All patients with an SI of ≥ 3 had a choledochal cyst, unlike patients with an SI of 1-2 or 2-3 (both P < 0.05). ROC analysis showed that an SI of 2.32 could serve as a cut-off value with a sensitivity of 68%, a specificity of 96.7%, and an area under the curve of 0.87. Of the 55 children with congenital CBDD, 34 (61.8%) had a SI of ≥ 2.32. By contrast, only one of 30 children (3.3%) with obstructive CBDD had such a high SI.
Despite the high specificity of the CBDD SI ≥ 2.32, its low sensitivity means that additional efforts are needed to distinguish between the two types of CBDD in children with SI < 2.32. We observed that anomalous union of pancreaticobiliary duct (APBDU) was very common in congenital CBDD: of the 21 children with congenital CBDD and SI < 2.32, 15 (71.4%) had APBDU. Multivariate analysis revealed that SI ≥ 2.32 (OR = 2.4, 95%CI: 1.2-5.52) and APBDU comorbidity (OR= 5.7, 95%CI: 1.92-24.81) were independent factors that predicted congenital CBDD (Table 2).
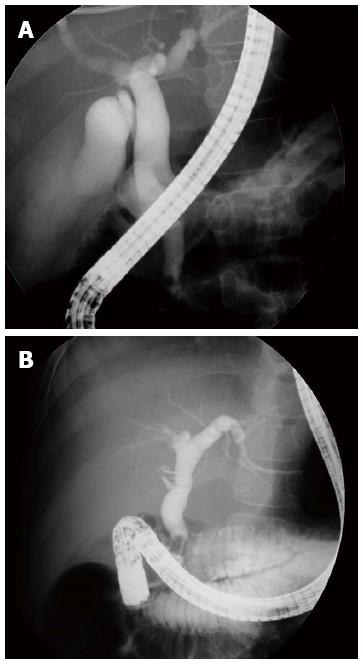
The two groups did not differ significantly in terms of any of the other cholangiographic findings. The patients with obstructive CBDD tended to have CBDs with cylindrical-fusiform features more frequently than the patients with congenital CBDD (93.3% vs 78.2%) but this difference did not achieve statistical significance. Moreover, patients with congenital CBDD tended to have cystic features more frequently than the patients with obstructive CBD (21.8% vs 6.7%) but this too did not achieve statistical significance. The two cases of obstructive CBDD with cystic features had had severe choledolithiasis, which had normalized after endoscopic removal of the stone; recurrence was not observed during the follow-up period. Notably, although choledolithiasis occurred in three-quarters of the children with obstructive CBDD (76.6%), more than half of the congenital CBDD cases (61.8%) also had choledolithiasis. Thus, choledolithiasis was not useful for differential diagnosis in CBDD. It was also difficult to differentiate between the obstructive and congenital CBDD patients with SI < 2.32 on the basis of their CBDD features: there were 46 patients with SI < 2.32, of whom 14 had congenital CBDD and 28 had obstructive CBDD. Ten of the 14 congenital CBDD patients (71.4%) and 21 of the 28 obstructive CBDD patients (75%) had cylindrical-fusiform CBDD features. Indeed, in our experience, it was sometimes difficult to differentiate between congenital and obstructive CBDD by only measuring their CBDD diameters (Figure 2).
The present study indicates that several cholangiographic features may be helpful for assessing CBDD, especially for the differential diagnosis of CBDD in children. Firstly, the SI of CBDD was helpful for discriminating congenital CBDD from obstructive CBDD. Secondly, APBDU comorbidity was also an important factor for this differential diagnosis. This close relationship between congenital CBDD and APBDU is already well-known in the literature. Thirdly, the presence of choledolithiasis was not useful for the differential diagnosis of the two CBDD types.
This is the first time that CBDD has been expressed as an SI that incorporated an age-corrected reference. In the present study, the SI of CBDD showed high specificity in terms of differentiating congenital CBDD from obstructive CBDD. This indicated the importance of using age-related CBD reference diameters to assess CBDD in children. Several other studies have also noted this. In a pediatric study of APBDU, most cases of the non-dilated CBD type of APBDU were found to actually have CBDD when the CBD diameter was corrected by an age-related reference[24]. Two TUS studies also provided cut-off CBD diameter references that would allow the identification of CBDD: 2 mm in neonates and 3 mm in children under 13 years of age[25,26]. However, when we employed these TUS references in the present study, the congenital CBDD group could not be differentiated from the obstructive CBDD group.
It is generally known that APBDU is often accompanied by congenital CBDD[12,27]. Although Todani’s classification system divides congenital CBDD into five types[22,23], most patients with choledochal cysts have types Ia, Ic, and IVa CBDD, and all of these types are accompanied by APBDU in almost all adults with this condition[12]: 50%-80% have type I congenital CBDD while 15%-35% have type IV congenital CBDD[28]. Moreover, 76% of children with types I and IV CBDD had APBDU[29]. In the present study, APBDU played a critical role in the differential diagnosis of children with cylindrical-fusiform CBDD. Of the 21 children with congenital CBDD with SI < 2, 20 (71.4%) had APBDU, which facilitated the differential diagnosis of our patients with cylindrical-fusiform CBDD.
No consensus has yet been reached regarding the best approach for identifying APBDU and CBDD in pediatric patients. In addition, APBDU has not been defined in children in relation to the age-corrected size of the common channel[30]. In adults, however, MRCP and endoscopic ultrasonography have been shown to be useful for diagnosing APBDU[31,32]. One study showed that MRCP diagnosed APBDU in adults with a sensitivity of 83% and a specificity of 90%[16]. Since much less is known about APBDU in children, it is unclear whether ERCP can be replaced by MRCP in these patients[33,34]. Indeed, in a study of children with known or suspected APBDU, only 70% were identified by MRCP[33]. The use of endoscopic ultrasonography in children has not been widely established[35]. As a result, ERCP remains the standard diagnostic modality for biliary disease in children, even though its usefulness is limited in neonates and by ERCP-associated complications.
While choledolithiasis with congenital CBDD was initially thought to be rare in Japan, it was then found to be more common than originally believed when it was assessed on the basis of local referral patterns[6,9,10,33]. The present study also showed a high prevalence of choledolithiasis among congenital CBDD children (61.8%). The rate of this comorbidity may depend on the age at diagnosis and the degree of pathological progression, as evidenced by the fact that adult patients with congenital CBDD have CBD stones more frequently than pediatric patients (50% vs 28.6%)[9].
In conclusion, the SI of CBDD, as measured by ERCP, together with APBDU comorbidity, may aid the differential diagnosis of congenital CBDD and obstructive CBDD. However, the study has several limitations. Firstly, it was not adequately powered because of the small cohort size, its retrospective study design, the discrepancy between the numbers of patients in each group, and the etiological heterogeneity of the obstructive CBDD group. In addition, this study may have been limited by selection bias, namely, patients with more complications tend to be referred to a tertiary hospital. Therefore, it is not yet possible to state conclusively that ERCP-measured CBD diameter is useful for differentially diagnosing congenital CBDD in children. However, further study is warranted given that the accurate diagnosis of children with choledochal cyst on the basis of bile duct measurements would facilitate their early and appropriate surgical management and thus result in low morbidity rates and a good prognosis. Given the current scarcity of related studies, a collaborative study that investigates the usefulness of ERCP for diagnosing children with CBDD is needed.
While the investigation of common bile duct dilatation (CBDD) in adults focuses mainly on causes of secondary obstruction of common bile duct (CBD), congenital CBDD must be prioritized in the diagnosis of CBDD in children. ERCP remains the standard diagnostic modality for CBDD in children. However, no consensus has been reached regarding the best approach for identifying CBDD and the diagnosis of CBDD is based on the morphology of CBD.
No study to measure the diameter of CBD and to adjust its degree of CBDD according to age has been done in children.
This study shows that rather than simple measurement of dilatation of the CBD, a calculated index adjusted for age would be more specific for differentiating obstructive form congenital pathology.
Not only evaluating the morphology of the CBD, but also measuring the diameter of CBD may be helpful in the differential diagnosis of CBDD in children.
CBDD is mainly caused by a congenital anomaly in children, while in adults it is caused by obstruction secondary to cancer and choledolithiasis.
This is an interesting concept regarding discriminating between congenital pathology and secondarily obstructive causes by utilizing a calculated index of the CBD diameter adjusted for age. However, this concept remains in need of further study to validate the applicability in children with bile duct dilatation.
| 1. | Coss A, Enns R. The investigation of unexplained biliary dilatation. Curr Gastroenterol Rep. 2009;11:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Holm AN, Gerke H. What should be done with a dilated bile duct? Curr Gastroenterol Rep. 2010;12:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 3. | Brown CW, Werlin SL, Geenen JE, Schmalz M. The diagnostic and therapeutic role of endoscopic retrograde cholangiopancreatography in children. J Pediatr Gastroenterol Nutr. 1993;17:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Cheng CL, Fogel EL, Sherman S, McHenry L, Watkins JL, Croffie JM, Gupta SK, Fitzgerald JF, Lazzell-Pannell L, Schmidt S. Diagnostic and therapeutic endoscopic retrograde cholangiopancreatography in children: a large series report. J Pediatr Gastroenterol Nutr. 2005;41:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Otto AK, Neal MD, Slivka AN, Kane TD. An appraisal of endoscopic retrograde cholangiopancreatography (ERCP) for pancreaticobiliary disease in children: our institutional experience in 231 cases. Surg Endosc. 2011;25:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Jang JY, Yoon CH, Kim KM. Endoscopic retrograde cholangiopancreatography in pancreatic and biliary tract disease in Korean children. World J Gastroenterol. 2010;16:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Ashida K, Nagita A, Sakaguchi M, Amemoto K, Tada H. Endoscopic retrograde cholangiopancreatography in paediatric patients with biliary disorders. J Gastroenterol Hepatol. 1998;13:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Miyano T, Ando K, Yamataka A, Lane G, Segawa O, Kohno S, Fujiwara T. Pancreaticobiliary maljunction associated with nondilatation or minimal dilatation of the common bile duct in children: diagnosis and treatment. Eur J Pediatr Surg. 1996;6:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Huang CS, Huang CC, Chen DF. Choledochal cysts: differences between pediatric and adult patients. J Gastrointest Surg. 2010;14:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Matsumoto Y, Uchida K, Nakase A, Honjo I. Congenital cystic dilatation of the common bile duct as a cause of primary bile duct stone. Am J Surg. 1977;134:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Matsumoto Y, Fujii H, Itakura J, Matsuda M, Yang Y, Nobukawa B, Suda K. Pancreaticobiliary maljunction: pathophysiological and clinical aspects and the impact on biliary carcinogenesis. Langenbecks Arch Surg. 2003;388:122-131. [PubMed] |
| 12. | Kamisawa T, Ando H, Suyama M, Shimada M, Morine Y, Shimada H. Japanese clinical practice guidelines for pancreaticobiliary maljunction. J Gastroenterol. 2012;47:731-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Youn HS, Seo JH, Park CH, Cho JM, Park JJ. An infantile case of cholelithiasis initially misdiagnosed as choledochal cyst. Pediatr Int. 2012;54:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Singh S, Kheria LS, Puri S, Puri AS, Agarwal AK. Choledochal cyst with large stone cast and portal hypertension. Hepatobiliary Pancreat Dis Int. 2009;8:647-649. [PubMed] |
| 15. | Aggarwal S, Kumar A, Roy S, Bandhu S. Massive dilatation of the common bile duct resembling a choledochal cyst. Trop Gastroenterol. 2001;22:219-220. [PubMed] |
| 16. | Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, Seo DW, Won HJ, Kim MY. Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc. 2005;62:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Karvonen J, Kairisto V, Grönroos JM. The diameter of common bile duct does not predict the cause of extrahepatic cholestasis. Surg Laparosc Endosc Percutan Tech. 2009;19:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Grönroos JM, Haapamäki MM, Gullichsen R. Effect of the diameter of the common bile duct on the incidence of bile duct stones in patients with recurrent attacks of right epigastric pain after cholecystectomy. Eur J Surg. 2001;167:767-769. [PubMed] |
| 19. | Grönroos JM, Karvonen J, Hurme S, Salminen P. Stone or stricture: does the calibre of intrahepatic bile ducts predict the diagnosis? ANZ J Surg. 2012;82:89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Witcombe JB, Cremin BJ. The width of the common bile duct in childhood. Pediatr Radiol. 1978;7:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Low VH. The normal retrograde cholangiogram: a definition of normal caliber. Abdom Imaging. 1997;22:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 851] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Todani T, Watanabe Y, Toki A, Morotomi Y. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg. 2003;10:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 24. | Ono Y, Kaneko K, Tainaka T, Sumida W, Ando H. Pancreaticobiliary maljunction without bile duct dilatation in children: distinction from choledochal cyst. J Pediatr Gastroenterol Nutr. 2008;46:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Carroll BA, Oppenheimer DA, Muller HH. High-frequency real-time ultrasound of the neonatal biliary system. Radiology. 1982;145:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Hernanz-Schulman M, Ambrosino MM, Freeman PC, Quinn CB. Common bile duct in children: sonographic dimensions. Radiology. 1995;195:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Ono S, Fumino S, Iwai N. Diagnosis and treatment of pancreaticobiliary maljunction in children. Surg Today. 2011;41:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 1 of 3: classification and pathogenesis. Can J Surg. 2009;52:434-440. [PubMed] |
| 29. | Stringer MD, Dhawan A, Davenport M, Mieli-Vergani G, Mowat AP, Howard ER. Choledochal cysts: lessons from a 20 year experience. Arch Dis Child. 1995;73:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 103] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Goldman M, Pranikoff T. Biliary disease in children. Curr Gastroenterol Rep. 2011;13:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Songür Y, Temuçin G, Sahin B. Endoscopic ultrasonography in the evaluation of dilated common bile duct. J Clin Gastroenterol. 2001;33:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 32. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Tipnis NA, Werlin SL. The use of magnetic resonance cholangiopancreatography in children. Curr Gastroenterol Rep. 2007;9:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Attila T, Adler DG, Hilden K, Faigel DO. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ramesh J S- Editor: Yu J L- Editor: Logan S E- Editor: Zhang DN