Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.661
Peer-review started: June 3, 2014
First decision: June 27, 2014
Revised: July 17, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 14, 2015
Processing time: 229 Days and 23.7 Hours
AIM: To determine the efficacy of two quintuple regimens for eradication of Helicobacter pylori (H. pylori) in patients who failed previous therapies.
METHODS: This prospective, open-label, randomized controlled trial was a phase II study conducted from April 2011 to March 2012 at the Gastrointestinal and Liver Diseases Research Center in Rasht, Iran. A total of 208 patients with dyspepsia who failed previous H. pylori eradication with a ten-day quadruple therapy were enrolled. A random block method was used to assign patients to one of two treatment groups. Patients in the first group were treated with 240 mg bismuth subcitrate, 20 mg omeprazole, 1000 mg amoxicillin, 500 mg clarithromycin and 500 mg tinidazole (BOACT group). Patients in the second group received a regimen containing 240 mg bismuth subcitrate, 20 mg omeprazole, 500 mg tetracycline, 500 mg metronidazole and 200 mg ofloxacin (BOTMO group). Both regimens were given twice daily for a duration of seven days. The eradication was confirmed by a 14C urea breath test 12 wk after completion of therapy. Patient compliance and drug side effects were evaluated at the end of the treatment period. The success rates were calculated by intention-to-treat and per-protocol analyses.
RESULTS: A total of 205 patients completed the course of treatment, with three patients excluded due to drug intolerance. The mean age of patients did not differ between the BOACT and BOTMO groups (41.6 ± 12.2 years vs 39.6 ± 11.8 years), and no significant differences were found between the two groups in terms of age, sex, smoking habits or the initial eradication regimen. The intention-to-treat and per-protocol eradication rates were significantly higher in the BOTMO group (86.5%, 95%CI: 0.85-0.87 and 86.7%, 95%CI: 0.80-0.89, respectively) compared with the BOACT group (75.5%, 95%CI: 0.73-0.76 and 76%, 95%CI: 0.69-0.80, respectively) (P < 0.05). Univariate analyses for both groups did not show any association of sex, smoking and initial therapeutic regimen with eradiation rate (P > 0.05 for all). Significantly more patients experienced side effects in the BOACT group compared to the BOTMO group (77.4% vs 36.6%, P < 0.01). This difference was exemplified by increases in headache and taste disturbance (P < 0.05).
CONCLUSION: Quintuple therapy with a BOTMO regimen is an alternative second-line rescue therapy for Iranian patients with failed first-line eradication treatment of H. pylori.
Core tip: Due to increasing antibiotic resistance, eradication of Helicobacter pylori has become more challenging. Antibiotic resistance exhibits a regional pattern and treatments typically involve 14-d medication periods, which are not always effective. This study compared two 7-d quintuple regimens and identified a regimen of bismuth subcitrate, omeprazole, tetracycline, metronidazole, and ofloxacin as an effective alternative second-line rescue therapy with minimal side effects for Iranian patients who failed a course of first-line treatment.
-
Citation: Mansour-Ghanaei F, Joukar F, Naghipour MR, Forouhari A, Seyed Saadat SM. Seven-day quintuple regimen as a rescue therapy for
Helicobacter pylori eradication. World J Gastroenterol 2015; 21(2): 661-666 - URL: https://www.wjgnet.com/1007-9327/full/v21/i2/661.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.661
Helicobacter pylori (H. pylori) infection is a global health problem associated with chronic gastritis, peptic ulcer disease and gastric cancer, which affects 20%-50% of people in Western nations and up to 80% of the population in developing countries[1,2]. Therefore, the eradication of the pathogen is of great importance to reduce H. pylori-related complications[3,4]. However, treatment failures resulting from antimicrobial resistance and poor compliance have become an increasing concern. This is especially important in regions with a high prevalence of H. pylori infection, such as Iran, where the prevalence, re-infection rate and resistance to standard therapeutic regimens are much higher than in Western countries[4-7]. Treatment with triple therapy, which is the most frequently recommended, fails to eradicate H. pylori in approximately 20% of cases[8]. Treatment with quadruple rescue therapy is still insufficient to reduce the failure rate below 20%[9,10]. Bacterial culture and microbial susceptibility tests are recommended by European guidelines for selection of third-line treatment regimens, but these methods are hindered by low sensitivity, high cost, unavailability and their invasive nature[11,12]. Therefore, designing a novel rescue regimen that achieves greater than 80% eradication rate is a target of current research[11,13].
Recently, several multidrug rescue regimens against refractory H. pylori infection have been studied, though an ideal therapeutic regimen has not yet been identified[12,14-18]. In Iran, the most common regimen for the first-line treatment is a 14-d quadruple therapy containing bismuth subcitrate, omeprazole, metronidazole and either tetracycline or amoxicillin[19]. Mousavi et al[20] showed that a 14-d quadruple therapy (including amoxicillin) resulted in an eradication rate of 70.4% based on an intention-to-treat (ITT) analysis, and 75.7% based on a per-protocol (PP) analysis. Similarly, Agah et al[5] reported a 68% eradication rate using the same regimen. A higher eradication rate of 84% by ITT analysis was reported by Fakheri et al[21] with quadruple therapy including bismuth subcitrate, omeprazole, amoxicillin and clarithromycin. Despite the benefit, clarithromycin exhibits resistance that varies over time and based on the geographic region. In Iran, there is a high prevalence of clarithromycin and metronidazole resistance, indicating that Western eradication regimens are not ideal in this region[22]. Our previous study in an antibiotic-sensitive area of Iran using 7- and 14-d furazolidone-based quadruple regimens failed to show acceptable eradication rates by ITT analysis (71% and 65%, respectively)[23]. Therefore, rescue regimens should be chosen based on the regional pattern of antibiotic resistance, taking into account patient compliance, drug efficacy and safety[5,22]. The aim of this study was to compare two quintuple rescue therapy regimens with regard to compliance, safety and efficacy in patients who had failed an initial quadruple course of therapy.
This phase II study was a prospective, open-label, randomized controlled trial conducted from April 2011 to March 2012 at the Gastrointestinal and Liver Diseases Research Center of Guilan University of Medical Sciences, in Rasht, Iran. The study was approved by the ethics committee of the research center, and was in accordance with the Helsinki declaration for use of human subjects. This study is registered in the Iranian Registry of Clinical Trials (identification number: IRCT201103011155N11, Available from: URL: http://www.irct.ir).
Patients with H. pylori infection who failed previous eradication with a ten-day quadruple therapy comprised of bismuth subcitrate, omeprazole, amoxicillin and clarithromycin or bismuth subcitrate, omeprazole, amoxicillin and metronidazole were consecutively recruited for this study (n = 208). The patients were referred from the outpatient gastroenterology clinics and private offices to our referral University center. Twelve weeks after completion of therapy, the diagnosis of H. pylori infection was made using a Heliprobe 14C urea breath test (Kibion AB, Uppsala, Sweden), which shows 94% sensitivity and 100% specificity[24]. Patients under 15 or over 65 years of age, and those with co-existing serious illnesses such as liver cirrhosis, uremia and gastrointestinal malignancies were excluded from the study. Other exclusion criteria were pregnancy/lactation and having contraindication or allergy to any of the study drugs. The objectives of the study and potential side effects of drugs were explained to each patient, and informed written consent was obtained.
Patients were randomized according to classification guidelines of the Federal Drug Administration/World Health Organization for individually randomized trials for the testing of drugs or devices[25]. The random block method was used to assign patients into randomly permuted treatment blocks to ensure an equal number of subjects for each treatment. The first group consisted of 104 patients who received 240 mg bismuth subcitrate, 20 mg omeprazole, 1000 mg amoxicillin, 500 mg clarithromycin and 500 mg tinidazole twice daily for seven days (BOACT group). The second group of 104 patients was treated with 240 mg bismuth subcitrate, 20 mg omeprazole, 500 mg tetracycline, 500 mg metronidazole and 200 mg ofloxacin twice daily for seven days (BOTMO group). Demographic and clinical variables, including age, sex, smoking status and type of previous treatment regimen, were recorded. Patients were instructed to take their prescribed medications at the scheduled times and advised to avoid smoking, drinking alcoholic or caffeinated beverages, eating spicy foods or taking non-steroidal anti-inflammatory drugs or medications containing a monoamine oxidase inhibitor.
The primary outcome measured was the H. pylori eradication rate as assessed by the 14C urea breath test. Successful eradication of H. pylori was confirmed by a negative result. The secondary outcomes were the incidence of adverse effects and patient compliance. Adverse effects from the treatments were assessed using a 0-10 scale system (mild: 0-3, moderate: 4-6, severe: 7-10), and patient compliance was defined as a consumption of > 80% of the prescribed drugs.
ITT and PP analyses were performed to assess the efficacy of the treatment regimens for H. pylori eradication. The ITT analysis included all patients who were initially randomized into one of the treatment groups and took at least one treatment dose. The PP analysis excluded patients who refused to continue the treatment, or those with poor compliance to therapy. H. pylori eradication percentages, odds ratios and 95%CI were assessed for each group. Demographic variables, previous treatments, eradication rates, adverse events and patient compliance were compared between the groups using χ2 and Student’s t analyses. Statistical analyses were performed using SPSS, version 16.0 software (SPSS Inc., Chicago, IL, United States), and P < 0.05 was considered to be statistically significant.
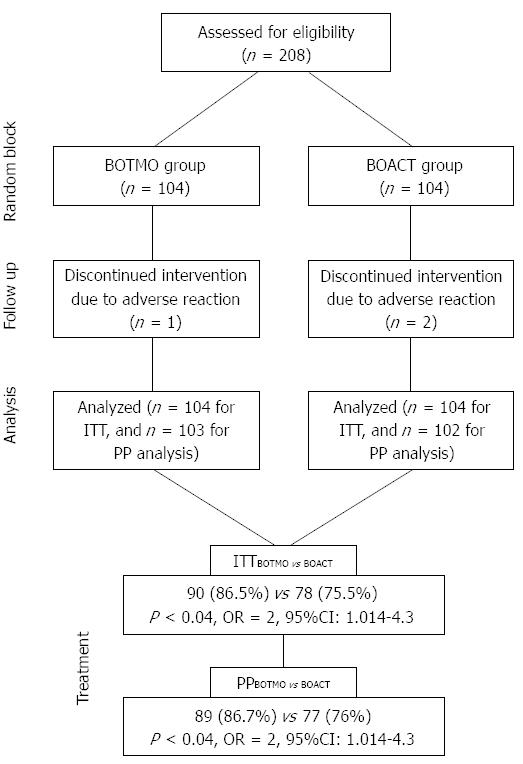
A total of 208 patients with persistent H. pylori infection were enrolled in this study. Of the 104 assigned to each group, two patients (females with severe epigastric pain and headache) in the BOACT group and one patient (male with severe nausea) in the BOTMO group were excluded from the study due to drug intolerance (Figure 1).
Basic demographic and clinical characteristics of the study population and their initial eradication therapy regimen are shown in Table 1. The mean age was 41.6 ± 12.2 years for patients treated with BOACT and 39.6 ± 11.8 years for those receiving the BOTMO regimen. Most of the patients were female (BOACT: 72/104, 69.2%; BOTMO: 65/104, 62.5%). No significant differences were found between the two groups in terms of age, sex, smoking habits or initial regimen.
| Characteristic | BOACT (n = 104) | BOTMO (n = 104) |
| Male/Female | 32/72 | 39/65 |
| Age (yr) | 41.6 ± 12.2 | 39.6 ± 11.8 |
| Smoking, n (%) | ||
| Yes | 28 (26.9) | 27 (26.0) |
| No | 76 (73.1) | 77 (74.0) |
| Initial eradication regimen, n (%) | ||
| BOAC | 82 (78.8) | 75 (72.1) |
| BOAM | 22 (21.2) | 29 (27.9) |
On ITT analysis, the eradication rate was 75.5% (95%CI: 0.73-0.76) in the BOACT group and 86.5% (95%CI: 0.85-0.87) in the BOTMO group; the difference between the two groups was statistically significant (OR = 2, 95%CI: 1.014-4.300; P < 0.04). In the PP analysis, H. pylori was eradicated in 76% of patients in the BOACT group (95%CI: 0.69-0.80) and 86.7% of patients in the BOTMO group (95%CI: 0.80-0.89); the difference between the two groups was statistically significant (OR = 2, 95%CI: 1.014-4.300; P < 0.04).
Univariate analyses for both groups did not show any association of sex, smoking and initial therapeutic regimen with eradiation rate (P > 0.05 for all).
Despite the discontinuation of treatment by two patients in the BOACT group and one patient in the BOTMO group, both regimens were well-tolerated by the majority of patients. A total of 71 side effects were reported in 59 patients (28.8%), which were rated as mild (Table 2). A significantly greater proportion of patients reported adverse side effects in the BOACT group compared to the BOTMO group (77.4% vs 36.6%; P < 0.01). This corresponded with 55 side effects in 35 BOACT patients and 26 side effects in 20 BOTMO patients. Specifically, significantly more reports of headache and taste disturbance occurred in the BOACT group than in the BOTMO group (P < 0.05).
| Side effect | Regimen | |
| BOACT (n = 1021) | BOTMO (n = 1032) | |
| Headache | 17 (17.6)a | 7 (7.8) |
| Taste disturbance | 14 (15.7)a | 6 (5.8) |
| Nausea | 5 (4.9) | 3 (2.9) |
| Epigastric pain | 4 (3.9) | 2 (1.9) |
| Diarrhea | 4 (3.9) | 2 (1.9) |
| Heartburn | 3 (2.9) | 2 (1.9) |
| Stool color change | 3 (2.9) | 2 (1.9) |
| Urine color change | 2 (1.9) | 1 (0.9) |
| Anorexia | 3 (2.9) | 1 (0.9) |
| Total | 55 (77.4)b | 26 (36.6) |
The results of the present study show a higher H. pylori eradication rate with a 7-d quintuple therapy with BOTMO compared to BOACT in patients who initially failed quadruple therapies. Although both regimens demonstrated good patient compliance, fewer side effects were reported in patients receiving BOTMO therapy. These findings are consistent with those of the only other study comparing the efficacy of a quintuple regimen, comprised of bismuth subcitrate, tetracycline, metronidazole, roxithromycin and lansoprazole, with triple and quadruple treatment regimens[26]. In that study, a significantly higher rate of H. pylori eradication was found with the quintuple regimen, though the length of treatment was 14 d and side effects were not evaluated.
At the present, triple therapy suggested by both Canadian and European guidelines is the most preferred first-line regimen in clinical practice[3,27]. However, the success rate of this eradication regimen is decreasing[10,17]. Even the most commonly recommended quadruple rescue therapy regimen fails to eradicate infection in more than 20% of patients[6,28]. In one study of patients with peptic ulcers who failed to respond to previous eradication regimens, an eradication rate of 69% was obtained after treatment with a 7-d course of therapy with bismuth subcitrate, a high-dose of furazolidone (200 mg, b.i.d), amoxicillin and a proton-pump inhibitor[29]. A similar eradication rate (63% by ITT analysis) was achieved in another study using a 7-d rescue quadruple regimen containing bismuth subcitrate, omeprazole, tetracycline and a high-dose of furazolidone (200 mg, b.i.d)[30].
Iranian patients show an increasing resistance to metronidazole, clarithromycin[5,22,23] and furazolidone[23]. In order to overcome the challenge of H. pylori eradication failure, several maiden rescue regimens have recently been proposed[14,16,18]. Furthermore, Sardarian et al[31] compared the efficacy of a hybrid therapy (40 mg pantoprazole and 1000 mg amoxicillin for 14 d, with 500 mg clarithromycin and 500 mg tinidazole for the last 7 d, b.i.d) with sequential therapies (40 mg pantoprazole for 10 d with 1000 mg amoxicillin for the first 5 d and 500 mg clarithromycin and 500 mg tinidazole for the last 5 d, all twice daily) for H. pylori eradication in 396 Iranian patients[31]. The rates of compliance were 96.7% and 98.6% for the hybrid and sequential groups, respectively. The eradication rate for the hybrid group was significantly higher than that of the sequential group by both ITT (89.5% vs 76.7%) and PP (92.9% vs 79.9%) analyses. Severe side effects were observed in 2.4% of patients in the hybrid group and 3.8% of those in the sequential group.
According to the results of our study, the quintuple BOTMO regimen was successful in eradicating H. pylori in 86.5% and 86.7% of patients by ITT and PP analyses, respectively. Although a cure rate of > 80% was achieved, which is acceptable by the standards of Maastricht and other guidelines for successful eradication[32], none of the regimens achieved the target threshold for an ideal eradication regimen of more than 90%. It is possible that the efficacy of the clarithromycin-based BOACT regimen used in the present study was affected by the use of clarithromycin in the failed initial eradication therapies.
In addition to being effective and compatible with regional microbial resistance patterns, a suitable anti-H. pylori regimen should be cost-effective, easy to administer and well-tolerated[3,18]. In the present study, approximately one-third of patients experienced adverse events, which were reported as mild to moderate. Of the total study population, only three patients discontinued treatment due to severe side effects. Generally, both treatment regimens were well-tolerated and had a good compliance (98.7% vs 99.04% in BOACT and BOTMO regimens, respectively).
A limitation of this study was the lack of regional estimates of eradication rates with regard to antibiotic resistance. Furthermore, the results of this study may not be applicable to patients who failed other therapies. H. pylori is an actively dividing spiral bacterium that assumes a coccoid morphology under stressful conditions such as antibiotic exposures[33-35], which could contribute to treatment failures and relapse of infection[35-38]. Faghri et al[35] suggested that a therapy must eradicate viable coccoids in addition to the spiral forms, in order to be successful.
In conclusion, quintuple rescue therapy using a BOTMO regimen provided higher eradication rates than the BOACT regimen. Furthermore, the drugs used in the BOTMO regimen induced fewer side effects and are widely available in regions of Iran where culturing of H. pylori is difficult. Thus, the BOTMO regimen could be an alternative second-line rescue therapy for Iranian patients who failed previous eradication treatment. However, the regional pattern of antimicrobial resistance necessitates that more studies in other populations be conducted. Moreover, treatment regimens of longer than seven days should also be evaluated.
Helicobacter pylori (H. pylori) infection is associated with chronic gastritis, peptic ulcer disease and gastric cancer. Therefore, the eradication of the pathogen is of great importance in order to reduce H. pylori-related complications. As no new drugs to treat H. pylori have been developed, eradication requires multiple-drug therapies. If a drug regimen fails to eradicate the bacteria, an appropriate second-line therapy should be selected.
H. pylori resistance to antibiotics is the most important factor in treatment failure. This necessitates the development of new, alternative protocols for successful treatment.
This is the first study to evaluate the efficacy of two seven-day quintuple rescue regimens including bismuth subcitrate, omeprazole and either amoxicillin, clarithromycin and tinidazole, or tetracycline, metronidazole and ofloxacin as a second-line treatment for H. pylori following the failure of first-line regimens in Iranian patients.
This study indicates that quintuple therapy with bismuth subcitrate, omeprazole, tetracycline, metronidazole and ofloxacin for seven days is an effective alternative second-line rescue therapy for Iranian patients who failed first-line treatment of H. pylori infection.
Quintuple therapy for H. pylori eradication involves treatment with bismuth subcitrate, three antibiotics and a proton-pump inhibitor.
This study provides useful information and suggestions for future research evaluating treatment regimens for H. pylori eradication. The authors show that tetracycline-containing quintuple rescue therapy is highly effective in treating H. pylori eradication failures of first-line regimens in Iran.
| 1. | Georgopoulos SD, Papastergiou V, Karatapanis S. Helicobacter pylori Eradication Therapies in the Era of Increasing Antibiotic Resistance: A Paradigm Shift to Improved Efficacy. Gastroenterol Res Pract. 2012;2012:757926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1936] [Article Influence: 80.7] [Reference Citation Analysis (3)] |
| 3. | Malfertheiner P, Mégraud F, O’Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 845] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 4. | Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1497-1499. [PubMed] |
| 5. | Agah S, Shazad B, Abbaszadeh B. Comparison of azithromycin and metronidazole in a quadruple-therapy regimen for Helicobacter pylori eradication in dyspepsia. Saudi J Gastroenterol. 2009;15:225-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ghadir MR, Shafaghi A, Iranikhah A, Pakdin A, Joukar F, Mansour-Ghanaei F. Furazolidone, amoxicillin and omeprazole with or without bismuth for eradication of Helicobacter pylori in peptic ulcer disease. Turk J Gastroenterol. 2011;22:1-5. [PubMed] |
| 7. | Gatta L, Zullo A, Perna F, Ricci C, De Francesco V, Tampieri A, Bernabucci V, Cavina M, Hassan C, Ierardi E. A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Aliment Pharmacol Ther. 2005;22:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Gisbert JP, González L, Calvet X, García N, López T, Roqué M, Gabriel R, Pajares JM. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Cho DK, Park SY, Kee WJ, Lee JH, Ki HS, Yoon KW, Cho SB, Lee WS, Joo YE, Kim HS. [The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy]. Korean J Gastroenterol. 2010;55:368-375. [PubMed] |
| 10. | Yun SP, Seon HG, Ok CS, Yoo KH, Kang MK, Kim WH, Kwon CI, Ko KH, Hwang SG, Park PW. Rifaximin Plus Levofloxacin-Based Rescue Regimen for the Eradication of Helicobacter pylori. Gut Liver. 2012;6:452-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hsu PI, Wu DC, Chen A, Peng NJ, Tseng HH, Tsay FW, Lo GH, Lu CY, Yu FJ, Lai KH. Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest. 2008;38:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (95)] |
| 14. | Gisbert JP. Rescue Therapy for Helicobacter pylori Infection 2012. Gastroenterol Res Pract. 2012;2012:974594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Mirzaee V, Rezahosseini O. Randomized control trial: Comparison of Triple Therapy plus Probiotic Yogurt vs. Standard Triple Therapy on Helicobacter Pylori Eradication. Iran Red Crescent Med J. 2012;14:657-666. [PubMed] |
| 16. | Kuo CH, Kuo FC, Hu HM, Liu CJ, Wang SS, Chen YH, Hsieh MC, Hou MF, Wu DC. The Optimal First-Line Therapy of Helicobacter pylori Infection in Year 2012. Gastroenterol Res Pract. 2012;2012:168361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Hsu PI, Peng NJ. H. pylori Eradication Therapy. Gastroenterol Res Pract. 2013;2013:935635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1358] [Article Influence: 71.5] [Reference Citation Analysis (1)] |
| 19. | Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion. 2001;64:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Mousavi S, Toussy J, Yaghmaie S, Zahmatkesh M. Azithromycin in one week quadruple therapy for H pylori eradication in Iran. World J Gastroenterol. 2006;12:4553-4556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ, Massarrat S. Clarithromycin vs. furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Malekzadeh R, Mohamadnejad M, Siavoshi F, Massarrat S. Treatment of Helicobacter Pylori Infection in Iran: Low Efficacy of Recommended Western Regimens. Arch Iranian Med. 2004;7:1-8. |
| 23. | Mansour-Ghanaei F, Yousefi Mashhour M, Heidarzadeh A, Jafarshad R, Joukar F, Purrasuli Z, Hamami P. Helicobacter Pylori Ran Away Furazolidone-based Quadruple Therapy! Middle East J Dig Dis. 2009;1:2-6. |
| 24. | Mansour-Ghanaei F, Sanaei O, Joukar F. Clinical Validation of an Office-Based C-UBT (Heliprobe) for H. pylori Diagnosis in Iranian Dyspeptic Patients. Gastroenterol Res Pract. 2011;2011:930941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Ronald G. Randomized Trials. Johns Hopkins Bloomberg School of Public Health. Available from: http://ocw.jhsph.edu/courses/fundamentalsprogramevaluation/PDFs/Lecture12.pdf. |
| 26. | Daskalopoulos G, Ho YY, Lian XX. Does pentuple therapy offer any advantage over triple therapy or quadruple therapy in the eradication of H. pylori [abstract]. Gut. 1997;41 suppl 1:A105. |
| 27. | Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, Thomson A. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori--an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547-554. [PubMed] |
| 28. | Ma HJ, Wang JL. Quadruple therapy for eradication of Helicobacter pylori. World J Gastroenterol. 2013;19:931-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Felga GE, Silva FM, Barbuti RC, Navarro-Rodriguez T, Zaterka S, Eisig JN. Quadruple therapy with furazolidone for retreatment in patients with peptic ulcer disease. World J Gastroenterol. 2008;14:6224-6227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 30. | Eisig JN, Silva FM, Rodriguez TN, Hashimoto CL, Barbuti RC. A furazolidone-based quadruple therapy for Helicobacter pylori retreatment in patients with peptic ulcer disease. Clinics (Sao Paulo). 2005;60:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol. 2010;16:4357-4362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Poursina F, Faghri J, Moghim S, Zarkesh-Esfahani H, Nasr-Esfahani B, Fazeli H, Hasanzadeh A, Safaei HG. Assessment of cagE and babA mRNA expression during morphological conversion of Helicobacter pylori from spiral to coccoid. Curr Microbiol. 2013;66:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | She FF, Su DH, Lin JY, Zhou LY. Virulence and potential pathogenicity of coccoid Helicobacter pylori induced by antibiotics. World J Gastroenterol. 2001;7:254-258. [PubMed] |
| 35. | Faghri J, Poursina F, Moghim SH, Zarkesh H, Bahram E, Esfahani N, Fazeli H. Morphological and Bactericidal Effects of Different Antibiotics on Helicobacter pylori. Jundishapur J Microbiol. 2014;7:e8704. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (6)] |
| 36. | Can F, Karahan C, Dolapci I, Demirbilek M, Tekeli A, Arslan H. Urease activity and urea gene sequencing of coccoid forms of H. pylori induced by different factors. Curr Microbiol. 2008;56:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Chen TS. Is the coccoid form of Helicobacter pylori viable and transmissible? J Chin Med Assoc. 2004;67:547-548. [PubMed] |
| 38. | Costa K, Bacher G, Allmaier G, Dominguez-Bello MG, Engstrand L, Falk P, de Pedro MA, García-del Portillo F. The morphological transition of Helicobacter pylori cells from spiral to coccoid is preceded by a substantial modification of the cell wall. J Bacteriol. 1999;181:3710-3715. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bao Z, Safaei HG S- Editor: Qi Y L- Editor: A E- Editor: Wang CH