Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.600
Peer-review started: May 10, 2014
First decision: July 8, 2014
Revised: July 29, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: January 14, 2015
Processing time: 253 Days and 13.8 Hours
AIM: To assess the long-term effects of physical activity on irritable bowel syndrome (IBS) symptoms and on quality of life, fatigue, depression and anxiety.
METHODS: Seventy-six patients from a previous randomized controlled interventional study on increased physical activity in IBS were asked to participate in this long-term follow-up study. The included patients attended one visit in which they filled out questionnaires and they underwent a submaximal cycle ergometer test. The primary end point was the change in the IBS Severity Scoring System (IBS-SSS) at baseline, i.e., before the intervention and at follow-up. The secondary endpoints were changes in quality of life, fatigue, depression and anxiety.
RESULTS: A total of 39 [32 women, median age 45 (28-61) years] patients were included in this follow-up. Median follow-up time was 5.2 (range: 3.8-6.2) years. The IBS symptoms were improved compared with baseline [IBS-SSS: 276 (169-360) vs 218 (82-328), P = 0.001]. This was also true for the majority of the dimensions of psychological symptoms such as disease specific quality of life, fatigue, depression and anxiety. The reported time of physical activity during the week before the visit had increased from 3.2 (0.0-10.0) h at baseline to 5.2 (0.0-15.0) h at follow-up, P = 0.019. The most common activities reported were walking, aerobics and cycling. There was no significant difference in the oxygen uptake 31.8 (19.7-45.8) mL per min per kg at baseline vs 34.6 (19.0-54.6) mL/min per kg at follow-up.
CONCLUSION: An intervention to increase physical activity has positive long-term effects on IBS symptoms and psychological symptoms.
Core tip: Increased physical activity for 12 wk has been shown to improve irritable bowel syndrome (IBS) symptoms. This follow-up study found that the patients included in an intervention to increase physical activity show improvements in IBS symptoms, as well as different aspects of the disease specific quality of life, fatigue, depression and anxiety on the long term. The study supports the evidence for the positive effects of physical activity in IBS and defends physical activity as a treatment option for IBS.
- Citation: Johannesson E, Ringström G, Abrahamsson H, Sadik R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol 2015; 21(2): 600-608
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/600.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.600
In a recent randomized controlled study moderately increased physical activity for 12 wk was tested as an intervention in irritable bowel syndrome (IBS)[1]. Improvements in IBS symptoms as well as in some aspects of the disease specific quality of life were observed in the intervention group. However, there were no significant improvements in fatigue, depression or anxiety. These data demonstrated that moderately increased physical activity for 12 wk improves IBS symptoms without a general improvement of other associated symptoms.
Physical activity has been found to improve a wide range of diseases like fibromyalgia, depression, hypertension and diabetes mellitus. In general these conditions were improved in the short-term but the level of physical activity usually decreases after 12 mo and the benefits of the intervention may be difficult to maintain[2-4]. In some patient groups it is difficult to motivate the patients to change their life style[2,4].
The aim of this study was to assess the long-term effects of the previous intervention to increase physical activity in IBS patients. The primary end point was to assess the change in the IBS Severity Scoring System (IBS-SSS). The secondary endpoints were changes in quality of life, fatigue, depression and anxiety. Moreover, assessments of the level of physical activity and oxygen uptake at follow-up were included.
In the previous study the patients were randomized to a physical activity group or to a control group. The physical activity group were instructed by a physiotherapist to increase their physical activity and the control group were instructed to maintain their lifestyle[1]. Patients in the physical activity group were given individual advice depending on their previous level of physical activity and experience of exercise. The activities suggested could be any activity depending on individual factors, such as time, opportunities, or costs. After 12 wk the control group was also instructed by a physiotherapist to increase their physical activity as in the intervention group and they followed the same protocol as the intervention group during the next 12 wk. Both the intervention group and the control group in the previous study were therefore instructed to increase their physical activity before the end of the previous study. Thus all patients completing the previous study had an intervention and were evaluated at the end of the intervention.
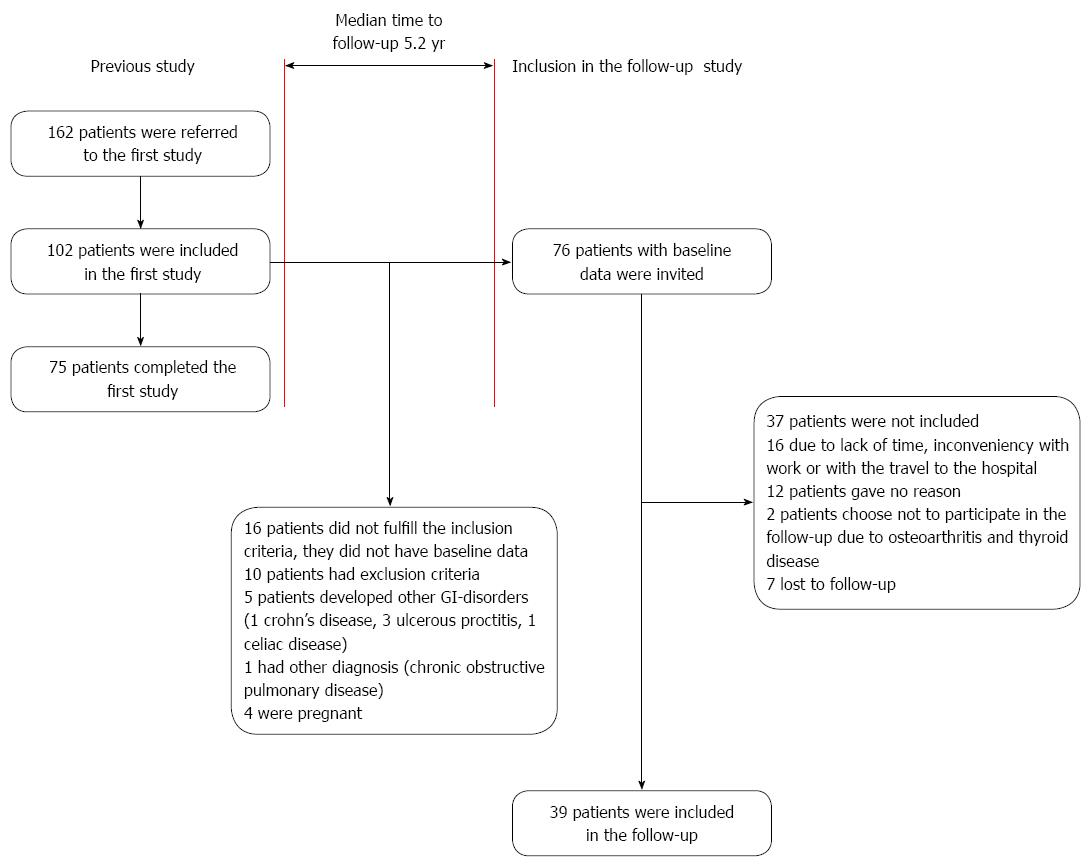
In the present study all eligible patients from the previous study were invited to participate in this long-term follow-up. The inclusion criterion was that the patients had baseline data. Baseline data was the data from the first visit of each patient in the previous study before the start of the 12 wk intervention or control period. The exclusion criteria were pregnancy, organic GI disorders or other organic disease hindering physical activity. Seventy-six patients had baseline data and were invited to participate as shown in the flow chart (Figure 1).
The patients were invited by mail in July 2011 and by telephone in August and September 2011. The visits were conducted at the Sahlgrenska University Hospital in Gothenburg, Sweden, during September and October 2011. The subjects attended one visit in which they filled out questionnaires, underwent a submaximal cycle ergometer test and their body weight was registered. The week before the visit the patients registered their bowel movements and physical activities in a paper diary. They were also asked about other IBS related treatments in the years between the baseline visit and the follow-up. The results were compared with the baseline data and the data from the end of the intervention, i.e., at 12 wk. All the subjects gave informed consent, and the study was approved by the Regional Ethical Review Board of the University of Gothenburg, Dnr 091-05.
IBS-SSS: The IBS-SSS[5] consists of visual analogue scales and is divided into two subscales, an overall IBS score and an extra colonic score. The IBS score contains questions regarding pain severity, pain frequency, abdominal bloating, bowel habit dissatisfaction, and life interference. The extra colonic score contains questions regarding vomiting, gas, belching, satiety, headache, fatigue, musculoskeletal pain, heartburn, dysuria and urgency. Each subscale ranges from 0 to 500, with higher scores meaning more severe symptoms. A reduction of 50 in the IBS-score is considered to be adequate to detect a clinical improvement[5].
Bristol stool form scale: Bristol stool form scale was used to record bowel movements during the week before the visits to the laboratory. The patients recorded all bowel movements and its consistency according to the Bristol stool form scale[6]. The scale ranges from 1 to 7, where type 1 and 2 is hard and lumpy stools and type 6 and 7 is loose and watery stools.
The hospital anxiety and depression scale: The hospital anxiety and depression scale (HADS) is a reliable instrument that was developed for medical outpatients[7] and consists of 14 items, each using a 4-graded Likert scale (0-3). The scale is divided into two subscales, anxiety and depression. Each subscale ranges from 0 to 21, where high score indicates more severe symptoms. Cut-off scores can be used to identify cases of clinically significant mood disorder for both subscales. A score of up to 7 indicates no mood disorder while scores of 8-10 show a borderline mood disorder and a score above 10 shows a case of mood disorder. In our analysis scores of 8 and above are considered to indicate clinical symptoms of depression and anxiety.
IBS-quality of life: The IBS-quality of life (IBS-QOL) is a disease specific instrument measuring health-related quality of life (HRQOL). It consists of 30 items which measures nine QOL dimensions: emotional functioning, mental health, sleep, energy, physical functioning, diet, social role, physical role and sexual relations. For each subscale the scores are transformed to range from 0 to 100; 100 representing the best possible disease specific quality of life[8,9].
Short form-36: Short form-36 (SF-36) was used to assess the general HRQOL. SF-36 includes 36 items which are divided into eight subscales: physical functioning, physical role, bodily pain, general health perceptions, vitality, social functioning, emotional role and mental health. For each subscale the raw scores are transformed into a scale from 0 to 100, with 100 representing the best possible HRQOL[10].
Fatigue impact scale: This scale was initially developed for patients with chronic fatigue syndrome[11] and has previously been used in studies in IBS patients[12,13]. The scale consists of 40 questions divided into three subscales: physical functioning (10 items), cognitive functioning (10 items) and psychosocial functioning (20 items). The subjects are asked to rate to which extent fatigue has caused problems for them during the previous month. Each item consists of a statement and the subject should rate 0 to 4 where 0 means “no problem” and 4 means “extreme problem”.
Weight was measured to the nearest 0.1 kg and oxygen uptake was calculated from a submaximal cycle (Monark Ergomedic 839) ergometer test according to Astrand et al[14,15]. A training diary was used to register physical activity for one week before the visit. The patients reported the type of physical activity and the duration and intensity of the activity. The patients were instructed to record the time they started the activity and the time they finished. The intensity was rated on Borgs rating of perceived exertion scale[16] which starts at 6, no exertion at all and goes to 20, maximal exertion.
The primary endpoint was changes in IBS-SSS, at baseline compared with follow-up. The secondary endpoints were to assess changes in other questionnaires assessing QOL, anxiety, depression, fatigue and bowel movements and changes in oxygen uptake, weight, and time of physical activity reported in the training diary.
As an exploratory endpoint, changes in the above-mentioned parameters in the period between the end of the intervention and the follow-up visit were assessed.
The results are presented as median, percentile 10 and 90. Paired t test was only used for oxygen uptake and body weight. The results from the questionnaires were considered as ordinal data, and analyses were performed using Wilcoxon’s signed rank test. Significance was accepted at the 5% level (P < 0.05). For statistical analysis we used SPSS version 20 (SPSS, IBM; Corporation, NY).
A total of 39 [32 women, median age 45 (28-61) years] patients were included in the follow-up as shown in the flow chart (Figure 1). According to the Rome II criteria[17] 13 patients were classified as diarrhea-predominant IBS, 11 were classified as constipation-predominant IBS and 15 as alternating IBS. Patients who were not included in the follow-up are reported in Figure 1. In total 33 patients attended the follow-up visit while six patients participated by completing the questionnaires due to inconveniency for them to come to the visit.
Thirty-seven of the included patients participated in the previous intervention either as a part of the intervention group (19 patients) or as a control group and were then included in the intervention (18 patients). One control discontinued the previous intervention because of pregnancy and one due to lack of time. These two patients received instructions to increase their level of physical activity at their last visit. In the study group 30 patients were married or cohabitant and 9 were single. Seventeen patients (44%) stated that they had a physically demanding work. The median follow-up time was 5.2 years and the total range of time between baseline and follow-up was 3.8-6.2 years.
Between the end of the previous intervention and the follow-up seven of the included patients participated in one or two other IBS related interventions. Four patients participated in a short IBS school, three sessions[18]. Three patients underwent treatment with nurse-administered gut-directed hypnotherapy. One of these patients also had one consultation visit to a dietitian. Medications that were used by the patients only at the follow-up and not at the start of the previous study are presented in Table 1.
| Drug | Patients (n) |
| Antacids | 1 |
| Neuroleptic drug | 1 |
| Drug against neuropathic pain | 1 |
| Anti epileptic drug | 1 |
| Acetaminophen | 3 |
| Antidepressant | 3 |
| B12 vitamine | 3 |
| Post climacteric hormone | 1 |
| Anticonceptive | 2 |
| NSAIDs | 2 |
| Sedative | 1 |
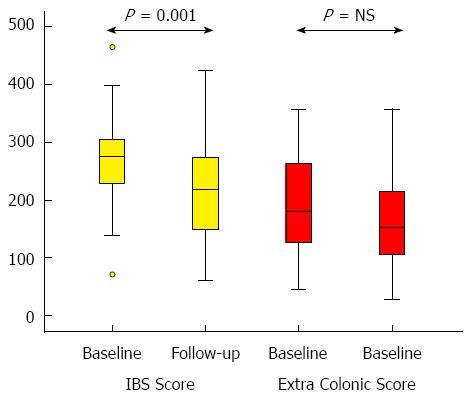
The IBS-score (IBS-SSS) improved significantly as illustrated in Figure 2. Fifty-four percent of the patients had an improvement of more than 50 points, which is considered a clinically significant improvement[5]. Ten percent of the patients had their symptoms worsened with more than 50 points according to the IBS-score. The extra colonic score showed no significant changes (Figure 2).
A separate analysis of the IBS-SSS in the 24 [19 women, median age 50.5 (30.5-62.5) years] patients who had had no change in medication and had received no other IBS related intervention detected also an improvement in IBS-SSS, IBS-score 282 (184-379) vs 218 (85-342), P = 0.002. There was no significant change in the Extra colonic score.
The stool consistency had become firmer, from 4.5 (2.3-5.8) at baseline to 3.8 (1.5-4.8) at follow-up, P = 0.004. There was no significant change in the stool frequency per week at baseline 11 (5-21) compared with follow-up 9 (4-25), P = NS.
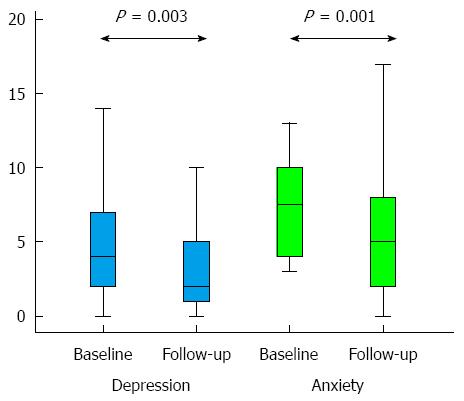
The group demonstrated low levels of anxiety and depression in HADS at baseline, these low levels were reduced further at follow-up, and this was true for both the depression and the anxiety subscale (Figure 3). At baseline 10 patients had depressive symptoms and 20 patients had anxiety as indicated by their scores. At follow-up this was reduced to 3 patients with depressive symptoms and 8 patients with anxiety.
There were significant improvements in five out of nine dimensions in the disease specific quality of life (IBS-QOL) (Table 2). Improved quality of life was also demonstrated on the subscales general health perceptions, emotional role and mental health in the SF-36 (Table 3).
| Dimension | Baseline | Follow-up | P value |
| Emotional functioning | 56 (31-88) | 69 (44-100) | 0.001 |
| Mental health | 80 (50-100) | 90 (45-100) | NS |
| Sleep | 67 (33-100) | 83 (42-100) | 0.008 |
| Energy | 50 (38-88) | 75 (50-100) | 0.005 |
| Physical functioning | 67 (33-100) | 92 (49-100) | 0.002 |
| Diet | 60 (47-87) | 73 (40-93) | NS |
| Social role | 69 (43-94) | 81 (50-100) | 0.009 |
| Physical role | 69 (31-100) | 81 (38-100) | NS |
| Sexual relations | 60 (18-80) | 60 (20-80) | NS |
| Subscale | Baseline | Follow-up | P value |
| Physical functioning | 90 (65-100) | 95 (64-100) | NS |
| Physical role | 50 (0-100) | 100 (0-100) | NS |
| Bodily pain | 51 (31-84) | 51 (22-100) | NS |
| General health perceptions | 54 (25-83) | 67 (32-97) | 0.006 |
| Vitality | 45 (20-80) | 55 (25-85) | NS |
| Social functioning | 75 (38-100) | 75 (49-100) | NS |
| Emotional role | 67 (0-100) | 100 (0-100) | 0.027 |
| Mental health | 72 (32-92) | 74 (52-96) | 0.016 |
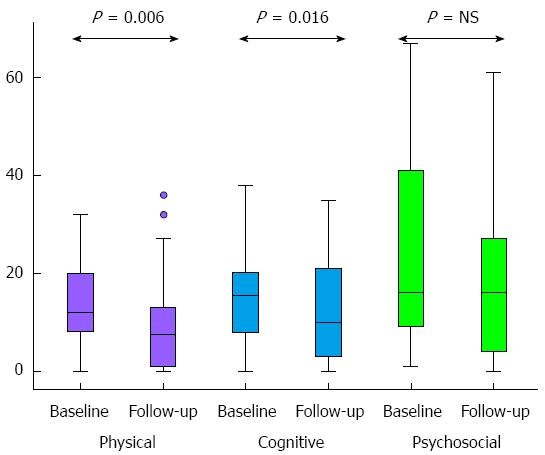
Fatigue was significantly reduced according to two out of three subscales on the fatigue impact scale, namely the physical and the cognitive subscale, whereas there was no change on the psychosocial subscale (Figure 4).
There was no significant difference in the oxygen uptake 31.8 (19.7-45.8) mL/min per kg at baseline and 34.6 (19.0-54.6) mL/min per kg at follow-up, P value is not significant. In the training diary the patients reported 3.2 (0.0-10.0) h of physical activity during the week before the baseline visit and 5.2 (0.0-15.0) h of physical activity during the week before the follow-up visit, P = 0.019. The range of intensity was rated from 9, very light exertion to 18, very hard exertion. The most common activity was walking followed by aerobics and cycling. The body weight had increased significantly from 66.6 (53.7-97.9) kg at baseline to 73.3 (52.6-95.7) kg at follow-up, P = 0.037.
This analysis includes 33 patients [26 women, median age 47 (28-62) years] who participated in the total 12 wk of intervention and completed the last visit in the first study. Significant improvements were detected on HADS on both subscales between the end of intervention and the follow-up. When the intervention was ended the results of the depression subscale was 5 (1-9) compared with 2 (0-7) at follow-up, P = 0.004. For anxiety the results were 8 (1.4-12.6) at end of intervention and 5 (1-11) at follow-up, P = 0.004.
The HRQOL was improved in four subscales of SF-36 namely bodily pain, general health perceptions, vitality and mental health (Table 4).
| Subscale | End of intervention | Follow-up | P value |
| Physical function | 90 (47-100) | 95 (56-100) | NS |
| Physical role | 75 (0-100) | 100 (0-100) | NS |
| Bodily pain | 51 (26-72) | 61 (22-100) | 0.030 |
| General health perceptions | 60 (22-85) | 63 (30-97) | 0.013 |
| Vitality | 45 (15-78) | 55 (22-83) | 0.012 |
| Social function | 75 (43-100) | 88 (50-100) | NS |
| Emotional role | 100 (0-100) | 100 (0-100) | NS |
| Mental health | 64 (35-92) | 76 (52-94) | 0.006 |
The stool consistency had become firmer, from 4.4 (3.1-5.5) at the end of intervention to 3.8 (1.8-4.8) at follow-up, P = 0.001. The body weight increased significantly from 66.3 (51.8-97) kg at end of intervention to 72.9 (53.2-96.5) kg at follow-up, P = 0.015. No other significant changes were observed on the other parameters (data not shown).
In a previous study the novel finding was described that a moderate increase in physical activity improves IBS symptoms and some aspects of the disease specific quality of life. However, there were no significant effects on fatigue, depression or anxiety directly after a 12 wk increase of physical activity[1]. The present work demonstrates that a 12 wk intervention followed by a continued moderate increase in physical activity, shows long term positive effects on IBS symptoms, quality of life, fatigue, anxiety and depression.
Our results are encouraging and confirm that a moderate increase of physical activity could be included in the initial management of patients with IBS. The mechanisms behind the improvement are probably complex and are not merely related to the level of maximum oxygen uptake. The maximal oxygen uptake declines with about 1% per year in normally active people[19]. The findings of maintained oxygen uptake and increased duration of physical activity per week in our group after 5 years is positive. The results are in contrast to results from patients with obesity, diabetes mellitus type 2 and hypertension showing that these groups are not easily motivated to increase and maintain their physical activity over a long period of time[2-4]. The reason for IBS patients to maintain a relatively stable oxygen uptake after 5 years can be due to the increase in the duration of physical activity per week. We may speculate that IBS patients may be well motivated to internalize and adopt the acquired experience of feeling better when they are physically active. The discomfort or pain these patients otherwise experience may enhance their motivation. Being physically active in order to improve the disease may also generate a feeling of control over the disease. A questionnaire study has shown a higher acceptance for lifestyle changes among IBS patients younger than 55 years[20]. Our patient group had a median age of 45 years and may easier adapt to lifestyle changes compared with elderly patients, which may be an additional explanation behind our results.
There are probably multiple mechanisms involved in the improvement of symptoms. Both physical factors as well as psychological factors are likely to play a role. The change of gas transit and colonic transit due to increased physical activity may contribute to these improvements. Villoria et al[21] showed that mild physical activity enhances intestinal gas clearance and reduces symptoms in patients complaining of abdominal bloating. The same group[22] showed earlier that physical activity improves gas transit as well as abdominal distension in healthy subjects, but not the perception of bloating. This may contribute to the improvement of symptoms shown in the present study. The improvement in patients with constipation-predominant IBS can be due to the positive effects of physical activity on symptoms of constipation[23,24].
The present work shows that 54% of the patients had a clinically significant improvement of the IBS symptoms compared with 43% in the previous study after a 12 wk intervention. This implies that the effects of physical activity cannot be explained only by a placebo effect. A pure placebo effect should have declined at the time of follow-up. However, a placebo effect is probably also involved. Brain-gut interactions may play a role in the processes leading to our results. Stress induces exaggeration of the neuroendocrine response and visceral perceptual alterations[25]. Physical activity counteracts the effects of stress and can therefore favourably influence brain plasticity[26]. General effects seem to be detected first after a longer period of increased physical activity and may explain the improvements in fatigue, depression and anxiety and quality of life. Factors like high levels of illness behavior, anxiety, sleep problems, and somatic symptoms are independent predictors of IBS onset[27]. Physical activity has a protective effect on depressive symptoms and may therefore protect against IBS symptom deterioration[1,28].
Our data also demonstrate a significant improvement in five aspects of the disease specific quality of life. In the previous study only two aspects of the IBS-QOL were improved after a 12 wk intervention. Earlier studies have shown that long term physical activity is an important determinant of health related quality of life in women[29]. Therefore it is important to consider the aspect of time when studies to assess the effect of physical activity on HRQOL are designed.
The physical and cognitive aspects of fatigue were improved in this study while fatigue was not affected in the previous study. Chronic fatigue and IBS often occur together[30] and fatigue is a symptom that is difficult to influence in other chronic diseases like liver disease[31]. Therefore the finding that fatigue in IBS can be affected by increased physical activity on the long-term is important. According to the review article by Puetz[32], there is an agreement among studies suggesting a strong, dose-response relationship between physical activity and the reduction of feelings of low energy and fatigue. This knowledge as well as our data may be used to motivate patients suffering from fatigue to be more active.
Clinically significant improvement was shown in depression and anxiety although the group had low scores at baseline. Studies on depression have previously demonstrated reduced symptoms secondary to increased physical activity[28]. The data presented in this study confirms previous data and reveals that a time with increased physical activity longer than 12 wk is needed to improve low levels of anxiety and depression[29].
One clear limitation in our study is that we do not have a control group in this part of the study. This is due to ethical concerns expressed by the ethics committee. The original design of the previous study was an intervention of 12 mo to improve physical activity. The committee did not accept a longer intervention than 12 wk because the control group would wait too long for the intervention.
Thirty-nine patients out of 76 were assessed on the follow-up after about five years. Given the long time to follow-up and given the fact that the patients were not compensated for loss of income during the visit, this is an expected proportion of patients. Fifty one percent have therefore participated. A larger proportion would have strengthen the study even more. However, the majority of patients not participating gave plausible reasons not to participate. The general impression when the patients were contacted was that their participation was related to their practical ability to participate and not to positive or negative experience on physical activity in IBS. One included patient expressed spontaneously the impression that physical activity was not effective for her and she stated that this was the reason for her to participate. A negative impression from the previous interventions seems not to stop the patients from participating.
There are some studies addressing the long term natural history of IBS or functional gastrointestinal disorders. Halder et al[33] studied the long term changes in functional gastrointestinal disorders and noticed that the prevalence is stable over time but there was a turnover of symptoms over time rather than total symptom resolution. Agréus et al[34] conducted a population based study demonstrating that more than 50% of the patients with IBS reported the same symptom profile after one and seven years. Symptom resolution in IBS was not observed in these studies indicating that physical activity in the present study had a positive influence on the course of the disease.
One of the findings in this study is that patients with IBS included in the previous study on increased physical activity had maintained an increased level of physical activity at follow-up. The patients reported a longer duration of physical activities in the training diary during the week before the follow up visit compared with baseline. This reflects the ambition of these patients to be physically active. We hypothesized that the increase of the physical activity would increase the oxygen uptake. At the time of the follow-up the most common activity was walking. Walking is in most cases not challenging enough to increase cardiorespiratory fitness. When designing future studies we would consider using a pedometer as a complement to the ergometer cycle test to measure physical activity. There is also a need of more studies on physical activity in IBS investigating the effect of different types of activities. Duration, intensity and frequency of the physical activity also have to be analyzed with a dose-response perspective considering the fact that endurance athletes report GI symptoms[35-37].
An important observation in the present study is the wide spectrum of positive clinical effects shown after a long time of follow up of about 5 years, which is unusual for interventional studies.
In conclusion, an intervention to increase physical activity improves IBS symptoms, as well as different aspects of the disease specific quality of life, fatigue, depression and anxiety on the long term. The present study supports the evidence for the positive effects of physical activity in IBS and defends physical activity as a treatment option for IBS. However, further research and larger studies are needed to elucidate the effects of different physical activities in the IBS subgroups.
We would like to thank Professor Magnus Simrén for his support, interesting discussions and ideas.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disease. There is no overall treatment for IBS know today. In a recent randomized controlled study moderately increased physical activity for 12 wk was tested as an intervention in IBS. Improvements in IBS symptoms as well as in some aspects of the disease specific quality of life were observed in the intervention group but not in the control group.
In IBS several other non-pharmacological treatments has been studied such as patient education, hypnotherapy and other psychological treatments. These treatments often show good results however they are not available to the majority of patients.
A moderate increase of physical activity has been shown to improve gastrointestinal symptoms in IBS. This long-term follow-up shows that there is an enhanced improvement about 5 years after participating in an intervention to increase physical activity. At follow-up there was an improvement in IBS symptoms, quality of life, fatigue, depression and anxiety, some of these improvements were not as evident in the previous study.
The study supports the evidence for the positive effects of physical activity in IBS and defends physical activity as a treatment option for IBS. Advice on increased physical activity can be given to patients with IBS in both primary care and secondary care.
IBS is a very common functional bowel disorder. The patients suffer from diarrhea, constipation or both. Abdominal pain or discomfort and bloating are common symptoms of IBS. Other symptoms as impaired quality of life, depression and fatigue can accompany the gastrointestinal symptoms. Physical activity is used in medical care in both treatment and prevention of diseases. Physical activity has been found to improve a wide range of diseases like fibromyalgia, depression, hypertension and diabetes mellitus.
The physical activity intervention is manageable in a clinical setting in both primary and secondary care, with low costs and a low risk of potential harmful effects. The authors have chosen an adequate set of questionnaires to evaluate their participants.
| 1. | Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2011;106:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow-up. J Clin Rheumatol. 2011;17:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005;CD003180. [PubMed] |
| 5. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1297] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 6. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2222] [Article Influence: 76.6] [Reference Citation Analysis (3)] |
| 7. | Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28548] [Cited by in RCA: 32889] [Article Influence: 764.9] [Reference Citation Analysis (0)] |
| 8. | Hahn BA, Kirchdoerfer LJ, Fullerton S, Mayer E. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Watson ME, Lacey L, Kong S, Northcutt AR, McSorley D, Hahn B, Mangel AW. Alosetron improves quality of life in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Sullivan M, Karlsson J, Ware JE. The Swedish SF-36 Health Survey--I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18 Suppl 1:S79-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1103] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 12. | Simrén M, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in patients with irritable bowel syndrome seen in referral centers versus primary care: the impact of gender and predominant bowel pattern. Scand J Gastroenterol. 2001;36:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Peyron JF, Czerucka D, Cherikh F, Filippi J. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218-221. [PubMed] |
| 15. | Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1-92. [PubMed] |
| 16. | Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7211] [Cited by in RCA: 8184] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 17. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 833] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 18. | Ringström G, Störsrud S, Simrén M. A comparison of a short nurse-based and a long multidisciplinary version of structured patient education in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2012;24:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Wilmore JH, Costill DL, Kenney WL. Aging in Sport and Exercise. Physiology of Sport and Exercise. 4th ed. Champaign: Human Kinetics 2008; 402-421. |
| 20. | Harris LR, Roberts L. Treatments for irritable bowel syndrome: patients’ attitudes and acceptability. BMC Complement Altern Med. 2008;8:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 21. | Villoria A, Serra J, Azpiroz F, Malagelada JR. Physical activity and intestinal gas clearance in patients with bloating. Am J Gastroenterol. 2006;101:2552-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Dainese R, Serra J, Azpiroz F, Malagelada JR. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med. 2004;116:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Song BK, Cho KO, Jo Y, Oh JW, Kim YS. Colon transit time according to physical activity level in adults. J Neurogastroenterol Motil. 2012;18:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | De Schryver AM, Keulemans YC, Peters HP, Akkermans LM, Smout AJ, De Vries WR, van Berge-Henegouwen GP. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand J Gastroenterol. 2005;40:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Posserud I, Agerforz P, Ekman R, Björnsson ES, Abrahamsson H, Simrén M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 26. | Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH. Neurobiology of exercise. Obesity (Silver Spring). 2006;14:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 596] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 27. | Nicholl BI, Halder SL, Macfarlane GJ, Thompson DG, O’Brien S, Musleh M, McBeth J. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain. 2008;137:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Herring MP, Puetz TW, O’Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Wolin KY, Lee IM, Colditz GA, Glynn RJ, Fuchs C, Giovannucci E. Leisure-time physical activity patterns and risk of colon cancer in women. Int J Cancer. 2007;121:2776-2781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Dansie EJ, Furberg H, Afari N, Buchwald D, Edwards K, Goldberg J, Schur E, Sullivan PF. Conditions comorbid with chronic fatigue in a population-based sample. Psychosomatics. 2012;53:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kalaitzakis E, Josefsson A, Castedal M, Henfridsson P, Bengtsson M, Hugosson I, Andersson B, Björnsson E. Factors related to fatigue in patients with cirrhosis before and after liver transplantation. Clin Gastroenterol Hepatol. 2012;10:174-81, 181.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Puetz TW. Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Med. 2006;36:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Halder SL, Locke GR, Schleck CD, Zinsmeister AR, Melton LJ, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799-807. [PubMed] |
| 34. | Agréus L, Svärdsudd K, Talley NJ, Jones MP, Tibblin G. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol. 2001;96:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Peters HP, Bos M, Seebregts L, Akkermans LM, van Berge Henegouwen GP, Bol E, Mosterd WL, de Vries WR. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: prevalence, medication, and etiology. Am J Gastroenterol. 1999;94:1570-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 37. | Strid H, Simrén M, Störsrud S, Stotzer PO, Sadik R. Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scand J Gastroenterol. 2011;46:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Thompson JR, Wensaas KA S- Editor: Gou SX L- Editor: A E- Editor: Ma S