Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5961
Peer-review started: October 20, 2014
First decision: December 26, 2014
Revised: January 9, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: May 21, 2015
Processing time: 212 Days and 17.6 Hours
AIM: To assess the prognostic significance of immunological and nutritional-based indices, including the prognostic nutritional index (PNI), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio in gastric cancer.
METHODS: We retrospectively reviewed 632 gastric cancer patients who underwent gastrectomy between 1998 and 2008. Areas under the receiver operating characteristic curve were calculated to compare the predictive ability of the indices, together with estimating the sensitivity, specificity and agreement rate. Univariate and multivariate analyses were performed to identify risk factors for overall survival (OS). Propensity score analysis was performed to adjust variables to control for selection bias.
RESULTS: Each index could predict OS in gastric cancer patients in univariate analysis, but only PNI had independent prognostic significance in multivariate analysis before and after adjustment with propensity scoring (hazard ratio, 1.668; 95% confidence interval: 1.368-2.035). In subgroup analysis, a low PNI predicted a significantly shorter OS in patients with stage II-III disease (P = 0.019, P < 0.001), T3-T4 tumors (P < 0.001), or lymph node metastasis (P < 0.001). Canton score, a combination of PNI, NLR, and platelet, was a better indicator for OS than PNI, with the largest area under the curve for 12-, 36-, 60-mo OS and overall OS (P = 0.022, P = 0.030, P < 0.001, and P = 0.024, respectively). The maximum sensitivity, specificity, and agreement rate of Canton score for predicting prognosis were 84.6%, 34.9%, and 70.1%, respectively.
CONCLUSION: PNI is an independent prognostic factor for OS in gastric cancer. Canton score can be a novel preoperative prognostic index in gastric cancer.
Core tip: This is the first study to compare the prognostic significance of different immuno-nutritional indices including prognostic nutritional index (PNI), neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in gastric cancer. We found that PNI was an independent prognostic factor for overall survival in gastric cancer before and after the propensity score analysis, especially in patients with advanced disease, deep tumors, or lymph node metastasis. We also proposed that a new index-Canton score (a combination of PNI, NLR and PLT) is a superior prognostic factor compared to PNI, NLR, or PLR alone, as it better represents the relative contribution of each of these indices.
- Citation: Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H, Peng JJ, Chen CQ, Guo P, Hao YT, He YL. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol 2015; 21(19): 5961-5971
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5961
Gastric cancer continues to be a major cause of morbidity and mortality worldwide, especially in developing countries. Despite an improvement in survival over recent years due to the development of better endoscopic and imaging techniques, surgical skills, and medical treatments, its prognosis remains unfavorable[1]. Surgery continues to be the most effective therapy for gastric cancer. Pathological results after surgery are widely used to evaluate the long-term postoperative prognosis. However, the indices used to evaluate the optimal timing of surgery or to predict survival preoperatively are still limited.
Many researchers have reported that the postoperative prognosis of gastric cancer is associated not only with tumor behavior, but also with the general condition of patients, especially their immunological and nutritional status[2-4]. The prognostic nutritional index (PNI), which is simple to calculate and easy to interpret, has been widely used to assess the preoperative immunological and nutritional status of patients undergoing gastrointestinal and cardiac surgery[5,6]. Its application as a prognostic marker was recently suggested by some researchers, and it was recently used to predict prognosis in a number of malignancies, including pancreatic, hepatocellular, and colorectal carcinoma. However, its prognostic significance in gastric cancer has not been fully studied, and the mechanisms that link PNI to outcome remain unclear[7-9]. In addition to PNI, markers of systematic inflammation, such as the number of white blood cells, neutrophils, platelets, and lymphocytes, and the indices derived from these, including the neutrophil-lymphocyte ratio (NLR)[10,11] and platelet-lymphocyte ratio (PLR), have also been used as prognostic markers. NLR was found to be associated with survival in lung and ovarian cancers, while PLR was found to be associated with prognosis in pancreatic cancer[12-15].
For gastric cancer, it remains unclear whether these parameters are independent prognostic factors in different disease stages, which of them have the highest prognostic value, and whether there is an advantage to combining them. We therefore retrospectively investigated the associations between PNI and clinicopathological features, as well as the predictive significance of PNI, NLR, and PLR, either alone or in combination, for overall survival (OS) in gastric cancer patients. A reliable prognostic index could help in making key clinical decisions such as the timing of surgery and the correct postoperative medical treatment.
We enrolled 632 patients with histologically proven gastric cancer who underwent gastrectomy between January 1998 and December 2008 at the First Affiliated Hospital of Sun Yat-sen University. They were all aged over 18 years, and complete clinical and laboratory data were available in each case. Preoperative data were collected within 7 d before surgery and the blood samples were obtained from the first or the second day of patients’ admission when they did not receive any treatment. Gastrectomy was performed for all patients for whom this was indicated. Patients were routinely followed, every 3 mo in the first year, every 3-6 mo in the second and third year, and at least once a year thereafter. The latest follow-up was December 2013, and the average follow-up duration was 55.75 mo (range, 0.8-186 mo). Patients with a history of inflammatory disease, active concomitant infection, other malignancies or synchronous immune disease (e.g., syphilis or hyperthyroidism) that might have interfered with the results of baseline immunological and nutritional status were excluded, as were patients who underwent preoperative chemotherapy. Thus, a total of 632 patients (413 men and 219 women) with a mean age of 57 years were finally eligible and analyzed. The follow-up rate reached 93.2%. Written informed consent was obtained from all patients.
We retrospectively reviewed these patients’ medical records to retrieve specific data, such as general clinical information (age, sex, height, and body weight), coexisting comorbidities, surgical data (types of gastrectomy, bleeding, and durations of surgery), tumor depth, lymph node metastasis, distant metastasis, histopathological analysis of the resected specimen, resectability of the tumor, postoperative surgical and medical complications, postoperative chemotherapy, and survival. Gastric cancer stage was classified according to the 7th edition of the American Joint Committee on Cancer TNM classification system[16]. The degree of resectability was classified as R0, R1, or R2 [R0, radical resection (the tumor was cleared macroscopically and histologically); R1, remaining microscopic disease; and R2, remaining macroscopic disease]. Events occurring within 30 d after surgery were classified as postoperative complications or mortality. The Clavien-Dindo classification was applied to rate the severity of each postoperative complication[17]. The presence of postoperative complications in this study was defined as Clavien classification grade II or higher and the serious complications were defined as grade III or IV, as there were no grade V complications. Immunological and nutritional indices were generated from the data of preoperative blood tests, including the level of serum albumin (ALB) and carcinoembryonic antigen (CEA), the total lymphocyte count (TLC), white blood cell count, neutrophil count, platelet count, and monocyte count. The earliest set of measurements was used if there were more than one set for a given patient. PNI, NLR, and PLR were calculated as ALB (g/L) + 5 × TLC (109/L), neutrophil count/lymphocyte count, and platelet count/lymphocyte count, respectively[18].
PNI stratification according to nutritional significance has previously been suggested, whereby a value higher than 50 was normal, a value higher than 45 was considered mild malnutrition, a value higher than 40 was considered moderate malnutrition, and a value lower than 40 was considered severe malnutrition. However, there is no validated cut-off value for PNI, NLR, or PLR, and therefore, receiver operating characteristic (ROC) curve analysis was performed with 5-year OS as the outcome, and the Youden index was then estimated[19]. The optimal cut-off value is that which allows the prediction of 5-year OS with the best sensitivity and specificity[20]. According to the defined cut-off values for these three indices, patients were stratified into a PNI low or high group, a NLR low or high group, and a PLR low or high group (Table 1)[10,21]. Events occurring within 30 d after surgery were classified as postoperative complications or mortality.
| Combined marker | Score |
| Prognostic Nutritional Index | |
| ≥ 48.2 | 1 |
| < 48.2 | 2 |
| Neutrophil-lymphocyte ratio | |
| ≤ 1.83 | 1 |
| > 1.83 | 2 |
| Platelet-lymphocyte ratio | |
| ≤ 140 | 1 |
| > 140 | 2 |
| Canton score | |
| PNI ≥ 48, NLR ≤ 1.83 and PLT ≤ 3 × 1011/L | 0 |
| PNI ≥ 48, NLR ≤ 1.83 and PLT > 3 × 1011/L | 1 |
| PNI ≥ 48, NLR > 1.83 and PLT ≤ 3 × 1011/L | 1 |
| PNI < 48, NLR ≤ 1.83 and PLT ≤ 3 × 1011/L | 1 |
| PNI ≥ 48, NLR > 1.83 and PLT > 3 × 1011/L | 2 |
| PNI < 48, NLR ≤ 1.83 and PLT > 3 × 1011/L | 2 |
| PNI < 48, NLR > 1.83 and PLT ≤ 3 × 1011/L | 2 |
| PNI < 48, NLR > 1.83 and PLT > 3 × 1011/L | 3 |
The categorical variables are presented as numbers and percentages and the differences between groups were determined using the χ2 test. The survival curves were calculated by the Kaplan-Meier method and compared using the log-rank test. The areas under the ROC curve (AUC) of all the derived indices (any combination of PNI, NLR, PLR and PLT, n = 15) were calculated to compare the predictive ability of each index at different time points (the end of follow-up, 12 mo, 36 mo and 60 mo), respectively. Then, we further compared the predictive value of the index with the largest AUC to that of PNI by comparing their AUC using the Z-test. OS was calculated from the date of surgery to the date of death or the last follow-up. Mantel-Cox regression methodology was used for univariate analysis of the potential factors related to survival. Factors that showed significant prognostic value in univariate analysis were further analyzed in the final multivariate Cox proportional hazards model adjusted for a propensity score in four strata.
Propensity score analysis was performed to adjust variables to control for selection bias due to the non-randomization of patients allocated in two groups according to the corresponding cut-off value[22,23]. A propensity score that represents the probability of being allocated into different groups was estimated with a logistic regression model for all patients. Any potential factors involved in both group selection and survival in the univariate analysis were entered in the model. The percentage of correctly classified patients reached 60.1 in this model. According to the propensity score, patients were stratified in four strata with 25% of patients in each. All the calculations were performed using the SPSS statistical package (version 20.0; SPSS Inc., Chicago, IL, United States). A P value less than 0.05 was considered statistically significant.
The baseline patient characteristics are summarized in Table 2. There were 413 male and 219 female patients (65.3% vs 34.7%), with a mean age of 57 years (range, 19-89 years), and 187 patients were older than 65 years. Five hundred and twenty-five patients underwent radical gastrectomy: total gastrectomy, partial gastrectomy, and palliative resection in 262, 263 and 107 cases, respectively. Ninety-five, 112, 267, and 158 patients had stages I, II, III, and IV disease, respectively. Eighty-one (12.8%) patients had postoperative complications. Decreased serum ALB levels (< 35 g/L) and elevated platelet counts (> 300 × 109/L) were noted in 125 (19.8%) and 148 (23.4%) patients, respectively.
| Variable | n (%) | PNI-H | PNI-L | P value |
| Age (yr) | 0.407 | |||
| ≤ 65 | 445 (70.4) | 235 | 210 | |
| > 65 | 187 (29.6) | 92 | 95 | |
| Sex | 0.958 | |||
| Male | 413 (65.3) | 214 | 199 | |
| Female | 219 (34.7) | 113 | 106 | |
| Resectability | < 0.001 | |||
| 0 | 509 (80.5) | 285 | 224 | |
| 1, 2 | 123 (19.5) | 42 | 81 | |
| Tumor depth | 0.006 | |||
| T1, T2 | 126 (19.9) | 79 | 47 | |
| T3, T4 | 506 (80.1) | 248 | 258 | |
| Lymph node | 0.015 | |||
| N0 | 193 (30.5) | 114 | 79 | |
| N1-3 | 439 (69.5) | 213 | 226 | |
| Distant metastasis | < 0.001 | |||
| M0 | 474 (75.0) | 266 | 208 | |
| M1 | 158 (25.0) | 61 | 97 | |
| Pathological stage | 0.002 | |||
| I, II | 207 (32.8) | 125 | 82 | |
| III, IV | 425 (67.2) | 202 | 223 | |
| WBC | 0.279 | |||
| ≤ 11 × 109/L | 597 (94.5) | 312 | 285 | |
| > 11 × 109/L | 35 (5.5) | 15 | 20 | |
| ALB | < 0.001 | |||
| ≥ 35 g/L | 507 (80.2) | 325 | 182 | |
| < 35 g/L | 125 (19.8) | 2 | 123 | |
| PLT | 0.072 | |||
| ≤ 300 × 109/L | 484 (76.6) | 260 | 224 | |
| > 300 × 109/L | 148 (23.4) | 67 | 81 | |
| CEA | 0.353 | |||
| ≤ 5 ng/mL | 547 (86.6) | 287 | 260 | |
| > 5 ng/mL | 85 (13.4) | 40 | 45 | |
| Histological type | 0.958 | |||
| Well | 190 (30.1) | 98 | 92 | |
| Poor | 442 (69.9) | 229 | 213 | |
| Blood loss | 0.034 | |||
| ≤ 400 mL | 423 (66.9) | 233 | 190 | |
| > 400 mL | 209 (33.1) | 94 | 115 | |
| Operative time | 0.883 | |||
| ≤ 4 h | 253 (40.0) | 130 | 123 | |
| > 4 h | 379 (60.0) | 197 | 182 | |
| Postoperative chemotherapy | 0.666 | |||
| Absent | 237 (37.5) | 120 | 117 | |
| Present | 395 (62.5) | 207 | 188 | |
| Postoperative complications | 0.488 | |||
| Absent | 551 (87.2) | 288 | 263 | |
| Present | 81 (12.8) | 39 | 42 | |
| Severe complication | 0.045 | |||
| Absent | 580 (91.8) | 307 | 273 | |
| Present | 52 (8.2) | 20 | 32 |
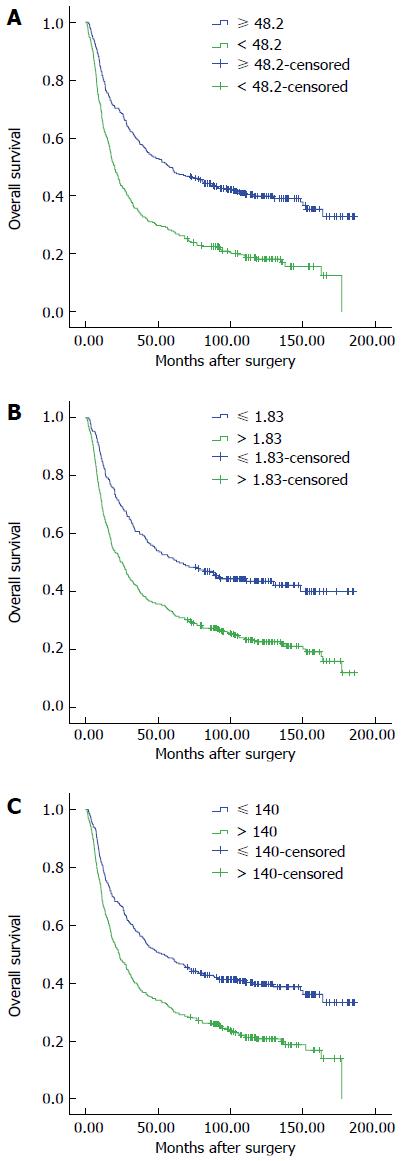
We performed ROC curve analysis to determine the optimal cut-off value with 5-year OS as an endpoint. The AUCs were 0.642, 0.636, and 0.614 for PNI, NLR, and PLR, respectively. Cut-off values of 48.2, 1.83, and 140 provided the maximal Youden index with sensitivities of 70.1%, 49.5%, and 73.2%, and specificities of 55.8%, 73.2%, and 60.3% for PNI, NLR, and PLR, respectively. Therefore, 327 (51.7%) and 305 (48.3%) patients were stratified into PNI high and low groups; 421 (66.6%) and 311 (34.4%) patients, into NLR high and low groups; and 340 (53.8%) and 292 (46.2%) patients, into PLR high and low groups, respectively.
Four-hundred and forty-eight (70.9%) patients died during follow up, with 1-, 3-, and 5-year OS rates of 72.9%, 47.6% and 39.1%, respectively. A decreased PNI and elevated NLR and PLR were associated with a reduced OS (P < 0.001 for all; Figure 1). The 1-, 3-, and 5-year OS rates were 80.7%, 59.3%, and 49.5% in patients with a PNI > 48, and 64.6%, 35.1%, and 28.9% in patients with a PNI ≤ 48 (P < 0.001 for all groups; Figure 1A).
In univariate analysis, multiple factors, including PNI, NLR, and PLR, were associated with a shorter OS (Table 3). Of these, a multivariate analysis adjusted for propensity score revealed that PNI, resectability, CEA levels, distant metastasis, pathological stage, postoperative complications, and age were independent prognostic factors for OS (Table 3).
| Variable | UnivariateP value | Multivariate | ||
| HR | 95%CI | P value | ||
| Age (yr) | 0.041 | 0.004 | ||
| ≤ 65 | 1 | |||
| > 65 | 2.323 | 1.299-4.156 | ||
| Sex | 0.299 | |||
| Male | ||||
| Female | ||||
| Resectability | < 0.001 | 0.018 | ||
| R0 | 1 | |||
| R1, R2 | 2.062 | 1.133-3.759 | ||
| Tumor depth | < 0.001 | |||
| T1, T2 | ||||
| T3, T4 | ||||
| Lymph node | < 0.001 | |||
| N0 | ||||
| N1-3 | ||||
| Distant metastasis | < 0.001 | < 0.001 | ||
| M0 | 1 | |||
| M1 | 10.505 | 6.540-16.874 | ||
| Pathological stage | ||||
| I | 1 | |||
| II | < 0.001 | 2.552 | 1.591-4.095 | < 0.001 |
| III | < 0.001 | 4.695 | 3.053-7.220 | < 0.001 |
| IV | < 0.001 | 10.505 | 6.540-16.874 | < 0.001 |
| WBC | 0.002 | |||
| ≤ 11 × 109/L | ||||
| > 11 × 109/L | ||||
| ALB | < 0.001 | |||
| ≥ 35 g/L | ||||
| < 35 g/L | ||||
| PLT | < 0.001 | |||
| ≤ 300 × 109/L | ||||
| > 300 × 109/L | ||||
| CEA | < 0.001 | 0.004 | ||
| ≤ 5 ng/mL | 1 | |||
| > 5 ng/mL | 1.457 | 1.126-1.883 | ||
| Histological type | < 0.001 | |||
| Well | ||||
| Poor | ||||
| Blood loss | < 0.001 | |||
| ≤ 400 mL | ||||
| > 400 mL | ||||
| Operative time | 0.067 | |||
| ≤ 4 h | ||||
| > 4 h | ||||
| Postoperative chemotherapy | 0.479 | |||
| Absent | ||||
| Present | ||||
| Postoperative complications | < 0.001 | 0.002 | ||
| Absent | 1 | |||
| Present | 1.516 | 1.164-1.974 | ||
| PNI | < 0.001 | < 0.001 | ||
| 1 | 1 | |||
| 2 | 1.668 | 1.368-2.035 | ||
| NLR | < 0.001 | 0.656 | ||
| 1 | 1 | |||
| 2 | 1.056 | 0.830-1.343 | ||
| PLR | < 0.001 | 0.113 | ||
| 1 | 1 | |||
| 2 | 1.190 | 0.960-1.475 | ||
| Propensity score | 0.398 | |||
Clinicopathological features such as resectability, tumor depth, lymph node metastasis, distant metastasis, pathological stage, ALB, blood loss, and severe postoperative complications differed significantly between the PNI low and high groups (Table 2).
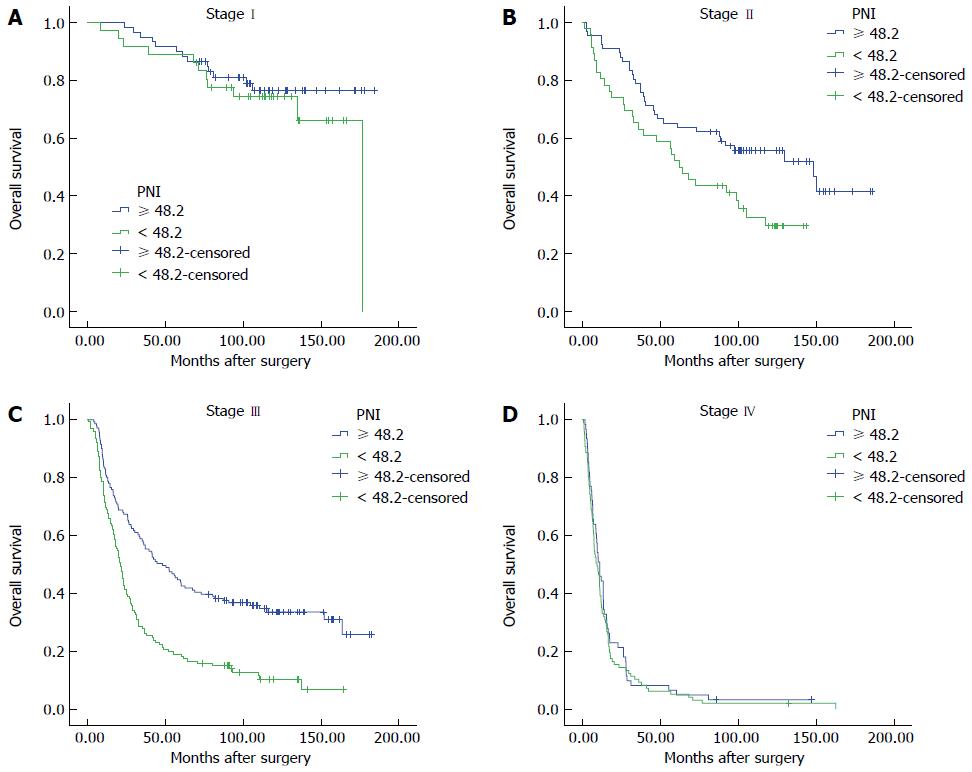
Immunological and nutritional status might vary according to disease stage, and we therefore classified patients into four groups according to pathological stage (Figure 2). The OS of patients with a low PNI was significantly shorter only if they had stage II or III disease (stage II: PNI-H vs PNI-L, 65.15% vs 52.17%, P = 0.019; stage III: 43.97% vs 19.05%, P < 0.001), but not stage I or IV disease (stage I: 89.83% vs 88.88%, P = 0.377; stage IV: 6.56% vs 5.15%, P = 0.471). Patients with a high NLR had a significantly shorter OS only if they had stage I disease (P = 0.007). However, in patients with stage III or IV disease, a high PLR was significantly associated with a shorter OS (P = 0.011, 0.031 vs P = 0.132, 0.556, respectively).
To further explore the association between PNI and gastric cancer progression, we performed subgroup analysis according to tumor depth and lymph node metastasis. Among patients with T1 or T2 tumors (n = 126 in total), those with a high PNI tended to have a longer (but not significantly longer) OS (P = 0.134), although those with a high PNI and a T3 or T4 tumor did have a significantly longer OS (n = 506, P < 0.001). Similarly, OS in the PNI high group was only significantly longer amongst those with lymph node metastasis (P < 0.001).
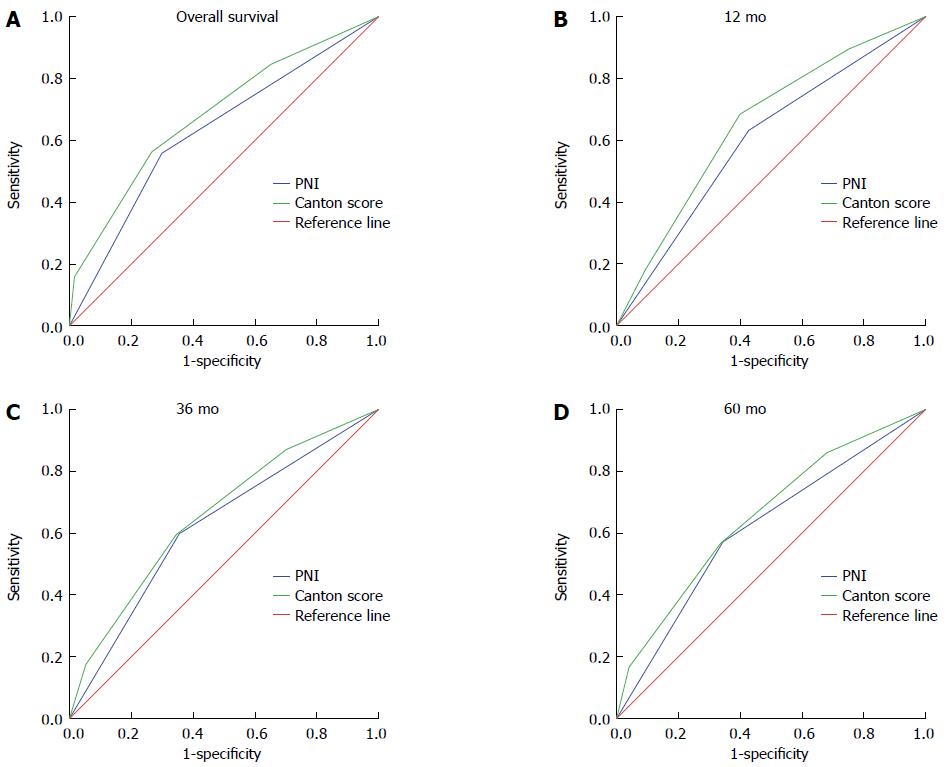
As one indicator might have limited predictive value, we combined a number of factors to generate a new preoperative prognostic index. After combining PNI, NLR, PLR, ALB, and PLT to generate several new indices and comparing them, we found two indices with the greatest prognostic significance (Table 4). They were the combination of PNI, NLR, and PLT and the combination of PNI, NLR, PLR and PLT. With the advantage of convenience, the combination of PNI, NLR and PLT, which we referred to Canton score, was chosen as the novel prognostic index considering there was no significant difference between these two derived indices. Canton score is defined as the number of the following prognostic indexes (PNI ≥ 48, NLR ≤ 1.83 and PLT ≤ 3 × 1011/L) and thus has a value of 0, 1, 2, or 3. Detailed definition of the value of Canton score is shown in Table 1. The AUC for Canton score with 5-year OS as an outcome was 0.684, with an obvious difference compared to that of PNI (P = 0.024). We then compared the AUCs of Canton score and PNI with 12-, 36-, 60-mo and overall OS as endpoints. The AUC of Canton score in each case was higher than that of PNI (P = 0.022, P = 0.030, P < 0.001, and P = 0.024, respectively) (Figure 3). Moreover, in univariate analysis, hazard ratios (HRs) for death were 1.414 [95% confidence interval (95%CI): 1.054-1.895), 2.341 (95%CI: 1.772-3.091), and 3.555 (95%CI: 2.545-4.966)] for patients with a Canton score of 1, 2, and 3, respectively, compared to those with a Canton score of 0 (Table 5). Multivariate analysis also revealed an independent prognostic role for Canton score (Canton score = 1: HR = 1.076; 95%CI, 0.796-1.454; Canton score = 2: HR = 1.554; 95%CI: 1.151-2.097; Canton score = 3: HR = 1.643; 95%CI: 1.142-2.364) (Table 5). The maximum sensitivity, specificity, and agreement rate of Canton score in predicting prognosis were 84.6%, 34.9%, and 70.1%, respectively, superior to these values for PNI, suggesting that Canton score is a novel and effective preoperative prognostic index (Table 6).
| Item | All survival | 12 mo | 36 mo | 60 mo | ||||
| AUC | P value | AUC | P value | AUC | P value | AUC | P value | |
| PNI | 0.630 | < 0.001 | 0.602 | < 0.001 | 0.621 | < 0.001 | 0.614 | < 0.001 |
| NLR | 0.613 | < 0.001 | 0.593 | < 0.001 | 0.587 | < 0.001 | 0.588 | < 0.001 |
| PLR | 0.611 | < 0.001 | 0.580 | 0.002 | 0.592 | < 0.001 | 0.593 | < 0.001 |
| PLT | 0.573 | 0.004 | 0.564 | 0.013 | 0.562 | 0.007 | 0.566 | 0.005 |
| PNI + NLR | 0.535 | 0.493 | 0.502 | 0.968 | 0.476 | 0.570 | 0.487 | 0.776 |
| PNI + PLR | 0.477 | 0.656 | 0.480 | 0.644 | 0.435 | 0.123 | 0.455 | 0.309 |
| PNI + PLT | 0.498 | 0.970 | 0.493 | 0.867 | 0.469 | 0.468 | 0.449 | 0.251 |
| NLR + PLR | 0.477 | 0.647 | 0.447 | 0.229 | 0.459 | 0.329 | 0.472 | 0.522 |
| NLR + PLT | 0.487 | 0.796 | 0.466 | 0.437 | 0.488 | 0.772 | 0.461 | 0.382 |
| PLR + PLT | 0.440 | 0.240 | 0.448 | 0.237 | 0.448 | 0.219 | 0.442 | 0.190 |
| PNI + NLR + PLR | 0.679 | < 0.001 | 0.638 | < 0.001 | 0.651 | < 0.001 | 0.647 | < 0.001 |
| PNI + NLR + PLT | 0.684 | < 0.001 | 0.655 | < 0.001 | 0.657 | < 0.001 | 0.654 | < 0.001 |
| PNI + PLR + PLT | 0.668 | < 0.001 | 0.634 | < 0.001 | 0.647 | < 0.001 | 0.646 | < 0.001 |
| NLR + PLR + PLT | 0.660 | < 0.001 | 0.627 | < 0.001 | 0.629 | < 0.001 | 0.632 | < 0.001 |
| PNI + NLR + PLR + PLT | 0.685 | < 0.001 | 0.647 | < 0.001 | 0.657 | < 0.001 | 0.655 | < 0.001 |
| Variable | Univariate P value | Multivariate | ||
| HR | 95%CI | P value | ||
| Age (yr) | 0.041 | 0.051 | ||
| ≤ 65 | 1 | |||
| > 65 | 1.238 | 0.999-1.536 | ||
| Sex | 0.299 | |||
| Male | ||||
| Female | ||||
| Resectability | < 0.001 | 0.002 | ||
| R0 | 1 | |||
| R1, R2 | 1.567 | 1.204-2.040 | ||
| Tumor depth | < 0.001 | |||
| T1, T2 | ||||
| T3, T4 | ||||
| Lymph node | < 0.001 | |||
| N0 | ||||
| N1-3 | ||||
| Distant metastasis | < 0.001 | |||
| M0 | ||||
| M1 | ||||
| Pathological stage | 0.234 | |||
| I, II | ||||
| III, IV | < 0.001 | |||
| WBC | 0.002 | |||
| ≤ 11× 109/L | ||||
| > 11× 109/L | ||||
| ALB | < 0.001 | |||
| ≥ 35 g/L | ||||
| < 35 g/L | ||||
| PLT | < 0.001 | 0.795 | ||
| ≤ 300 × 109/L | ||||
| > 300 × 109/L | ||||
| CEA | < 0.001 | |||
| ≤ 5 ng/mL | ||||
| > 5 ng/mL | ||||
| Histological type | < 0.001 | |||
| Well | ||||
| Poor | ||||
| Blood loss | < 0.001 | 0.407 | ||
| ≤ 400 mL | ||||
| > 400 mL | ||||
| Operative time | 0.067 | |||
| ≤ 4 h | ||||
| > 4 h | ||||
| Postoperative chemotherapy | 0.479 | |||
| Absent | ||||
| Present | ||||
| Postoperative complications | < 0.001 | |||
| Absent | ||||
| Present | ||||
| PLR | < 0.001 | 0.524 | ||
| PLR-L | ||||
| PLR-H | ||||
| Canton score | ||||
| 0 | 1 | |||
| 1 | 0.021 | 1.076 | 0.796-1.454 | 0.633 |
| 2 | < 0.001 | 1.554 | 1.151-2.097 | 0.004 |
| 3 | < 0.001 | 1.643 | 1.142-2.364 | 0.007 |
| Variable | UnivariateP value | Multivariate | ||
| HR | 95%CI | P value | ||
| Resectability | < 0.001 | 0.002 | ||
| R0 | 1 | |||
| R1, R2 | 1.567 | 1.204-2.040 | ||
| Canton score | ||||
| 0 | 1 | |||
| 1 | 0.021 | 1.076 | 0.796-1.454 | 0.633 |
| 2 | < 0.001 | 1.554 | 1.151-2.097 | 0.004 |
| 3 | < 0.001 | 1.643 | 1.142-2.364 | 0.007 |
In our study, we found that PNI, NLR, and PLR were associated with the OS of gastric cancer patients in univariate analysis, but only PNI was an independent prognostic factor in multivariate analysis before and after propensity score adjustment, together with resectability, postoperative complication, distant metastasis, pathological stage, and CEA levels. In addition, subgroup analysis showed that a low PNI predicted a significantly shorter OS in patients with stage II or III disease, T3 or T4 tumors, or lymph node metastasis. We developed a new index, Canton score, which is a better prognostic indicator for OS than PNI.
Preoperative immunological and nutritional conditions are associated with both the postoperative and long-term outcomes of malignant tumors[1,24]. Identifying prognostic factors before surgery to help determine the optimal preoperative therapy and timing of surgery is important. Previous attempts have focused on a number of immuno-nutritional indices, including PNI, NLR, PLR, PI, and GPS[4,6,7,9,15,21,25-27]. However, their reported prognostic value can vary between studies of the same cancer type; for example, in hepatocellular carcinoma, PLR was found to be a prognostic marker in one study, but another study suggested that only PNI was a prognostic marker[15]. It is therefore crucial to compare different indices to identify a more effective and convenient scoring system. However, to date, these three indices have not been analyzed together, nor has their association with OS been compared in gastric cancer. In this study, we found that PNI had the best predictive value, with the highest AUC for 5-year OS, and it was the only independent prognostic factor for OS. An additional consideration is the heterogeneity of patient cohorts among studies, which might account for their different findings. To reduce this bias, we performed propensity score analysis and found that PNI was an independent prognostic factor before and after adjustment.
The assessment of different prognostic factors depended on the pathological stage of gastric cancer; for example, PNI had greatest prognostic significance in advanced gastric cancer patients (stages II and III). Thus, error due to different proportions of patients with a given pathological stage among different studies cannot be excluded. For example, one study with 327 stage I patients found a significant difference in OS between stage I patients with low or high PNI, but our study with only 95 stage I patients found no significant association between PNI and OS[27]. A larger cohort of stage I patients may give a different result.
It remains unclear how the association between PNI and OS varied according to pathological stage. We found that for patients in stage I or III, 5-year OS was significantly shorter in the group of low PNI, however, for patients in stage I or IV, no significant association was found although the 5-year OS of patients with a low PNI was slightly shorter. This implies that the predictive significance of PNI might be greater in advanced gastric cancer, which is supported by the finding that PNI was only significantly associated with OS in patients with deeper tumor invasion and lymph node metastasis. This result is consistent with that of our meta-analysis and the findings of other studies[4,28]. This dependence on tumor invasion and spread might reflect the relatively good immunological and nutritional status of gastric cancer patients with stage I disease, and that gastric cancer was not always considered to be the major cause of death in these patients. Indeed, one study found that more than 50% of patients with stage I disease died from non-cancer causes, regardless of PNI[27]. Similarly, we found that age and postoperative complications were the two main factors influencing prognosis in stage I patients. Advanced age was also an independent factor for poor prognosis, but only after propensity score adjustment, and several other studies did not find age to be an independent factor. This suggests that the non-randomization of patients and the consequent compounding factors need to be accounted for; hence, our propensity score analysis made our results more reliable. Older patients with a decline in both biological and physiologic functions of the digestive system and accompanying disorders such as chronic diseases, malignancies, and psychological illness are often in a poor immunological and nutritional condition[29], and events such as respiratory failure consequent to pneumonia were common non-malignant causes of death in the elderly[30].
The mechanism by which low PNI may impact survival is not fully understood. Serum ALB is required for a number of key physiological functions, including the maintenance of serum osmolality, tissue repair, transport of extrinsic and intrinsic compounds such as drugs and nutrients, and modulating systematic inflammation[31]. Thus, hypoalbuminemia can result in postoperative complications, including anastomotic edema and fistula, delayed tissue repair, reduced therapeutic efficacy of drugs and nutrients, and more importantly, the activation of systematic inflammation and influencing host immunity. Consequently, low ALB levels could promote tumor growth and invasion and trigger infections, which worsen prognosis. In addition to ALB levels, the lymphocyte count reflects immunological status and the degree of systematic inflammation to some extent[10]. Yang et al[32] convincingly showed that the impairment of lymphocyte mediated antitumor response is an immunological determinant of prognosis in hepatocellular carcinoma. It has also been reported that a cascade of inflammatory mediators during systematic inflammation can lead to tumor progression. This results from the recruitment of inflammatory cells including lymphocytes by the activation of transcription factors and inflammatory mediators after activation of the extrinsic (pre-existing inflammation) or intrinsic pathway (oncogene activation)[33]. There is also an interaction between nutritional status and systematic inflammation response. Moreover, both malnutrition (hypoalbuminemia) and an inflammatory response (based on the TLC) may affect therapeutic compliance and in turn affect prognosis[34].
Subsequent prospective clinical studies failed to find any benefit for serum ALB supplementation and its preoperative use for cancer patients remains controversial although correcting the serum ALB level before surgery was found to improve survival in early studies. However, anti-inflammatory therapy has been shown to extend survival of gastric cancer patients in a recent trial[35]. Based on our findings, it might be possible to improve survival by boosting immunity and nutritional status. Indeed, previous studies have also found that improved preoperative immunological and nutritional status could reduce the length of hospital stay and improve prognosis[27,36]. If this is validated in further clinical trials, we would recommend preoperative medical treatment to achieve nutritional and immunological levels that optimize the PNI, and then perform surgery at the optimal time.
The new index that we propose here, Canton score, is a superior prognostic factor compared to PNI, NLR, or PLR alone, as it better represents the relative contribution of each of these indices. In addition to PNI and NLR, which include TLC and ALB levels, together with the neutrophil count, Canton score also includes the PLT level, which was recently found be associated with tumor development. Platelets can secrete angiogenic factors and hence promote tumor growth by stimulating angiogenesis and are also involved in tumor invasion by binding to tumor cells via the adhesion molecules found in their alpha-granules. Further studies are needed to fully evaluate the predictive value of Canton score in cancer.
Several limitations of our study need to be considered. This was a retrospective observational study in which we detected a significant association between PNI and OS in gastric cancer, but we could not prove this association. Additionally, patients with different disease stages were not equally distributed, with relatively few cases of stage I disease, thus probably skewing the results.
In conclusion, PNI, but not NLR or PLR, is an independent prognostic factor for OS in gastric cancer, especially amongst patients with advanced disease, deep tumor invasion, or lymph node metastasis. However, more studies with larger sample sizes are needed to explore the prognostic value of PNI in gastric cancer and the benefit of intervention to improve immunological and nutritional status in order to achieve a favorable PNI. Further, we showed that Canton score could be a novel preoperative prognostic index in gastric cancer.
Gastric cancer is a major cause of morbidity and mortality worldwide, especially in developing countries. However, the indices used to evaluate the optimal timing of surgery or to predict survival preoperatively are still limited. Prognostic nutritional index (PNI), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) were recently used to predict prognosis in a number of malignancies, including pancreatic, hepatocellular, and colorectal carcinomas. In this study, the authors aimed to assess the prognostic significance of these immunological and nutritional-based indices in gastric cancer.
For gastric cancer, it remains unclear about the prognostic significance of immuno-nutritional indices in patients with different disease stages. It is important to compare different indices to identify a more effective and convenient scoring system. And it is unknown whether there is an advantage to combining these prognostic indices.
PNI, but not NLR or PLR, is an independent prognostic factor for overall survival (OS) in gastric cancer, especially amongst patients with advanced disease, deep tumor invasion, or lymph node metastasis. Canton score, a combination of PNI, NLR and PLT, can be a novel preoperative prognostic index in gastric cancer for its better prognostic value than PNI.
PNI and canton score can be used to predict survival of patients with gastric cancer preoperatively, which helps in making key clinical decisions such as the timing of surgery and the correct preoperative medical treatment. More studies with larger sample sizes are needed to explore the prognostic value of these two indices in gastric cancer and the benefit of intervention to improve immunological and nutritional status.
Canton score is an innovative prognostic index which derives from the combination of PNI, NLR and PLT. It is defined as the number of the following prognostic indexes (PNI > 48, NLR < 1.83 and PLT < 3 × 1011/L) and thus has a value of 0, 1, 2, or 3. Higher value usually indicates better immuno-nutritional status.
Overall this is an interesting study and the work is generally clearly presented and described. The authors compared the prognostic significance of different immuno-nutritional indices including PNI, NLR and PLR in a large cohort of gastric cancer patients, and proposed a new index-Canton score, which is superior to other indexes in predicting OS. The article has an adequate bibliography, the manuscript is correctly written and the conclusions are justified by the results found in the study.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8:1041-1048. [PubMed] |
| 3. | Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383-5391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg. 2012;36:1632-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Wakita M, Fukatsu A, Amagai T. Nutrition assessment as a predictor of clinical outcomes for infants with cardiac surgery: using the prognostic nutritional index. Nutr Clin Pract. 2011;26:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 9. | Maeda K, Shibutani M, Otani H, Nagahara H, Sugano K, Ikeya T, Kubo N, Amano R, Kimura K, Muguruma K. Low nutritional prognostic index correlates with poor survival in patients with stage IV colorectal cancer following palliative resection of the primary tumor. World J Surg. 2014;38:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, Zhou J, Wang XC, Gong JF, Gao J. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945-950. [PubMed] |
| 11. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [PubMed] |
| 12. | Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805-5813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 14. | Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 17. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 18. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 19. | Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670-675. [PubMed] |
| 20. | Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM. 2006;8:19-20. [PubMed] |
| 21. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 617] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 22. | Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327-333. [PubMed] |
| 23. | He MM, Zhang DS, Wang F, Wang ZQ, Luo HY, Jin Y, Wei XL, Xu RH. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PLoS One. 2013;8:e83921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Yao ZH, Tian GY, Wan YY, Kang YM, Guo HS, Liu QH, Lin DJ. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139:2117-2123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 27. | Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 29. | Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging. 2010;5:207-216. [PubMed] |
| 30. | Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (2)] |
| 32. | Yang ZQ, Yang ZY, Zhang LD, Ping-Bie SG, Ma KS, Li XW, Dong JH. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8543] [Article Influence: 474.6] [Reference Citation Analysis (0)] |
| 34. | Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, Lee CK. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109:147-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1125] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kilgour E, Liu XE, Yu JR S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN