Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5794
Peer-review started: November 23, 2014
First decision: January 22, 2015
Revised: February 25, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: May 21, 2015
Processing time: 179 Days and 6.1 Hours
Pancreaticoduodenectomy (PD) will result in removal of important multiorgans in upper intestinal tract and subsequently secondary physiologic change. In the past, surgeons just focused on the safety of surgical procedure; however, PD is regarded as safe and widely applied to treatment of periampullary lesions. Practical issues after PD, such as, effect of duodenectomy, metabolic surgery-like effect, alignment effect of gastrointestinal continuity, and non-alcoholic fatty liver disease were summarized and discussed.
Core tip: In the past, pancreaticoduodenectomy (PD) should be avoided because of its extremely high morbidity and mortality. With the advance of surgical techniques and perioperative management, PD has been regarded as good choice for the treatment of periampullary pathologic conditions. In this moment, turning our interest to potential physiological change following PD may be necessary, because PD always results in removal of important internal organs in upper gastrointestinal tract and altering normal path of gastrointestinal flow. Well awareness of these “internal” changes will be helpful for proper management of the patients with PD.
- Citation: Kang CM, Lee JH. Pathophysiology after pancreaticoduodenectomy. World J Gastroenterol 2015; 21(19): 5794-5804
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5794
In the past, it was thought that pancreaticoduodenectomy (PD) should be avoided because of its extremely high rates of morbidity (greater than 70%) and mortality (greater than 30%)[1]. More recently, many surgeons have focused on technical innovation to reduce postoperative severe morbidity after PD. Based on advancements in surgical experiences, perioperative management and interventional radiology, it is thought that most complications related to PD can be managed in a conservative way. Based on the literature, mortality after PD is now considered to be 2%-5% and morbidity is reported to be 33%-64%[2-4]. PD recently has gained wide acceptance as a safe surgical method of choice for the treatment of periampullary pathological conditions.
PD consists of two surgical components: (1) resection phase: removal of pancreatic head, common bile duct, gallbladder, and duodenum along with some part of the proximal jejunum. Partial gastrectomy can be included; and (2) reconstruction phase: gastrointestinal continuity is created by pancreatico-enterostomy [pancreaticogastrostomy (PG) or pancreaticojejunostomy (PJ)], hepaticojejunostomy, and duodeno-or, gastro-jejunostomy.
When surgical technique is largely standardized, potential physiological changes following PD need to be concerned because PD results in the removal of important internal organs in the upper gastrointestinal tract and alters the normal path of the gastrointestinal flow. Therefore, surgeons who perform PD should be well aware of these “internal” challenges for proper management of patients with PD. Herein, the following issues will be discussed to understand the practical pathophysiological changes that occur after PD.
The duodenum is a source of various peptide hormones. Among them, motilin is a 22 amino acids peptide that is primarily localized in enterochromaffin cells of the duodenum and proximal jejunum[5], which is known to be responsible for phase III activity of the gastroduodenal migrating motor complex (MMC)[5]. It was found that exogenous motilin could induce premature phase III contraction in the upper gastrointestinal tract. Moreover, reduced plasma concentrations of motilin were associated with gastroparesis (Table 1). Therefore, PD can lead to the inevitable removal of the duodenum, which can reduce plasma levels of motilin, resulting in delayed gastric emptying (gastroparesis) by reducing coordinated stomach, duodenum and proximal jejunum movements.
| Ref. | Year | Study design and model | Primary end point | Observations |
| Tanaka et al[74] | 1987 | Normal dog vs Duodenectomized dog | Phase III contraction, plasma level of motilin | All control dogs showed characteristic MMC Duodenectomized dog showed non-typical, irregular and non-cyclic pattern of contraction Duodenectomized dog showed low plasma concentration of motilin without cyclical variation |
| Tanaka et al[75] | 1988 | Normal dog vs Duodenectomized dog | Inter-digestive gastric and small intestinal MMC plasma level of motilin and Polypeptide Y | MMC was abolished in duodenectomized dogs (3 out of 4 dogs) The other dogs showed intermittent cyclic, but markedly abnormal characteristics of gastric contraction Jejunal MMC appeared with short interval Duodenectomy abolished cyclic variation of plasma motilin and polypeptide Y |
| Suzuki et al[76] | 2001 | Conscious dog vs Duodenectomized dog | Phase III contraction, plasma level of insulin, and motilin | Duodenectomy resulted in no phase III contraction in upper GI tract Duodenectomy resulted in no fluctuation of plasma motilin (low level of motilin) Exogenous administration of motilin resulted in comparable response of phased III as shown in control |
| Malfertheiner et al[77] | 1989 | Normal dog vs | Pancreatic trypsin | In duodenectomized dog |
| Duodenectomized dog | GI motility plasma motilin, PPY | Trypsin secretion was not coordinated with inter-digestive motility, motilin, and PPY Inter-digestive motility was altered Plasma level of motilin and PPY were reduced, and showed no cyclic pattern | ||
| Itoh et al[78] | 1976 | Normal dog | GI motility plasma motilin | Gastrointestinal contractile activity in the conscious dog, Digestive states: motilin had no influence upon the motor activity Inter-digestive states: had influence upon the motor activity |
| Vantrappen et al[79] | 1979 | Human | GI motility plasma motilin level | The effect of exogenous motilin on interdigestive migrating motor complex Plasma motilin levels is one of the factor involved in the production of the activity front of the MMC in man |
| Sarna et al[80] | 1983 | Normal dog | Plasma motilin levels Migrating myoelectric complexes (MMCs) | Cause and effect relationship between plasma motilin levels and migrating myoelectric complexes Endogenous motilin does not initiate spontaneous mmcs MMC contractions release motilin |
Motilin is not yet available for clinical use. However, there is some clinical evidence to support these experiments and hypotheses. Naritomi et al[6] evaluated the first occurrence of MMC and motilin in patients with pylorus-preserving pancreaticoduodenectomy (PPPD) and duodenum-preserving pancreatic head resection (DPPHR). They found that the PPPD group required a longer amount of time for initial gastric phase III recovery, and the plasma levels of motilin were lower. Yeo et al[7] performed a prospective randomized placebo-controlled trial and found that erythromycin could significantly accelerate gastric emptying after PD and reduce the incidence of delayed gastric emptying (DGE) by 37%. Indeed, erythromycin can act as a motilin agonist by binding motilin receptors, and its clinical benefit to improve gastric emptying has been demonstrated in diabetic gastroparesis[8] and postvagotomy gastroparesis[9]. Matsunaga et al[10] also showed manometric evidence of improved early gastric stasis by erythromycin after PPPD. Administration of saline caused no changes in gastric or jejunal motility; however, erythromycin could induce phase III-like gastric contraction and reduce the amount of gastric juice output in all patients.
Duodenectomy also influences on the secretion of other gastrointestinal hormones. Malfertheiner et al[11] showed that plasma levels of pancreatic polypeptide (PP) were altered with no cyclic pattern in duodenectomized dogs. Müller et al[12] evaluated changes in CCK, PP, and gastrin in PPPD and DPPHR patients. They found that PP was significantly reduced in both PPPD and DPPHR, and cholecystokinin (CCK) was reduced in an early postoperative period after PPPD. Tangoku et al[13], and Kingsnorth et al[14] evaluated plasma gastrin and CCK responses between standard PD and PPPD. Basal plasma levels of gastrin and CCK were significantly higher in controls compared with patients with standard PD (P < 0.05), suggesting that preservation of the stomach and part of the duodenum (pylorus-preserving) appeared to be a more physiological procedure for performing PD.
Regarding reduced gastrin levels following PD, it has been proposed that postoperative atrophic changes in the remnant pancreas after PD can be derived from removal of the duodenum and distal stomach because these organs are a source of gastric stimulation[15]. Jang et al[16] investigated the effects of induced hypergastrinemia on the prevention of pancreatic atrophy after PPPD. They performed a randomized control study and successfully demonstrated that induced hypergastrinemia by Lansoprazole could prevent postoperative volume change of the remnant pancreas and preserve long-term exocrine and endocrine function in patients with PPPD. This study is a good example to show how potential physiological changes can be translated into clinical practice for proper management of patients who undergo PD.
Furthermore, Chung et al[17] investigated the role of vagal and efferent adrenergic innervation to coordinate the gastric and small intestinal MMCs after removing the pylorus, duodenum, and upper jejunum in three dogs. They concluded that duodenectomy could reestablish gastric MMC-like activity without motilin, showing a peak after 1-4 mo, and it appeared to require extrinsic innervation. PD sometimes (depending on the surgeons’ preference and disease extent) requires extensive soft tissue dissection around a major arterial system, including the celiac axis, common hepatic artery, and superior mesenteric artery for margin-negative resection. Too much dissection of soft tissue (for example, extended PD) can result in surgical denervation of visceral autonomic nerves and can be one of the reasons for transient delayed gastric emptying in a clinical setting[18,19].
Based on this brief review of the literature, it can be noted that duodenectomy not only disrupts the coordination of gastric and intestinal MMC but also disrupts the coordination between inter-digestive motility and pancreatic secretion and abolishes the inter-digestive cyclic variations in plasma gastrointestinal hormones, such as motilin, CCK, gastrin, and pancreatic polypeptide (PP). Additionally, extensive soft tissue dissection-induced disconnection of neural stimulation and secondary postoperative inflammatory insults can cause pathophysiological changes after PD, which can be attributed to a clinical delay in postoperative recovery.
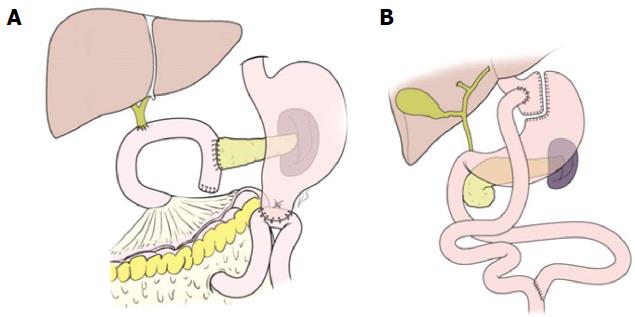
The bariatric surgical procedures were attempted to promote weight loss by restricting food intake and promoting malabsorption. The most commonly performed procedures were Roux-en-Y gastric bypass (46.6%), vertical sleeve gastrectomy (27.8%), adjustable gastric banding (17.8%), and biliopancreatic diversion with duodenal switch (2.2%)[20]. Interestingly, when looking at schematic figures showing PD, it could be noted that PD is somewhat similar in appearance to Roux-en-Y gastric bypass (Figure 1). The food passage after PD could be similar to that after Roux-en-Y gastric bypass, bypassing duodenum and passing directly into distal jejunum. Natural bile and pancreatic flow can be thought of as a Roux-en-Y loop in PD. Therefore, PD might cause the physiological changes that appear after bariatric surgery.
Notably, glucagon-like peptide-1 (GLP-1) is an interesting gastrointestinal hormone. After Roux-en-Y gastric bypass, GLP-1 is secreted by L cells of the small bowel, with higher concentrations in the distal ileum and colon. This peptide is produced in response to a meal and decreases food intake through its effects on the hypothalamus and brainstem. Additionally, GLP-1 is known to slow gastric emptying, inhibit glucagon release and stimulate the pancreas to secrete insulin (incretin effect)[21,22]. Recently, You et al[23] showed that about 30% of patients with PD were found to have hypertrophic changes in the remnant pancreas, and Wu et al[24] also reported resolution of diabetes after PD. They observed resolution of long-standing diabetes after PD in patients with (3, 9.1% of 33 patients, P = 0.005) and without (6, 9.8% of 61 patients) pancreatic cancer, suggesting that PD-associated anatomical changes might play an important role in the resolution of DM after PD.
Despite conflicting observations about GLP-1 levels after PD[25], several studies have investigated changes in plasma GLP-1 levels after PD. Ohtsuka et al[26] previously showed that improved glucose metabolism after PD was mainly influenced by improved insulin resistance. They observed significantly increased plasma GLP-1 levels after PD; however, even after removal of the pancreatic head (reduced pancreatic volume), β-cell function did not change. Muscogiuri et al[27] evaluated the effect of duodenectomy on GLP-1 secretion after PD. They found that PPPD was associated with a remarkable increase in GLP-1 levels, which reached levels comparable with those observed after gastric bypass[28]. Harmuth et al[29] reported that conventional PD was associated with accelerated gastric emptying, enhanced postprandial GLP-1 release, and improved insulin sensitivity. The rapid transport of unabsorbed nutrients to the distal bowel triggers enhanced release of GLP-1, resulting in improved glycemic control.
Notably, GLP-1 agents used to control diabetes have been associated with an increased risk of pancreatic cancer in patients with type 2 diabetes[30]. However, a recent study demonstrated that GLP-1 could harbor anticancer properties against pancreatic cancer. GLP-1 receptor activation has anti-tumor effects on human pancreatic cancers via inhibition of the PI3K/Akt pathway[31]. Additionally, activation of the GLP-1 receptor was found to inhibit growth and promote apoptosis of human pancreatic cancer cells[32]. PD-induced GLP-1 release can be used for future treatment of resected pancreatic head cancer, although further investigations are warranted.
In addition to the direct effects of removing organ by resection, pathophysiological changes after PD will also be influenced by how the gastrointestinal alignment is rearranged in the reconstructive phase. Various methods for reconstruction, similar to gastrointestinal alignment, have been reported in PD, such as Billroth I (the Imanaga method)[33], Billroth II (the Whipple and/or Child method)[34], Roux-en-Y loop fashion[35], an additional Braun anastomosis[36], and retrocolic/antecolic reconstruction[37]. In clinical practice, DGE appears to represent the pathophysiological changes that occur after PD. Conflicting observations have been reported about the incidence of DGE, and the exact mechanisms to explain the occurrence of DGE according to different reconstruction method remain to be determined. However, robust evidence is accumulating about the incidence of DGE according to different gastrointestinal reconstructive methods following PD (Table 2).
| Ref. | Year | Study design | Primary end point | Observations |
| Eshuis et al[81] | 2014 | In PPPD Antecolic (n = 125) vs Retrocolic (n = 121) | DGE | No differences in DGE (45 patients (36%) vs 41 (34%), absolute risk difference: 2.1% (95%CI: -9.8-14.0) No differences in need for postoperative nutritional support, other complications, hospital mortality, and median length of hospital stay |
| Tamandl et al[82] | 2014 | In PPPD, antecolic (n = 36) vs retrocolic (n = 28) | DGE | No differences in DGE (17.6% vs 23.1%, P = 0.628) No differences in length of hospital stay [13.0 (10.0-17.5) vs 12.5 (11.0-17.0) days; P = 0.446], time to regular diet [5 (5-7) d vs 5 (4-6) d, P = 0.353], and NG tube requirement [4 (3-7) d vs 3 (3-5) d, P = 0.600] |
| Imamura et al[83] | 2014 | In PPPD, antecolic (n = 58) vs vertical retrocolic (n = 58) | DGE | No difference in DGE (12.1% vs 20.7%, P = 0.316) At postoperative 6 mo, DGE was accelerated in antecolic group At postoperative 12 mo, better postoperative weight recovery in vertical retrocolic group (93.8% ± 1.2% vs 98.5 % ± 1.3%, P = 0.015) |
| Tani et al[84] | 2014 | In PD, Conventional (n = 76) vs Isolated Roux-en-Y (n = 77) | POPF/DGE | No differences in DGE and POPF POPF: conventional (34%) vs isolated Roux-en-Y (33%), P = 0.909 DGE: conventional (12%) vs isolated Roux-en-Y (15%), P = 0.609 |
| Shimoda et al[85] | 2013 | In SSPPD, Billroth II (n = 52) vs Roux-en-Y (n = 49) | DGE | Lower DGE in Billroth II: (5.7% vs 30.4%, P = 0.028) Shorter hospital stay in Billroth II (31.6 ± 15.0 d vs 41.4 ± 20.5 d, P = 0.037) Significant association between POPF and DGE (P = 0.037) |
| Ke et al[86] | 2013 | In PD | DGE/POPF | No differences in DGE and POPF |
| Continuous loop (n = 109) vs Roux-en-Y (n = 107) | POPF: continuous loop (17.6%) vs Roux-en-Y (15.7%), P > 0.05 DGE: continuous loop (25%) vs Roux-en-Y (23%), P > 0.05 | |||
| Gangavatiker et al[87] | 2011 | In conventional PD and PPPD Antecolic (n = 32) vs Retrocolic (n = 36) | DGE | No difference in DGE (34.4% vs 27.8%, P = 0.6) |
| Kurahara et al[88] | 2011 | In SSPPD, Antecolic (n = 24) vs retrocolic (n = 22) | DGE | Lower incidence of DGE in the antecolic group [20.8% vs 50%, P = 0.0364, especially in the incidence of DGE grade B/C (4.2% vs 27.3%, P = 0.0234)] Significantly shorter time to full resumption of diet in antecolic group No significant difference in other postoperative complications |
| Chijiiwa et al[89] | 2009 | In PPPD, Antecolic (n = 17) vs retrocolic (n = 18) | DGE | No difference in DGE DGE: 6% vs 22%, P = 0.34 |
Short-term perioperative outcomes, such as postoperative complications, length of hospital stay, and resuming of acceptable diet, are the main concerns after PD. Miyakawa et al[38] demonstrated that fat absorption after Billroth I PG (PG-I) is superior to that after Billroth II PJ (PJ-II) in patients with disordered exocrine function of the pancreatic remnant, suggesting that PG-I allows for more effective utilization of the exocrine enzymes of the pancreatic remnant because of elimination of the blind loop characteristic of the PJ-II. Ohtsuka et al[39] evaluated nutritional status and quality of life after PD, and compared these data between 18 patients with end-to-end (Imanaga) and 13 patients with end-to-side (Traverso) gastrointestinal reconstruction. They found that the scores of psychosocial conditions remained low, even over a long-term, in both groups. However, the values of nutritional parameters showed no significant difference between the two groups at each time point, suggesting that the postoperative quality of life and nutritional status were not different between Imanaga and Traverso reconstructions after PPPD. However, a paucity of high-level evidence exists about long-term outcomes, including nutritional outcomes and quality of patients’ life, which could be influenced by potential pathophysiological changes after PD according to reconstruction methods.
Some recent trials showed that removal of the pylorus could result in a lower incidence of DGE. Matsumoto et al[40] performed a prospective randomized comparison between PPPD and modified classical PD, and assessed the effects stomach-preserving PD on postoperative DGE occurrence and long-term nutritional status. They observed that the incidence of DGE, as assessed by the International Study Group of Pancreatic Surgery, was similar (20% vs 12%, P = 0.414), and long-term nutritional status indicated by serum albumin levels, serum total cholesterol levels, and body mass index during the 3-year follow-up) were also comparable between the two groups. Similarly, Kawai et al[41] reported their prospective, randomized, controlled study comparing PPPD and Pylorus-resecting PD (PrPD), showing that PrPD was associated with a low incidence of DGE; however, during a 6-mo follow-up period, comparable outcomes for quality of life, weight loss, and nutritional status between the two groups were observed.
Previously, most concerns after PD were postoperative pancreatic fistula, because it was one of the main causes of significant morbidity and mortality related to PD. However, with advances in surgical techniques, perioperative management, and interventional radiology, most PD-related complications can now be managed by conservative methods, and surgeons have begun to focus on long-term functional outcomes after PD.
Several reports have shown a potential relationship between morphologic changes (pancreatic atrophy, stricture, and main pancreatic duct dilatation) and remnant pancreatic function after PD[42-46]. Notably, Lemaire et al[47] evaluated pancreatic function, pancreatic atrophy, and main pancreatic duct dilation in the remnant pancreas after PD. They found a significant reduction in pancreatic parenchymal thickness and increased dilation of the main pancreatic duct in remnant pancreas. Finally, pancreatic atrophy tended to develop over time, and all patients were reported to have reduced levels of fecal-1 elastase. Nakamura et al[48] also demonstrated reduced pancreatic parenchymal thickness (atrophy), which indicated pancreatic exocrine insufficiency after PD. Therefore, this morphological change can indirectly show the some aspects of exocrine function in the remnant pancreas remain after PD. Tomimaru et al[49] reported a significant atrophy of the pancreatic parenchyma that occurred postoperatively in the PG and PJ groups (P < 0.0001), but these changes were more severe in the PG group than in the PJ group (P = 0.0018), suggesting that PJ was preferable to PG after PD. Fang et al[50] evaluated the long-term morphological and functional outcomes of the remnant pancreas after PD. The pancreatic duct diameter in the remnant pancreas usually increased, but there was no significant difference in the pancreatic duct diameter in both the PJ and PG groups, indicating that there was no significant difference in pancreatic exocrine or endocrine insufficiency, or pancreatic morphological changes. This evidence strongly suggests that the remnant pancreas following PD will have a chance to undergo atrophic changes and deteriorating exocrine pancreatic function after a long period of time.
Generally, there are two methods for remnant pancreatic reconstruction; PJ and PG. Several theoretical concerns exist regarding the functional outcome of the remnant pancreas following PD, which are as follows: (1) because of the absence of ampullary function, the remnant pancreas is thought to be vulnerable to regurgitation of gastrointestinal fluid into the main pancreatic duct. Most notably in PG, reflux of ingested food and low pH-gastric juice to the pancreatic duct can result in chronic inflammation, stenosis, and inactivation of pancreatic enzymes, leading to insufficiency of the remnant pancreas[51,52]; (2) in PJ, the easy activation of pancreatic enzymes can occur by intestinal enterokinase and an alkaline pH, resulting in irritating the remnant pancreas and clinically relevant pancreatic fistula[53]; and (3) reduced plasma levels of gastrin resulting from removal of the duodenum and distal part of stomach can affect atrophic changes of the remnant pancreas[15,16].
Interestingly, no significant difference in postoperative morbidity has been observed, even for postoperative pancreatic fistula[54] (POPF, Table 3), between PG and PJ[55-58]. However, a recent meta-analysis[59] demonstrated that PG was associated with lower postoperative pancreatic and biliary fistula rates in PD. One RCT dataset[60] showed that PG was related not only to a lower POPF rate but also to lower weight loss and better exocrine pancreatic function compared with PJ, suggesting that the “battle” between PG and PJ is ongoing. Most available reports on the functional outcome of the remnant pancreas following PD were based on retrospective study designs and limited numbers of patients. Most RCTs that tested PG and PJ focused on short-term perioperative outcomes, such as morbidity and mortality. Therefore, further evidence-based clinical investigations about remnant pancreatic function following PD should be performed.
| Postoperative pancreatic fistula | |||
| Grade | A | B | C |
| General appearance (clinical condition) | Well | Often Well | Ill appearing, Bad |
| Medical or interventional approach | No | Yes or No | Yes |
| Postoperative radiologic finding (US/CT) | Negative | Negative or Positive | Positive |
| Long-time drainage (≥ 21 d) | No | Usually Yes | Yes |
| Reoperation | No | No | Yes |
| Mortality related to POPF | No | No | Possibly yes |
| Sign of infection | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Readmission | No | Yes or No | Yes or No |
Non-alcoholic fatty liver disease (NAFLD) is thought to be associated with excessive nutrition and is one of the most common forms of chronic liver disease[61]. This disease started to be reported in late 1980[62], and a few clinical investigations correlating fatty liver and PD reported that PD can influence hepatic fat content, which was associated with frequent hepatic steatosis[63,64]. In severe cases, even steatohepatitis leading to hepatic decompression can develop because of malnutrition after PD[65]. Therefore, surgeons need to be concerned about this condition, especially in patients expecting long-term survival following PD. Recent clinical studies of fatty liver after PD are summarized in Table 4.
| Ref. | Year | Patient number | Follow-up period | Definitions of NAFLD | Incidence of fatty liver, n (%) | Risk factors/observation |
| (mo) | ||||||
| Song et al[90] | 2011 | 228 | 16 | When CTS-L was equal to or less than 10 HU When CTL/S was equal to or less than 0.9 HU | 15 (7.8) | In multivariate analysis, Pancreatic fistula (HR = 3.332, P = 0.037) External pancreatic duct stent (HR = 4.530, P = 0.017) |
| Sato et al[91] | 2014 | 110 | 6 | Hepatic CT value of less than 40 HU | 44 (40) | In multivariate analysis, |
| Younger age (OR = 1.079, P = 0.002), Female (OR = 6.102, P < 0.001) | ||||||
| Small remnant pancreatic volume (< 10 mL), OR = 4.109, P = 0.009 | ||||||
| Suspicion infection on POD7-28 (OR = 3.109, P = 0.027) | ||||||
| Kato et al[92] | 2010 | 54 | 7.7 ± 2.1 | Hepatic CT value of less than 40 HU a | 20 (37.0) | In multivariate analysis, |
| Pancreatic adenocarcinoma (P < 0.05) | ||||||
| Pancreatic resection line (left side of SMA, SMA/PV) (P < 0.01) | ||||||
| Diarrhea (P < 0.05) | ||||||
| Nagai et al[71] | 2014 | 361 | 6 | When CTL/S was equal to or less than 0.9 HU | 30 (8.3) | In patients with NAFLD, CTL/S ratio was significantly improved by pancrelipase treatment |
| Nutritional status by total protein, albumin, and cholesterol was significantly improved by pancrelipase treatment | ||||||
| Severe diarrhea was improved | ||||||
| Malnutrition after PD might be cause for postoperative NAFLD | ||||||
| Ito et al[93] | 2014 | 100 | NA | When CTL/S was equal to or less than 0.9 HU | 12 (12) | In multivariate analysis, Blood loss (HR = 1.001, P = 0.016) |
| Nakagawa et al[94] | 2014 | 104 | Median 7.7 (2.5-23.6) | When CTS-L was equal to or less than 10 HU | 26 (25) | In multivariate analysis, Postoperative pancreatic exocrine insufficiency (HR = 4.16, P = 0.02) |
| Tanaka et al[72] | 2011 | 60 | 12 | When CTL/S was equal to or less than 0.9 HU When CTL/S was equal to or less than 0.9 HU | 14 (23) | In multivariate analysis, |
| Pancreatic head cancer (OR = 12.0, P = 0.006) | ||||||
| De novo NAFLD after PD was associated with body weight loss and decreases in serum levels of albumin, cholinesterase, and total cholesterol | ||||||
| After administration of pancreatic enzyme, body weight and serum concentrations of albumin, cholinesterase, and total cholesterol were markedly increased | ||||||
| In addition, hepatic steatosis and serum AST and ALT levels were also significantly improved by treatment | ||||||
| De novo NAFLD after PD was primarily | ||||||
| caused by pancreatic exocrine insufficiency |
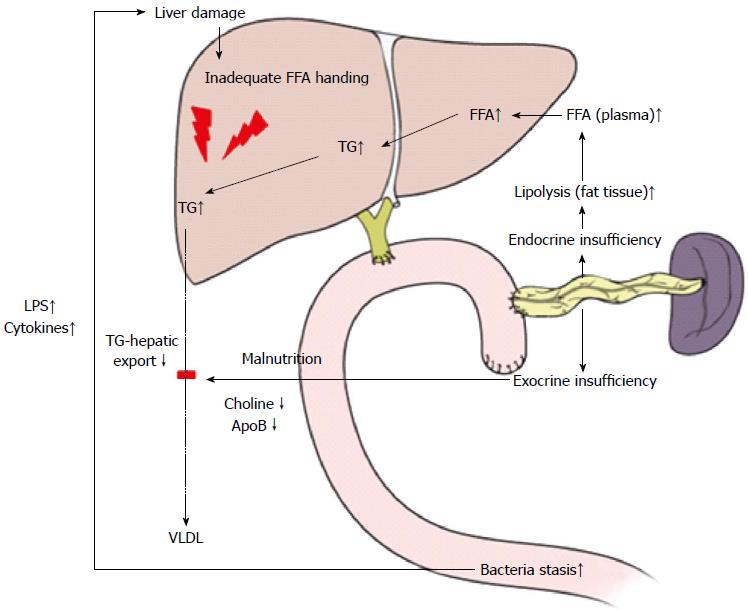
The mechanisms underlying NAFLD after PD (Figure 2) might differ from usual NAFLD associated with metabolic syndrome because NAFLD after PD was related to non-obese status, malnutrition, and a lack of hyperlipidemia or insulin resistance[66]. Most studies listed in Table 4 directly and indirectly suggest that malnutrition resulting from exocrine pancreatic insufficiency might cause NAFLD after PD. Pancreatic exocrine insufficiency induced malabsorption of essential amino acids, such as choline, which might result in the development of NAFLD after PD[67]. It has been shown that choline deficiency reduces plasma levels of apoprotein B[68], a major component VLDL, suggesting impaired hepatic export of TG in the form of very-low-density lipoprotein (VLDL). Insufficient secretion of insulin could play another role in the development of NAFLD after PD, which can enhance peripheral lipolysis and increase hepatic FFA uptake, and liver could have some difficulty in handling hepatic fat secretion by coupling triglyceride to apoprotein B[69], which plays an important role in secreting triglycerides from hepatocytes as VLDL particles. Overgrowth of small intestinal bacteria and hepatic stimulation of LPS[70] because of intestinal motor dysfunction and stasis can reduce the secretion of gastric juices and blind loops can also play an important role in NAFLD after PD. Therefore, NAFLD after PD represent the nutritional status of patients and is clinical reflection of the pathophysiological changes that occur after PD. Interestingly, NAFLD after PD is known to be associated with pancreatic cancer[71,72] and chemotherapy[73], so it will be interesting to investigate the potential correlation between the degree of post-hepatic steatosis and oncologic outcomes in resected pancreatic head cancer.
Previously, surgical techniques and safety were the only concerns regarding PD. This technique was regarded as one of the most complex and risky surgical procedures. However, as a consequence of advances in surgical experiences, techniques, and perioperative management, PD has become safer and the gold standard for treating periampullary pathologies. PD accompanies the removal of important organs and rearrangement of flow in the upper gastrointestinal tract, which can result in altered normal physiology and distinct clinical manifestations. In addition to proper surgical techniques, pancreatic surgeons need to understand these potential pathophysiological changes that can occur after PD for proper patients care in clinical practice. Further studies to link these potential pathophysiological changes with clinical outcomes will yield new insights to better understand how PD affects the lives of patients.
The authors would like to express special thanks to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his excellent illustrations in the papers.
| 1. | Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla and other related sites. Jpn J Surg. 1983;13:385-394. [PubMed] |
| 2. | Wellner UF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G, Bertrand C, Hubert C, Janssens M, Closset J, Belgian Section of Hepatobiliary and Pancreatic Surgery. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013;14:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Fernández-Cruz L, Cosa R, Blanco L, López-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Brown JC, Cook MA, Dryburgh JR. Motilin, a gastric motor activity-stimulating polypeptide: final purification, amino acid composition, and C-terminal residues. Gastroenterology. 1972;62:401-404. [PubMed] |
| 6. | Naritomi G, Tanaka M, Matsunaga H, Yokohata K, Ogawa Y, Chijiiwa K, Yamaguchi K. Pancreatic head resection with and without preservation of the duodenum: different postoperative gastric motility. Surgery. 1996;120:831-837. [PubMed] |
| 7. | Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, Cameron JL. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229-237; discussion 237-238. [PubMed] |
| 8. | Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, Muls E, Bouillon R. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 503] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 9. | Mozwecz H, Pavel D, Pitrak D, Orellana P, Schlesinger PK, Layden TJ. Erythromycin stearate as prokinetic agent in postvagotomy gastroparesis. Dig Dis Sci. 1990;35:902-905. [PubMed] |
| 10. | Matsunaga H, Tanaka M, Takahata S, Ogawa Y, Naritomi G, Yokohata K, Yamaguchi K, Chijiiwa K. Manometric evidence of improved early gastric stasis by erythromycin after pylorus-preserving pancreatoduodenectomy. World J Surg. 2000;24:1236-1241; discussion 1242. [PubMed] |
| 11. | Malfertheiner P, Sarr MG, Nelson DK, DiMagno EP. Role of the duodenum in postprandial release of pancreatic and gastrointestinal hormones. Pancreas. 1994;9:13-19. [PubMed] |
| 12. | Müller MW, Friess H, Beger HG, Kleeff J, Lauterburg B, Glasbrenner B, Riepl RL, Büchler MW. Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg. 1997;173:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Tangoku A, Nishikawa M, Adachi A, Suzuki T. Plasma gastrin and cholecystokinin response after pylorus-preserving pancreatoduodenectomy with Billroth-I type of reconstruction. Ann Surg. 1991;214:56-60. [PubMed] |
| 14. | Kingsnorth AN, Formela LJ, Chen D, Rehfeld JF. Plasma gastrin and cholecystokinin responses after pylorus-preserving pancreatoduodenectomy and defunctioned Roux loop pancreaticojejunostomy. Br J Surg. 1994;81:1356-1359. [PubMed] |
| 15. | Hashimoto N, Yasuda T, Haji S, Nomura H, Ohyanagi H. Comparison of the functional and morphological changes in the pancreatic remnant between pylorus-preserving pancreatoduodenectomy and pancreatoduodenectomy. Hepatogastroenterology. 2003;50:2229-2232. [PubMed] |
| 16. | Jang JY, Kim SW, Han JK, Park SJ, Park YC, Joon Ahn Y, Park YH. Randomized prospective trial of the effect of induced hypergastrinemia on the prevention of pancreatic atrophy after pancreatoduodenectomy in humans. Ann Surg. 2003;237:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Chung SA, Rotstein O, Greenberg GR, Diamant NE. Mechanisms coordinating gastric and small intestinal MMC: role of extrinsic innervation rather than motilin. Am J Physiol. 1994;267:G800-G809. [PubMed] |
| 18. | Iqbal N, Lovegrove RE, Tilney HS, Abraham AT, Bhattacharya S, Tekkis PP, Kocher HM. A comparison of pancreaticoduodenectomy with extended pancreaticoduodenectomy: a meta-analysis of 1909 patients. Eur J Surg Oncol. 2009;35:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1015] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 21. | Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257-270. [PubMed] |
| 22. | Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | You DD, Choi SH, Choi DW, Heo JS, Ho CY, Kim WS. Long-term effects of pancreaticoduodenectomy on glucose metabolism. ANZ J Surg. 2012;82:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Wu JM, Kuo TC, Yang CY, Chiang PY, Jeng YM, Huang PH, Tien YW. Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Mori Y, Ohtsuka T, Tsutsumi K, Yasui T, Ueda J, Takahata S, Nakamura M, Tanaka M. Different incretin responses after pancreatoduodenectomy and distal pancreatectomy. Pancreas. 2012;41:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ohtsuka T, Kitahara K, Kohya N, Miyoshi A, Miyazaki K. Improvement of glucose metabolism after a pancreatoduodenectomy. Pancreas. 2009;38:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Muscogiuri G, Mezza T, Prioletta A, Sorice GP, Clemente G, Sarno G, Nuzzo G, Pontecorvi A, Holst JJ, Giaccari A. Removal of duodenum elicits GLP-1 secretion. Diabetes Care. 2013;36:1641-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Harmuth S, Wewalka M, Holst JJ, Nemecek R, Thalhammer S, Schmid R, Sahora K, Gnant M, Miholić J. Distal gastrectomy in pancreaticoduodenectomy is associated with accelerated gastric emptying, enhanced postprandial release of GLP-1, and improved insulin sensitivity. J Gastrointest Surg. 2014;18:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36 Suppl 2:S245-S252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Zhao H, Wang L, Wei R, Xiu D, Tao M, Ke J, Liu Y, Yang J, Hong T. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes Metab. 2014;16:850-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Zhao H, Wei R, Wang L, Tian Q, Tao M, Ke J, Liu Y, Hou W, Zhang L, Yang J. Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. Am J Physiol Endocrinol Metab. 2014;306:E1431-E1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Imanaga H. A new method of pancreaticoduodenectomy designed to preserve liver and pancreatic function. Surgery. 1960;47:577-586. [PubMed] |
| 34. | Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102:763-779. [PubMed] |
| 35. | Kingsnorth AN, Berg JD, Gray MR. A novel reconstructive technique for pylorus-preserving pancreaticoduodenectomy: avoidance of early postoperative gastric stasis. Ann R Coll Surg Engl. 1993;75:38-42. [PubMed] |
| 36. | Hochwald SN, Grobmyer SR, Hemming AW, Curran E, Bloom DA, Delano M, Behrns KE, Copeland EM, Vogel SB. Braun enteroenterostomy is associated with reduced delayed gastric emptying and early resumption of oral feeding following pancreaticoduodenectomy. J Surg Oncol. 2010;101:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, Yamaue H. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Miyakawa S, Niwamoto N, Horiguchi A, Hanai T, Mizuno K, Ishihara S, Miura K. Fat absorption after pylorus-preserving pancreatoduodenectomy reconstructed with Billroth II pancreaticojejunostomy or Billroth I pancreaticogastrostomy. Hepatogastroenterology. 2000;47:264-268. [PubMed] |
| 39. | Ohtsuka T, Yamaguchi K, Chijiiwa K, Tanaka M. Effect of gastrointestinal reconstruction on quality of life and nutritional status after pylorus-preserving pancreatoduodenectomy. Dig Dis Sci. 2002;47:1241-1247. [PubMed] |
| 40. | Matsumoto I, Shinzeki M, Asari S, Goto T, Shirakawa S, Ajiki T, Fukumoto T, Suzuki Y, Ku Y. A prospective randomized comparison between pylorus- and subtotal stomach-preserving pancreatoduodenectomy on postoperative delayed gastric emptying occurrence and long-term nutritional status. J Surg Oncol. 2014;109:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Kawai M, Tani M, Hirono S, Okada K, Miyazawa M, Yamaue H. Pylorus-resecting pancreaticoduodenectomy offers long-term outcomes similar to those of pylorus-preserving pancreaticoduodenectomy: results of a prospective study. World J Surg. 2014;38:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Yoo D, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Jung BH, Kang SH. Pancreatic atrophy relative to external versus internal drainage of the pancreatic duct after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Kitamura T, Anaguchi-Hirao R, Kouhara H. Combination of type 2 diabetes and malnutrition worsened by anastomotic stenosis and pancreas atrophy following resection of pancreas head. Intern Med. 2008;47:1225-1230. [PubMed] |
| 44. | Kim JH, Yoo BM, Kim JH, Kim WH. Which method should we select for pancreatic anastomosis after pancreaticoduodenectomy? World J Surg. 2009;33:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Sato N, Yamaguchi K, Yokohata K, Shimizu S, Chijiiwa K, Tanaka M. Long-term morphological changes of remnant pancreas and biliary tree after pancreatoduodenectomy on CT. Int Surg. 1998;83:136-140. [PubMed] |
| 46. | Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Lemaire E, O’Toole D, Sauvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Reduced pancreatic parenchymal thickness indicates exocrine pancreatic insufficiency after pancreatoduodenectomy. J Surg Res. 2011;171:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Tomimaru Y, Takeda Y, Kobayashi S, Marubashi S, Lee CM, Tanemura M, Nagano H, Kitagawa T, Dono K, Umeshita K. Comparison of postoperative morphological changes in remnant pancreas between pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. Pancreas. 2009;38:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Fang WL, Su CH, Shyr YM, Chen TH, Lee RC, Tai LC, Wu CW, Lui WY. Functional and morphological changes in pancreatic remnant after pancreaticoduodenectomy. Pancreas. 2007;35:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Idezuki Y, Goetz FC, Lillehei RC. Late effect of pancreatic duct ligation on beta cell function. Am J Surg. 1969;117:33-39. [PubMed] |
| 52. | Ohshio G, Saluja A, Steer ML. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology. 1991;100:196-202. [PubMed] |
| 53. | Mackie JA, Rhoads JE, Park CD. Pancreaticogastrostomy: a further evaluation. Ann Surg. 1975;181:541-545. [PubMed] |
| 54. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3558] [Article Influence: 169.4] [Reference Citation Analysis (35)] |
| 55. | Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580-588; discussion 588-592. [PubMed] |
| 56. | Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767-771, discussion 771-773. [PubMed] |
| 57. | Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, Fingerhut A, Millat B, Radovanowic A, Fagniez PL, French Associations for Research in Surgery. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Wente MN, Shrikhande SV, Müller MW, Diener MK, Seiler CM, Friess H, Büchler MW. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Menahem B, Guittet L, Mulliri A, Alves A, Lubrano J. Pancreaticogastrostomy Is Superior to Pancreaticojejunostomy for Prevention of Pancreatic Fistula After Pancreaticoduodenectomy: An Updated Meta-analysis of Randomized Controlled Trials. Ann Surg. 2015;261:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Figueras J, Sabater L, Planellas P, Muñoz-Forner E, Lopez-Ben S, Falgueras L, Sala-Palau C, Albiol M, Ortega-Serrano J, Castro-Gutierrez E. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 61. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3742] [Article Influence: 155.9] [Reference Citation Analysis (4)] |
| 62. | Kita T, Nakamura K, Kida H, Kawarada Y, Mizumoto R. [Pathophysiology during follow-up after extensive pancreatectomy]. Nihon Geka Gakkai Zasshi. 1988;89:1426-1429. [PubMed] |
| 63. | Nirei K, Ogihara N, Kawamura W, Kang W, Moriyama M. Rapid recovery from acute liver failure secondary to pancreatoduodenectomy-related non-alcoholic steatohepatitis. Case Rep Gastroenterol. 2013;7:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Nomura R, Ishizaki Y, Suzuki K, Kawasaki S. Development of hepatic steatosis after pancreatoduodenectomy. AJR Am J Roentgenol. 2007;189:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Sim EH, Kwon JH, Kim SY, Jung SM, Maeng LS, Jang JW, Chung KW. Severe steatohepatitis with hepatic decompensation resulting from malnutrition after pancreaticoduodenectomy. Clin Mol Hepatol. 2012;18:404-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Satoh D, Yagi T, Nagasaka T, Shinoura S, Umeda Y, Yoshida R, Utsumi M, Tanaka T, Sadamori H, Fujiwara T. CD14 upregulation as a distinct feature of non-alcoholic fatty liver disease after pancreatoduodenectomy. World J Hepatol. 2013;5:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Tanaka N, Takahashi S, Fang ZZ, Matsubara T, Krausz KW, Qu A, Gonzalez FJ. Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2014;1841:1596-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Yao ZM, Vance DE. Reduction in VLDL, but not HDL, in plasma of rats deficient in choline. Biochem Cell Biol. 1990;68:552-558. [PubMed] |
| 69. | Soliman AT, Alsalmi I, Asfour M. Hypoinsulinaemia has an important role in the development of oedema and hepatomegaly during malnutrition. J Trop Pediatr. 1996;42:297-299. [PubMed] |
| 70. | Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 71. | Nagai M, Sho M, Satoi S, Toyokawa H, Akahori T, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Kinoshita S. Effects of pancrelipase on nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 74. | Tanaka M, Sarr MG. Total duodenectomy: effect on canine gastrointestinal motility. J Surg Res. 1987;42:483-493. [PubMed] |
| 75. | Tanaka M, Sarr MG. Role of the duodenum in the control of canine gastrointestinal motility. Gastroenterology. 1988;94:622-629. [PubMed] |
| 76. | Suzuki H, Mochiki E, Haga N, Shimura T, Itoh Z, Kuwano H. Effect of duodenectomy on gastric motility and gastric hormones in dogs. Ann Surg. 2001;233:353-359. [PubMed] |
| 77. | Malfertheiner P, Sarr MG, Spencer MP, DiMagno EP. Effect of duodenectomy on interdigestive pancreatic secretion, gastrointestinal motility, and hormones in dogs. Am J Physiol. 1989;257:G415-G422. [PubMed] |
| 78. | Itoh Z, Honda R, Hiwatashi K, Takeuchi S, Aizawa I, Takayanagi R, Couch EF. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl. 1976;39:93-110. [PubMed] |
| 79. | Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci. 1979;24:497-500. [PubMed] |
| 80. | Sarna S, Chey WY, Condon RE, Dodds WJ, Myers T, Chang TM. Cause-and-effect relationship between motilin and migrating myoelectric complexes. Am J Physiol. 1983;245:G277-G284. [PubMed] |
| 81. | Eshuis WJ, van Eijck CH, Gerhards MF, Coene PP, de Hingh IH, Karsten TM, Bonsing BA, Gerritsen JJ, Bosscha K, Spillenaar Bilgen EJ. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy: a randomized controlled trial. Ann Surg. 2014;259:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Tamandl D, Sahora K, Prucker J, Schmid R, Holst JJ, Miholic J, Goetzinger P, Gnant M. Impact of the reconstruction method on delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: a prospective randomized study. World J Surg. 2014;38:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Imamura N, Chijiiwa K, Ohuchida J, Hiyoshi M, Nagano M, Otani K, Kondo K. Prospective randomized clinical trial of a change in gastric emptying and nutritional status after a pylorus-preserving pancreaticoduodenectomy: comparison between an antecolic and a vertical retrocolic duodenojejunostomy. HPB (Oxford). 2014;16:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Tani M, Kawai M, Hirono S, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. Randomized clinical trial of isolated Roux-en-Y versus conventional reconstruction after pancreaticoduodenectomy. Br J Surg. 2014;101:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Shimoda M, Kubota K, Katoh M, Kita J. Effect of billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg. 2013;257:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 86. | Ke S, Ding XM, Gao J, Zhao AM, Deng GY, Ma RL, Xin ZH, Ning CM, Sun WB. A prospective, randomized trial of Roux-en-Y reconstruction with isolated pancreatic drainage versus conventional loop reconstruction after pancreaticoduodenectomy. Surgery. 2013;153:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Gangavatiker R, Pal S, Javed A, Dash NR, Sahni P, Chattopadhyay TK. Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled trial. J Gastrointest Surg. 2011;15:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Kurahara H, Shinchi H, Maemura K, Mataki Y, Iino S, Sakoda M, Ueno S, Takao S, Natsugoe S. Delayed gastric emptying after pancreatoduodenectomy. J Surg Res. 2011;171:e187-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Chijiiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, Kai M, Kondo K. Prospective randomized controlled study of gastric emptying assessed by (13)C-acetate breath test after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. J Hepatobiliary Pancreat Surg. 2009;16:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Song SC, Choi SH, Choi DW, Heo JS, Kim WS, Kim MJ. Potential risk factors for nonalcoholic steatohepatitis related to pancreatic secretions following pancreaticoduodenectomy. World J Gastroenterol. 2011;17:3716-3723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Sato R, Kishiwada M, Kuriyama N, Azumi Y, Mizuno S, Usui M, Sakurai H, Tabata M, Yamada T, Isaji S. Paradoxical impact of the remnant pancreatic volume and infectious complications on the development of nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Tabata M. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Ito Y, Kenmochi T, Shibutani S, Egawa T, Hayashi S, Nagashima A, Kitagawa Y. Evaluation of predictive factors in patients with nonalcoholic fatty liver disease after pancreaticoduodenectomy. Am Surg. 2014;80:500-504. [PubMed] |
| 94. | Nakagawa N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Sasaki H, Okano K, Sueda T. Nonalcoholic fatty liver disease after pancreatoduodenectomy is closely associated with postoperative pancreatic exocrine insufficiency. J Surg Oncol. 2014;110:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bove A, Cho A, Goetze TO, Marrelli D S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM