Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5685
Peer-review started: August 2, 2014
First decision: August 27, 2014
Revised: September 12, 2014
Accepted: November 30, 2014
Article in press: December 1, 2014
Published online: May 14, 2015
Processing time: 289 Days and 21.3 Hours
AIM: To evaluate the safety and efficacy of oral administration of Alequel™, an autologous protein-containing colon extract.
METHODS: A total of 43 patients were enrolled in a randomized, placebo-controlled, double-blind trial. Patients were orally administered with autologous protein-containing colon extract three doses of autologous study drug per week for 15 wk, for a total of 45 doses. Patients were followed for safety parameters. Remission was defined as a Crohn’s disease activity index (CDAI) score of less than or equal to 150. All patients were followed for changes in subsets of T cells by fluorescence-activated cell sorting analysis.
RESULTS: Analysis was performed on a total number of evaluable patients of 14 in the study drug group and 15 in the placebo group. Treatment was well tolerated by all patients. No major treatment-related adverse events were reported or observed in any of the treated patients during the feeding or follow-up periods. Between weeks 6 and 9 of the study, six of the 14 (43%) evaluable subjects who received the study drug achieved a CDAI of 150 or lower. In contrast, five of the 15 (33%) evaluable subjects in the placebo group achieved remission. Between weeks 9 and 12, the remission rates were 50% and 33% for the drug group and placebo group, respectively. Among the drug-treated subjects who achieved remission, the effect of the drug was judged as stable in eight of the 14 subjects as measured by at least two CDAI scores indicating remission in the 15-wk treatment period. A decreased percentage of peripheral natural killer T regulatory cells (a decrease of 28% vs an increase of 16%) and an increased ratio of CD4+/CD8+ T lymphocytes (an increase of 11% vs a decrease of 9%) were noted in subjects with a significant clinical response.
CONCLUSION: Oral administration of the autologous colonic extract could be a safe and effective for the treatment of patients with moderate to severe Crohn’s disease.
Core tip: Oral administration of the autologous colonic extract could be a safe and effective for the treatment of patients with moderate to severe Crohn’s disease (CD). Increased ratio of CD4+/CD8+ T lymphocytes was noted in subjects with a significant clinical response and may serve as a biomarker for response to therapy.
- Citation: Israeli E, Zigmond E, Lalazar G, Klein A, Hemed N, Goldin E, Ilan Y. Oral mixture of autologous colon-extracted proteins for the Crohn’s disease: A double-blind trial. World J Gastroenterol 2015; 21(18): 5685-5694
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5685
Crohn’s disease (CD) is an idiopathic immune-mediated disorder, resulting in chronic inflammation of the gut[1,2]. Although the pathogenesis of CD has not been adequately clarified, the current understanding is that transmural inflammation, the primary presentation of CD, is the result of a cascade of events and processes initiated by one or more antigens that remain unspecified. In the normal state, low-level physiological inflammation of the gut is kept in check through an active process of immune tolerance[3]. Evidence in humans points to an over-responsiveness and loss of tolerance of mucosal T-cells[4]. Current therapeutic approaches to treat CD are based on a relatively non-specific suppression of the immune system[5,6]. Undesirable side effects, some of which are severe, remain a major hurdle to the use of these therapies.
Oral tolerance is a natural immunologic process driven by the oral administration of an exogenous antigen[7-9]. Oral antigen administration can activate specific subsets of cells, suppress effector cells, and alleviate unwanted autoimmunity[6-8,10,11]. Multiple mechanisms of tolerance are induced by oral antigen administration. Due to their privileged access to the internal milieu, commensal bacteria and dietary Ag that continuously contact the mucosa represent a frontier between foreign and self-components. Low doses favor active suppression, whereas higher doses favor clonal anergy and clonal deletion. Oral Ag administration promotes regulatory T cells[12], including Th2 [interleukin (IL)-4+/IL-10+], Th3 [transforming growth factor (TGF)-beta] cells, CD4+CD25+ regulatory cells, and LAP+T cells[13-15]. Induction of oral tolerance is enhanced by IL-4, IL-10, anti-IL-12, TGF-beta, cholera toxin B subunit, Flt-3 ligand, anti-CD40 ligand and continuous Ag administration[8]. Thus, oral exposure to antigens from the bowel results in an active immune response and is an attractive physiologic approach for immunotherapy towards antigens presented in the gut mucosa[10]. Recent progress in mucosal immunology provides new insights into the potential use of oral tolerance in the clinic as a mechanism to induce regulatory T cells that may play a role in the suppression of inflammation[13,16-18]. This method of antigen-specific therapy is non-toxic and can be administered on a chronic basis[8,18].
The efficacy of mucosal tolerance has been clearly demonstrated in animal models of CD[19-22]. In humans, oral administration of AlequelTM, an extract of autologous colonic protein-derived antigens, was shown to be safe in patients with CD[23]. Ten patients with CD were treated orally with AlequelTM three times a week for 16 wk. Seven patients achieved clinical remission with an increase in their mean inflammatory bowel disease (IBD) questionnaire (IBDQ) score. High levels of colitis extracted protein-specific interferon (IFN)-gamma spot forming colonies were detected prior to treatment and a marked decrease in these colonies was observed following treatment. Furthermore, treatment altered the CD4+/CD8+ lymphocyte ratio and increased peripheral natural killer T (NKT) cell numbers. A significant increase in serum IL-10 and IL-4 levels was observed during the treatment period[23]. In a recently conducted randomized, double-blind, placebo-controlled trial, 31 patients with moderate to severe CD were enrolled in a 27-wk study[24]. Oral administration of AlequelTM resulted in clinical remission of CD in 58% of the patients in the treated group compared to clinical remission of 29% in the placebo group. A clinical response was seen in 67% and 43% of the patients receiving AlequelTM and placebo, respectively. An improved IBDQ score was seen in 43% of the patients receiving AlequelTM and only 12% of the patients receiving the placebo. A decrease in the number of subject-specific, antigen-directed, IFN-gamma spot-forming colonies and an increased percentage of peripheral blood NKT cells were only seen in the drug-treated cohort who achieved remission.
The gut epithelium has an ability to discriminate between pathogens and commensals and plays a role in mucosal immunology[25-28]. Dysfunctional interactions between microbes and epithelia play a role in IBD. Patients with IBD had altered microbiota, enhanced expression of inflammatory genes, and increased correlations between specific gene expression and microbes[25]. It was suggested that part of the effect of AlequelTM are mediated by an immune modulatory effects of bacterial antigens which are part of the mixture.
The aim of the phase II study reported here was to further evaluate the safety and efficacy of oral administration of this personalized drug in a more diverse cohort of CD patients in a randomized, double-blind, placebo-controlled format. Furthermore, we evaluated several markers that could be used to construct an immune profile to predict which of these individuals would be likely to respond to the administration of AlequelTM.
A randomized, double-blind, placebo-controlled, one-center trial was conducted comprising subjects with moderate to severe CD. The study was carried out in accordance with the guidelines of the Hebrew University-Hadassah Institutional Committee for Human Clinical Trials and with the approval of the Israel Ministry of Health Committee for Human Trials. NIH Gov, NCT02185183.
Participants (men and women older than 18 years of age) were evaluated for eligibility after they had signed a written informed consent form. The diagnosis of CD with clinical evidence of active (symptomatic) disease was based on clinical history, blood tests and/or histology, X-ray, or endoscopy. Subjects were required to have a Crohn’s disease activity index (CDAI) score between 220 and 400 as a condition for enrollment irrespective of endoscopic findings. Subjects receiving oral steroid therapy at the time of enrollment were required to be on a stable dose regimen of less than 10 mg of prednisone per day for four weeks prior to enrollment.
Patients falling into the following categories were ineligible for entry into the study: subjects who underwent bowel surgery within three months prior to the commencement of the trial; those who had experienced a prior colostomy, ileostomy, or colectomy with ileorectal anastamosis; subjects whose symptoms were believed to be due to the presence of fibrotic strictures; or individuals who were likely to require emergency surgery for persistent intestinal obstruction, bowel perforation, toxic megacolon, uncontrolled bleeding, or abdominal abscess or infection. Subjects with an infectious or neoplastic disease were also ineligible. Potential subjects on a dose regimen of oral steroid therapy greater than 10 mg of prednisone per day and those who were receiving an elemental diet or parenteral nutrition were also ineligible. In addition, subjects who had been treated with methotrexate, cyclosporine, or anti-tumor necrosis factor (TNF)-α or who had participated in another clinical trial within three months prior to enrollment were ineligible. However, patients on 6-mercaptopurine/azathioprine could be included.
Subjects who fulfilled the inclusion/exclusion criteria for participation in the study were scheduled for a colonoscopy. During the colonoscopy, colon biopsies were removed for preparation of the colon-specific antigen-containing extract (the study drug, AlequelTM). Each subject received a regimen of three doses of autologous study drug per week for 15 wk, for a total of 45 doses following an overnight fast. To prevent the possible effect of gastric acidity on the extract, patients also received Omeprazole together with the study drug at a dose of 20 μg throughout the trial.
Subjects were randomized by a computer-generated randomization program to receive either the study drug or the placebo. All subjects and investigators were blinded regarding treatment allocation. Confidentiality of the blinding code was ensured by an independent statistician.
Safety parameters: Study subjects were monitored by a variety of clinical, laboratory, and quality of life parameters during the treatment period (weeks 0-15) and during the follow-up period (weeks 16-21) after treatment. These terms were determined based on previous data from the phase I and II clinical trials[23,24]. Safety and tolerability of oral administration of the study drug was assessed by evaluating the subjects’ diary entries detailing adverse events and general health. A physical examination, record of vital signs, interim history and adverse events assessment was conducted every three weeks. Blood was drawn at each visit to obtain complete blood counts, sedimentation rate, and standard chemistries.
Efficacy parameters and surrogate markers: The effect of the study drug on the clinical status of the subjects was assessed by following the CDAI score for the week prior to the clinic visit. The primary end point was complete clinical remission, defined as a decrease in CDAI to 150 or lower for at least six consecutive weeks. As a means of identifying a possible surrogate marker to assess the clinical effect of the study drug, fluorescence-activated cell-sorting (FACS) analysis of peripheral blood T-cell populations were performed on specimens obtained at weeks 0, 9 and 15.
Blood samples were collected throughout the study period. Following lymphocyte isolation, duplicates of 2 × 104-5 × 104 cells in 500 μL PBS were deposited into Falcon 2052 tubes, incubated with 4 mL of 1% bovine serum albumin (BSA) for 10 min, and centrifuged at 1400 rpm for 5 min. Cells were suspended in 10 μL fetal bovine serum with 1:20 FITC-labeled anti-human CD3, CD4, CD8, CD16, or CD56 antibodies (Pharmingen and RD, Minneapolis, MN, United States). Cells were washed twice with 1% BSA, and 0.5 mL of 1% paraformaldehyde was added. For the control group, 5 μL of 1% BSA was added. Cell phenotyping was performed by a FACSTAR plus (Becton Dickinson, NJ). Only live cells were counted, and background fluorescence from non-antibody-treated lymphocytes was subtracted.
Sample size and power calculations were made based on the results of the phase I and II clinical trials. A total of 43 subjects were enrolled in the study, randomized, and treated according to the protocol. The study was not designed to detect rarely occurring treatment associated adverse events. Summary statistics at each time-point for all clinical and laboratory variables were calculated, and the statistical significance of differences from baseline were assessed by the Student’s t-test.
A total of 43 subjects were randomized after meeting all the inclusion and exclusion criteria. Of these subjects, 21 patients received the placebo and 22 patients received the study drug. After enrollment, two subjects in the study drug group withdrew consent and were not treated. The study was terminated prematurely by 11 subjects (five from the study drug and six from the placebo group). After the week 3 visit, two patients dropped out (one from the placebo group and one from the drug group), two more patients (both from the placebo group) dropped out after week 6; four patients dropped out (two from the placebo and two from the drug group) after week 9; and three dropped out (one from the placebo and two from the drug group) after week 12. Data from one additional subject was determined to be invalid due to a CDAI score at initiation of treatment below the required score of 220. Therefore, final analysis was performed on a total number of evaluable patients of 14 in the study drug group and 15 in the placebo group.
Table 1 summarizes the clinical data of the evaluable patients. The drug group included six males and eight females. The mean age of the patients in the drug group was 35 years old (range of 26 to 53 years old). The placebo group included six males and nine females, and the mean age of the patients in the placebo group was 30 years old (range of 19 to 48 years old). One subject in the drug group and three subjects in the placebo group were on a regimen of corticosteroids (less than or equal to a dose of 10 mg of prednisone) at initiation of treatment. Two patients in the drug group and four patients in the placebo group were receiving azathiopurine at initiation of treatment. Table 1 presents the average baseline CDAI score for each of the study groups, 281 vs 303, for patients in the placebo and study drug groups respectively (P value was not significant).
| Alequel (n = 14) | Placebo (n = 15) | |
| Sex (M:F) | 6:8 | 6:9 |
| Age (yr), mean (range) | 35 (26-53) | 30 (19-48) |
| Duration of disease, mean | 9.1 | 7.4 |
| Location of disease | ||
| Small bowel | 8 | 6 |
| Colon | 5 | 3 |
| Both | 1 | 6 |
| Steroid treatment | 1 (7) | 3 (20) |
| Thiopurine treatment | 2 (14) | 4 (27) |
| Baseline CDAI | 303 (223-394) | 281 (228-365) |
| Baseline IBDQ | 142 | 151 |
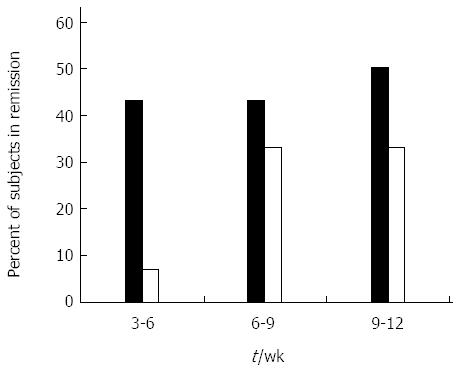
Clinical remission was defined as a decrease in CDAI score to 150 or lower at two consecutive visits during the study period. Clinical remission was used as the primary measure of treatment efficacy. Figure 1 shows the effect of oral administration of AlequelTM on clinical remission. The evaluable number of patients in each group was too small to reach a statistical significance. Between week 6 and week 9 of the study, six of the 14 (43%) evaluable subjects who received the study drug achieved a CDAI of 150 or lower. In contrast, five of the 15 (33%) evaluable subjects in the placebo group achieved remission. Between weeks 9 and 12, the remission rates were 50% and 33% for the drug group and placebo group, respectively. Among the drug-treated subjects who achieved remission, the effect of the drug was judged as stable in eight of the 14 subjects as measured by at least two CDAI scores indicating remission in the 15-wk treatment period.
Treatment was well tolerated by all patients. No major treatment-related adverse events were reported or observed in any of the treated patients during the feeding or follow-up periods. No major changes in any of the extra-intestinal systems monitored were reported in any of the patients during the study period.
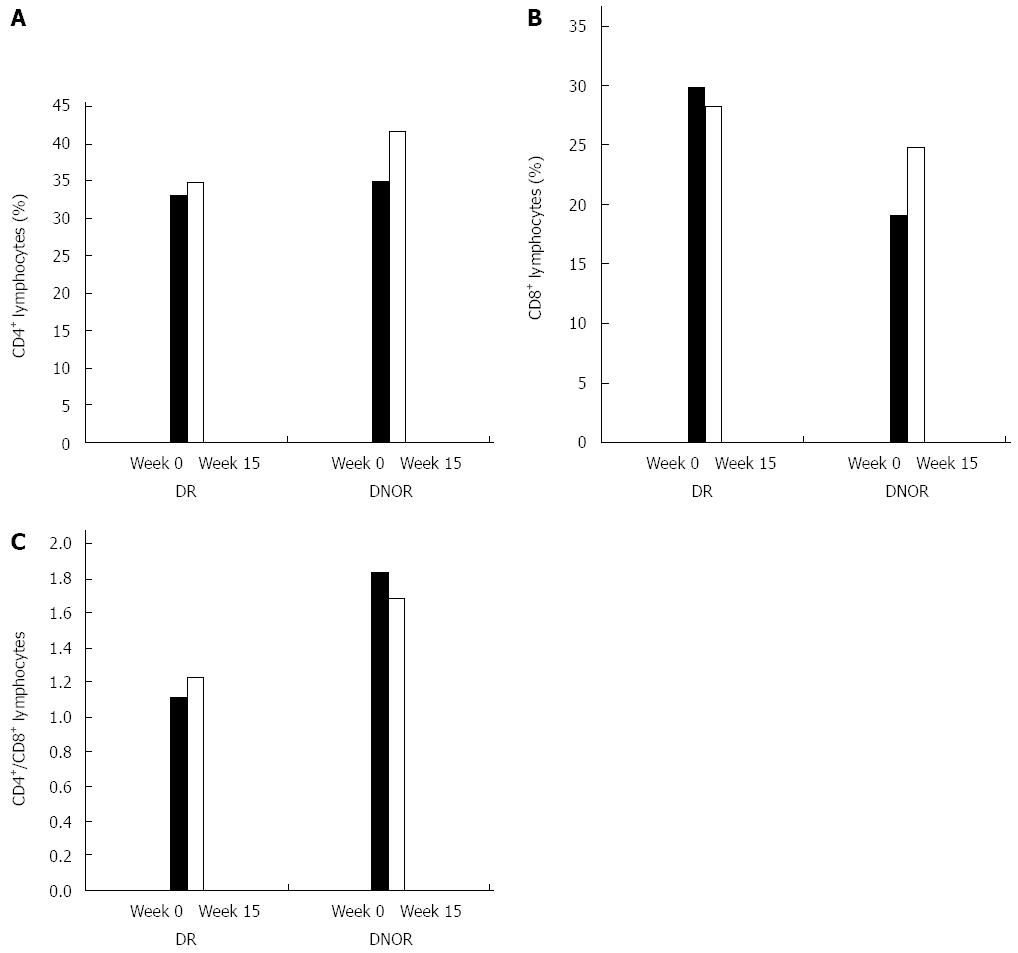
Analysis of the effect of treatment on peripheral blood lymphocytes revealed a difference between subjects in the drug treated group who achieved remission (DR) and those drug treated subjects who did not achieve remission (DNOR). The evaluable number of patients in each group was too small to reach a statistical significance. There was no difference between groups for the CD4+ lymphocytes at baseline or at end of treatment (Figure 2A). Figure 2B shows that at baseline the percentage of CD8+ lymphocytes was higher in the DR group vs the DNOR group (29.8% vs 19.1%, respectively). In the DR group there was a decrease of 6% of the CD8+ subset (from 29.8% to 28.2%) while in the DNOR group there was a 30% increase (from 19.1% to 24.7%).
The CD4/CD8 lymphocyte ratio was previously suggested to correlate with response in patients with CD[24]. Figure 2C demonstrates a distinct difference in the trend over time of the CD4+/CD8+ T lymphocyte ratio between the AlequelTM-treated DR patients compared to DNOR patients. In the DR-group, there was an 11% increase in the CD4+/CD8+ ratio between week 0 and week 12 (from 1.11 to 1.23). In the DNOR group, this ratio decreased by 9% (from 1.84 at week 0 to 1.68 at week 12).
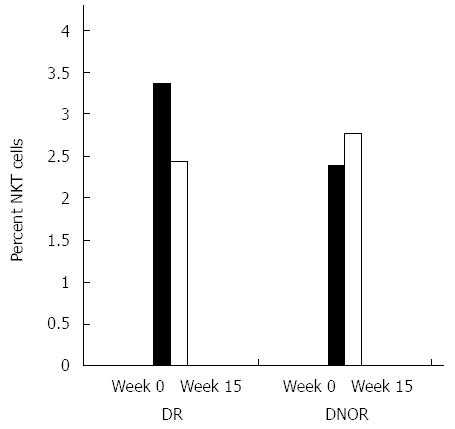
NKT cells were previously suggested to play a role in the regulation of the immune response in patients with CD[29,30]. Figure 3 shows the effect of AlequelTM treatment on NKT lymphocytes. A decrease in the percentage of peripheral NKT regulatory cells of 28% (from 3.36% to 2.43%) was noted in DR patients compared to an increase of 16% (from 2.38% to 2.76%) in the DNOR group.
This study examined the efficacy of AlequelTM, an autologous protein-containing extract of colon mucosal tissue. The results of the present study suggest that the induction of oral immune-regulation via oral administration of AlequelTM appears to be safe for the treatment of moderate to severe CD. Oral administration of Alequel™ resulted in an improved clinical remission rate (43% vs 33%) at weeks 6 to 9 in the drug treated group compared to the placebo group. From weeks 8 to 12, the clinical remission rates were 50% and 33% for the drug treated and placebo treated groups, respectively. Although the number of patients treated in the present study is small and did not enable the data to reach a level of statistical significance, the results support the induction of oral tolerance as an active response towards orally administered immunogenic material, involving the presentation of an epitope to cells in the gut-associated lymphoid tissue[16].
Phase I and Phase II clinical trials have suggested that oral administration of AlequelTM is safe and may be effective in patients with CD[23,24]. These data suggest that the beneficial clinical effect noted by oral administration of this mixture of autologous proteins may involve the induction of tolerance towards bystander proteins or may be associated with the presentation of the relevant antigens along with some mucosal adjuvants. Data presented here further support the safety of administration of AlequelTM to patients with CD and its efficacy in these subjects.
The present protocol was based on the results of previous studies and tested the effect of AlequelTM in a diverse group of patients with moderately to severe CD. Some of these patients had previously failed standard therapy (such as anti-TNF-α and/or thiopurines). The efficacy of treatments inducing mucosal tolerance have been clearly demonstrated in animal models of IBD[19,21,22,31] and other immune-mediated disorders[32-34]. Significant results have been observed in nonobese diabetic mice[35], experimental autoimmune encephalomyelitis (EAE)[32,36,37], hepatitis[6,7,11,38], type 2 diabetes[34], arthritis[35,39], graft vs host disease[12,40], metabolic syndrome[22], atherosclerosis[23,24], malignant disorders[6-8,10], allergies[41,42], and uveitis[36,43-54]. In several animal models, oral tolerance was more effective in preventing disease by treating an active immune response. These data suggest that induction of oral tolerance can be used to maintain disease remission rather than to induce remission of active disease.
Human studies aimed at suppression of unwanted immune responses have been conducted in patients with multiple sclerosis (MS)[32,37], myasthenia gravis[33], uveitis[34,35,39,40], thyroid disease[38], rheumatoid arthritis[46-48], Behcet’s disease[49], and type 1 diabetes[29-31,38]. Although these studies showed immune modulatory effects, most treatments did not lead to a profound suppression of disease activity[18]. Induction of oral tolerance towards keyhole limpet hemocyanin (KLH) was evaluated in normal individuals and in those with ulcerative colitis or CD[41]. Oral administration of KLH prior to systemic immunization decreased the magnitude of the T cell proliferative response, as well as skin test responses to KLH in normal individuals immunized with KLH. In individuals with ulcerative colitis, and to a greater extent, CD, prior oral administration of KLH led to an augmentation of the T cell proliferative response. However, this study did not measure any in vivo parameters, and there was only a two week interval between administration of the tolerance-inducing agent and the challenge by KLH. These results support the concept that oral administration of antigens can alter the systemic immune balance and show that such alterations occur in patients with IBD, although not necessarily in the same direction as in normal subjects[24].
Although it is clear that oral Ag administration can suppress autoimmunity and inflammatory diseases in animals, successful application of oral tolerance for the treatment of human diseases may depend on several factors. One important requirement is an improved formulation, including using adjuvants, optimizing the dose and frequency of administration, developing immune biomarkers to assess immunologic effects, and developing strategies to target the correct cells in the gut and liver and to target the right patient population. Early therapy is also an important factor since oral tolerance is mostly effective before or shortly after disease onset[18].
Data from clinical studies in patients with uveitis[40,49,50] and animal models of myasthenia[33,51] and EAE[52,53] suggest that protein mixtures may not be as effective oral tolerogens as purified proteins. Bystander suppression is a concept in which regulatory cells induced by oral Ag administration can suppress immune responses stimulated by other Ag, as long as the Ag is present in the anatomic vicinity[18,54,55]. During the course of chronic inflammatory autoimmune processes in animals, there is intra- and inter-antigenic spread of autoreactivity at the target organ[56,57]. Human patients with autoimmune diseases such as MS and type I diabetes are also reactive to multiple autoantigens in the target tissue[58,59]. As regulatory cells induced by oral Ag administration secrete nonspecific cytokines after being triggered by the fed Ag, they suppress inflammation in the microenvironment where the administered Ag is localized. Thus, it is not necessary to know the specific Ag that is the target of an autoimmune response in a human organ-specific inflammatory disease, but rather to feed an Ag capable of inducing regulatory cells that then migrate to the target tissue and suppress inflammation.
In the clinical study reported here, differences and changes in several immunological parameters were assayed during the treatment course, enabling the conclusion that there is a significant difference in the immune profile of subjects who respond to treatment. The data presented here show an increased ratio of CD4+/CD8+ T lymphocytes in subjects with a significant clinical response, compared with a decrease in the ratio in non-responders.
NKT cells are a unique lineage of T cells that share properties with both NK cells and memory T cells[60]. Their ability to generate both Th1 and Th2 responses indicates their importance as immunoregulatory cells[61,62], and they play a role in the immune regulation of colitis[29,30,63,64]. NKT cells have been suggested to be essential for induction of oral tolerance[57]. Oral tolerance is associated with promotion of NKT cells in both animal models and humans[19,20,38,54,55,61]. In an experimental model of colitis, induction of oral tolerance was associated with an increase in the number of NKT cells and a change in their function[20,65-67]. In the present study, we noted a 28% decrease and a 16% increase in the percentage of peripheral NKT regulatory cells in drug responders and non-responders, respectively. Previous studies suggested an increase in NKT cells in responders. Therefore, the decrease noted here may be associated with altered expression of NK1.1 on the surface of NKT cells over time[68]. As patients were tested at different time points compared to previous studies, these differences may suggest over-activation of NKT cells resulting in reduced expression of NK1.1.
Alternatively, some of the effects noted here can be explained by a potential beneficial immune effects of the gut microbiome[27,69]. Bacteria in the gut, and gut microbial products can exert an immune modulatory effects in animal models and humans[70-72]. AlequelTM contains bacterial products which may underline its immune modulatory effects.
Oral administration of the autologous colonic extract Alequel™ is a patient tailored approach that is safe and may be an effective method for the treatment of patients with moderate to severe CD. The level of peripheral NKT and the CD4/CD8 lymphocyte ratio may serve as surrogate markers to predict clinical response. Oral tolerance may thus provide a side effect-free, disease-antigen-specific approach for the treatment of patients with CD.
Oral tolerance is a natural immunologic process driven by the oral administration of an exogenous antigen. Recent progress in mucosal immunology provides new insights into the potential use of oral tolerance in the clinic as a mechanism to induce regulatory T cells that may play a role in the suppression of inflammation
The aim of the phase II study reported here was to further evaluate the safety and efficacy of oral administration of this personalized drug in a more diverse cohort of Crohn’s disease (CD) patients in a randomized, double-blind, placebo-controlled format.
This study examined the efficacy of AlequelTM, an autologous protein-containing extract of colon mucosal tissue. The results of the present study suggest that the induction of oral immune-regulation via oral administration of AlequelTM appears to be safe for the treatment of moderate to severe CD. Oral administration of Alequel™ resulted in an improved clinical remission rate (43% vs 33%) at weeks 6 to 9 in the drug treated group compared to the placebo group. From weeks 8 to 12, the clinical remission rates were 50% and 33% for the drug treated and placebo treated groups, respectively.
Oral administration of the autologous colonic extract Alequel™ is a patient tailored approach that is safe and may be an effective method for the treatment of patients with moderate to severe CD. The level of peripheral natural killer T and the CD4/CD8 lymphocyte ratio may serve as surrogate markers to predict clinical response. Oral tolerance may thus provide a side effect-free, disease-antigen-specific approach for the treatment of patients with CD.
The paper is quite of interest and also original in design, even if the sample size is small. Furthermore if the extract can be administerd at meal or fasting.
| 1. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1569] [Article Influence: 82.6] [Reference Citation Analysis (2)] |
| 2. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1384] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 3. | Elson CO. Genes, microbes, and T cells--new therapeutic targets in Crohn’s disease. N Engl J Med. 2002;346:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Egan LJ, Sandborn WJ. Advances in the treatment of Crohn’s disease. Gastroenterology. 2004;126:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci. 2004;1029:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ilan Y. Oral tolerance: can we make it work? Hum Immunol. 2009;70:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma growth via oral immune regulation towards tumor-associated antigens is associated with increased NKT and CD8+ lymphocytes. Oncology. 2004;66:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Safadi R, Israeli E, Papo O, Shibolet O, Melhem A, Bloch A, Rowe M, Alper R, Klein A, Hemed N. Treatment of chronic hepatitis B virus infection via oral immune regulation toward hepatitis B virus proteins. Am J Gastroenterol. 2003;98:2505-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mizrahi M, Ilan Y. The gut mucosa as a site for induction of regulatory T-cells. Curr Pharm Des. 2009;15:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med. 2006;12:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Wu HY, Center EM, Tsokos GC, Weiner HL. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus. 2009;18:586-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 539] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 19. | Ilan Y, Weksler-Zangen S, Ben-Horin S, Diment J, Sauter B, Rabbani E, Engelhardt D, Chowdhury NR, Chowdhury JR, Goldin E. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol. 2000;95:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Trop S, Samsonov D, Gotsman I, Alper R, Diment J, Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Dasgupta A, Ramaswamy K, Giraldo J, Taniguchi M, Amenta PS, Das KM. Colon epithelial cellular protein induces oral tolerance in the experimental model of colitis by trinitrobenzene sulfonic acid. J Lab Clin Med. 2001;138:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Dasgupta A, Kesari KV, Ramaswamy KK, Amenta PS, Das KM. Oral administration of unmodified colonic but not small intestinal antigens protects rats from hapten-induced colitis. Clin Exp Immunol. 2001;125:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Israeli E, Goldin E, Shibolet O, Klein A, Hemed N, Engelhardt D, Rabbani E, Ilan Y. Oral immune regulation using colitis extracted proteins for treatment of Crohn’s disease: results of a phase I clinical trial. World J Gastroenterol. 2005;11:3105-3111. [PubMed] |
| 24. | Margalit M, Israeli E, Shibolet O, Zigmond E, Klein A, Hemed N, Donegan JJ, Rabbani E, Goldin E, Ilan Y. A double-blind clinical trial for treatment of Crohn’s disease by oral administration of Alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol. 2006;101:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Hotte NS, Salim SY, Tso RH, Albert EJ, Bach P, Walker J, Dieleman LA, Fedorak RN, Madsen KL. Patients with inflammatory bowel disease exhibit dysregulated responses to microbial DNA. PLoS One. 2012;7:e37932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10:415-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 918] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 29. | El Haj M, Ben Ya’acov A, Lalazar G, Ilan Y. Potential role of NKT regulatory cell ligands for the treatment of immune mediated colitis. World J Gastroenterol. 2007;13:5799-5804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | van Dieren JM, van der Woude CJ, Kuipers EJ, Escher JC, Samsom JN, Blumberg RS, Nieuwenhuis EE. Roles of CD1d-restricted NKT cells in the intestine. Inflamm Bowel Dis. 2007;13:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kolker O, Klein A, Alper R, Menachem Y, Shibolet O, Rabbani E, Engelhardt D, Ilan Y. Early expression of interferon gamma following oral antigen administration is associated with peripheral tolerance induction. Microbes Infect. 2003;5:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Israeli E, Safadi R, Melhem A, Pappo O, Shibolet O, Klein A, Hemed N, Thalenfeld B, Engelhardt D, Rabbani E. Induction of oral immune regulation towards liver-extracted proteins for treatment of chronic HBV and HCV hepatitis: results of a phase I clinical trial. Liver Int. 2004;24:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | McFadden JP, White JM, Basketter DA, Kimber I. Does hapten exposure predispose to atopic disease? The hapten-atopy hypothesis. Trends Immunol. 2009;30:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43-52.e7. [PubMed] |
| 35. | Gregerson DS, Obritsch WF, Donoso LA. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993;151:5751-5761. [PubMed] |
| 36. | Singh VK, Nagaraju K. Experimental autoimmune uveitis: molecular mimicry and oral tolerance. Immunol Res. 1996;15:323-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, Hafler DA. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 429] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Miller A, al-Sabbagh A, Santos LM, Das MP, Weiner HL. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol. 1993;151:7307-7315. [PubMed] |
| 39. | Drachman DB, Okumura S, Adams RN, McIntosh KR. Oral tolerance in myasthenia gravis. Ann N Y Acad Sci. 1996;778:258-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Nussenblatt RB, Whitcup SM, de Smet MD, Caspi RR, Kozhich AT, Weiner HL, Vistica B, Gery I. Intraocular inflammatory disease (uveitis) and the use of oral tolerance: a status report. Ann N Y Acad Sci. 1996;778:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Thurau SR, Diedrichs-Möhring M, Fricke H, Arbogast S, Wildner G. Molecular mimicry as a therapeutic approach for an autoimmune disease: oral treatment of uveitis-patients with an MHC-peptide crossreactive with autoantigen--first results. Immunol Lett. 1997;57:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Thurau SR, Diedrichs-Möhring M, Fricke H, Burchardi C, Wildner G. Oral tolerance with an HLA-peptide mimicking retinal autoantigen as a treatment of autoimmune uveitis. Immunol Lett. 1999;68:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Lee S, Scherberg N, DeGroot LJ. Induction of oral tolerance in human autoimmune thyroid disease. Thyroid. 1998;8:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 456] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 46. | Weiner HL, Komagata Y. Oral tolerance and the treatment of rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:289-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, Fafard RD, Preuss HG. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 2002;22:101-110. [PubMed] |
| 48. | Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, Mizushima Y, Holmgren J, Lehner T. Oral tolerization with peptide 336-351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clin Exp Immunol. 2004;137:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | de Smet MD, Bitar G, Mainigi S, Nussenblatt RB. Human S-antigen determinant recognition in uveitis. Invest Ophthalmol Vis Sci. 2001;42:3233-3238. [PubMed] |
| 50. | Nussenblatt R. Orally and nasally induced tolerance studies in ocular inflammatory disease: guidance for future interventions. Ann N Y Acad Sci. 2004;1029:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Okumura S, McIntosh K, Drachman DB. Oral administration of acetylcholine receptor: effects on experimental myasthenia gravis. Ann Neurol. 1994;36:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Meyer AL, Benson JM, Gienapp IE, Cox KL, Whitacre CC. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. J Immunol. 1996;157:4230-4238. [PubMed] |
| 53. | Whitacre CC, Song F, Wardrop RM, Campbell K, McClain M, Benson J, Guan Z, Gienapp I. Regulation of autoreactive T cell function by oral tolerance to self-antigens. Ann N Y Acad Sci. 2004;1029:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Gotsman I, Shlomai A, Alper R, Rabbani E, Engelhardt D, Ilan Y. Amelioration of immune-mediated experimental colitis: tolerance induction in the presence of preexisting immunity and surrogate antigen bystander effect. J Pharmacol Exp Ther. 2001;297:926-932. [PubMed] |
| 55. | Shlomai A, Trop S, Gotsman I, Jurim O, Diment J, Alper R, Rabbani E, Engelhardt D, Ilan Y. Immunomodulation of experimental colitis: the role of NK1.1 liver lymphocytes and surrogate antigens--bystander effect. J Pathol. 2001;195:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 917] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 57. | Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 688] [Article Influence: 20.8] [Reference Citation Analysis (16)] |
| 58. | Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 386] [Article Influence: 12.1] [Reference Citation Analysis (19)] |
| 59. | Zhang J, Raus J. Myelin basic protein-reactive T cells in multiple sclerosis: pathologic relevance and therapeutic targeting. Cytotechnology. 1994;16:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1031] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 61. | Zigmond E, Preston S, Pappo O, Lalazar G, Margalit M, Shalev Z, Zolotarov L, Friedman D, Alper R, Ilan Y. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut. 2007;56:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 63. | Meyer EH, DeKruyff RH, Umetsu DT. iNKT cells in allergic disease. Curr Top Microbiol Immunol. 2007;314:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW. CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(-/-) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res. 2008;28:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Ilan Y. Immune downregulation leads to upregulation of an antiviral response: a lesson from the hepatitis B virus. Microbes Infect. 2002;4:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Shibolet O, Alper R, Avraham Y, Berry EM, Ilan Y. Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1+ T cells. Clin Immunol. 2002;105:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Trop S, Ilan Y. NK 1.1+ T cell: a two-faced lymphocyte in immune modulation of the IL-4/IFN-gamma paradigm. J Clin Immunol. 2002;22:270-280. [PubMed] |
| 68. | Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 69. | Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 70. | Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006-16.e4. [PubMed] |
| 71. | Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4607-4614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 416] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 72. | Hammer HF. Gut microbiota and inflammatory bowel disease. Dig Dis. 2011;29:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Caviglia R, Sacco R, van Langenberg DR, Wasko-Czopnik D S- Editor: Gou SX L- Editor: A E- Editor: Liu XM