Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5668
Peer-review started: October 29, 2014
First decision: November 26, 2014
Revised: December 17, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: May 14, 2015
Processing time: 201 Days and 21 Hours
AIM: To develop models to predict hepatitis B e antigen (HBeAg) seroconversion in response to interferon (IFN)-α treatment in chronic hepatitis B patients.
METHODS: We enrolled 147 treatment-naïve HBeAg-positive chronic hepatitis B patients in China and analyzed variables after initiating IFN-α1b treatment. Patients were tested for serum alanine aminotransferase (ALT), hepatitis B virus-DNA, hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen, HBeAg, antibody to hepatitis B e antigen (anti-HBe), and antibody to hepatitis B core antigen (anti-HBc) at baseline and 12 wk, 24 wk, and 52 wk after initiating treatment. We performed univariate analysis to identify response predictors among the variables. Multivariate models to predict treatment response were constructed at baseline, 12 wk, and 24 wk.
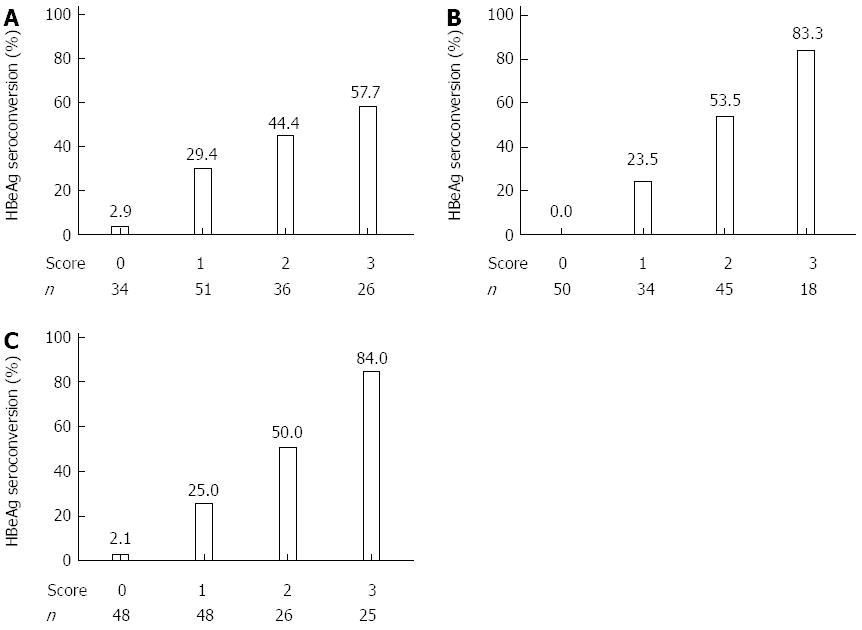
RESULTS: At baseline, the 3 factors correlating most with HBeAg seroconversion were serum ALT level > 4 × the upper limit of normal (ULN), HBeAg ≤ 500 S/CO, and anti-HBc > 11.4 S/CO. At 12 wk, the 3 factors most associated with HBeAg seroconversion were HBeAg level ≤ 250 S/CO, decline in HBeAg > 1 log10 S/CO, and anti-HBc > 11.8 S/CO. At 24 wk, the 3 factors most associated with HBeAg seroconversion were HBeAg level ≤ 5 S/CO, anti-HBc > 11.4 S/CO, and decline in HBeAg > 2 log10 S/CO. Each variable was assigned a score of 1, a score of 0 was given if patients did not have any of the 3 variables. The 3 factors most strongly correlating with HBeAg seroconversion at each time point were used to build models to predict the outcome after IFN-α treatment. When the score was 3, the response rates at the 3 time points were 57.7%, 83.3%, and 84.0%, respectively. When the score was 0, the response rates were 2.9%, 0.0%, and 2.1%, respectively.
CONCLUSION: Models with good negative and positive predictive values were developed to calculate the probability of response to IFN-α therapy.
Core tip: The response to interferon (IFN)-α therapy in chronic hepatitis B (CHB) patients varies significantly among individuals. This study of 147 patients evaluated multiple serological variables in hepatitis B e antigen (HBeAg)-positive CHB patients treated with IFN-α1b at baseline, 12 wk, and 24 wk, and then developed predictive models for HBeAg seroconversion at each of the 3 time points. The results suggest that models with good negative and positive predictive values were developed to calculate the probability of response to IFN-α therapy.
- Citation: Wang CT, Zhang YF, Sun BH, Dai Y, Zhu HL, Xu YH, Lu MJ, Yang DL, Li X, Zhang ZH. Models for predicting hepatitis B e antigen seroconversion in response to interferon-α in chronic hepatitis B patients. World J Gastroenterol 2015; 21(18): 5668-5676
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5668
Hepatitis B virus (HBV) infection is a worldwide heath problem. An estimated 350 million people are chronically infected with HBV, leading to complications such as chronic hepatitis, cirrhosis, and liver carcinoma, and accounting for one million deaths annually[1-5]. The aim of treatment for chronic hepatitis B (CHB) patients is to decrease cirrhosis and hepatocellular carcinoma with the ultimate aim of improving survival[6].
Currently, two types of antiviral therapies, including interferon (IFN)-α and nucleo(s)tide analogues, have been approved worldwide for the treatment of CHB[4,5]. IFN-α therapy can include conventional (IFN-α) and pegylated IFN-α (PegIFN-α). The advantages of IFN-α therapy include a limited treatment course, fewer cases of resistance, and sometimes clinical cure, with hepatitis B surface antigen (HBsAg) seroconversion in a few patients[7]. However, IFN-α therapy results in hepatitis B e antigen (HBeAg) seroconversion in only 30%-40% of patients[4]. The molecular mechanism behind why more patients do not respond to IFN-α therapy is unknown[8,9]. Many CHB patients in developing countries use IFN-α, not PegIFN-α therapy, for economic reasons. For example, in China, approximately 80% of patients are still using IFN-α[10]. To reduce unnecessary exposure to IFN-α and its potential side effects, as well as to reduce costs, early predictive parameters must be developed to determine whether initiation and continuation of treatment have a reasonable chance of success in an individual patient.
Factors associated with a favorable response to IFN-α therapy include serum alanine aminotransferase (ALT) level, serum HBV DNA level, serum HBsAg and HBeAg levels, antibody to hepatitis B core antigen (anti-HBc), and HBV genotype[9,11-19]. However, further studies are needed to confirm these associations.
Several models have been established for assessing IFN-α response[20-26]. Buster et al[20] reported that patients with genotype A HBV, high ALT levels, and/or low HBV DNA levels had a high predicted probability (> 30%) of a sustained response to treatment with PegIFN-α. The model may be used to select patients for IFN-α therapy, but it does not predict whether to continue treatment. Hansen et al[23] designed a dynamic model taking into account decline of HBV DNA during treatment, which provides more accurate predictions of response to PegIFN-α. This model has a high negative predictive value (NPV), but the positive predictive value (PPV) is not good. Clinical usefulness of model is poor, and the study concentrated on response to PegIFN-α. A multi-parameter model shows relatively poor capability for predicting HBeAg seroconversion in CHB patients treated with IFN-α.
In this study, we followed Chinese CHB patients treated with IFN-α1b and evaluated the HBeAg seroconversion achieved by 52 wk. We analyzed factors such as sex, age, HBV genotype, serum ALT level, serum HBV DNA level, serum HBsAg and HBeAg levels, and anti-HBc at baseline, 12 wk, and 24 wk to determine which factors were predictive for HBeAg seroconversion. We used a multivariate logistic regression analysis to determine predictive factors, and then developed predictive models for HBeAg seroconversion at each of the 3 time points.
A total of 164 treatment-naïve HBeAg-positive CHB patients receiving IFN-α1b antiviral therapy were enrolled in an open, prospective study, all of whom were followed at the First Affiliated Hospital of Anhui Medical University from March 2008 to June 2013. The study was formally approved by the ethics committee of Anhui Medical University and written informed consent was obtained from each participant.
Inclusion criteria were as follows: Patients with positive results for serum HBsAg for longer than 6 mo; HBV DNA > 10000 copies/mL; persistently elevated ALT level for 3 mo prior to treatment where the upper limit of normal (ULN) in this study was 40 U/mL; IFN-α treatment-naive. Exclusion criteria were: contraindications to IFN-α treatment, treatment with nucleotide analogues, infection with human immunodeficiency virus or hepatitis C or D, autoimmune chronic liver disease, heritable disorders, alcoholism, or drug abuse; patients were excluded if they did not reach 24 wk of IFN-α1b treatment.
HBeAg seroconversion was defined as the loss of HBeAg (≤ 1 S/CO using the test from Abbott, Chicago, IL, United States), and positive antibody to hepatitis B e antigen (anti-HBe) (≤ 1 S/CO using the test from Abbott) at 52 wk. Patients not fulfilling these criteria were considered non-responders. Patients with missing data at 52 wk were classified as non-responders at the end of treatment[27,28].
Treatment consisted of IFN-α1b (Shenzhen Kexing Biotech, Shenzhen, China) at a dose of 5 MU administered once every other day by a subcutaneous injection for 52 wk. When ALT level was greater than 10 × ULN, ALT-reducing and liver-protecting drugs were used until ALT level dropped to < 10 × ULN.
Patient histories were obtained, physical examinations were conducted, and laboratory assessments were performed at baseline, 12 wk, 24 wk, and at the end of treatment (52 wk). Clinical evaluation was performed to record general characteristics of the patients such as sex, age, and treatment duration. The HBV genotype was tested. During the follow-up period, patients were tested for serum ALT, HBV DNA, HBsAg, antibody to hepatitis B surface antigen (anti-HBs), HBeAg, anti-HBe, and anti-HBc. Serum samples collected at each visit were stored at -80 °C until tested.
Serum HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc were tested using commercially available kits (Abbott, Chicago, IL, United States). The sensitivity of the HBsAg assay ranged from 0.05-250 IU/mL. Samples with higher concentrations of HBsAg were diluted 1:500 with ARCHITECT diluents according to the manufacturer’s instructions. Serum ALT levels were measured at the time of sampling using an automatic biochemical analyzer (Roche, Switzerland). An ALT level ≤ 1 × ULN (40 U/L) was considered normal. Serum HBV DNA levels were measured using a TaqMan real-time PCR assay (Shanghai ZJ BioTech, Shanghai, China) with a lower detection limit of 1000 copies/mL. The HBV genotype was determined using a real-time PCR kit (Shanghai ZJ BioTech, Shanghai, China).
Quantitative variables are expressed as the mean ± SD, while categorical variables are expressed as a number (percentage). Statistical analysis of differences between groups was performed using the Student’s t-test, analysis of variance (ANOVA), the χ2 test, Fisher’s exact test, the Kruskal-Wallis test, or the Mann-Whitney test, as appropriate. A receiver operating characteristic (ROC) curve and area under the curve (AUC) were applied to assess the optimal parameter cut-off values through maximizing the Youden’s index (J), where J = sensitivity + specificity - 1. Spearman’s correlation coefficient was used to examine relationships among the parameters. We performed univariate logistic regression analysis to identify response predictors among the variables. The variables identified in the univariate analysis entered multivariate binary stepwise forward (Wald) logistic regression analysis in order to construct predictive models. A two-tailed P-value less than 0.05 was considered statistically significant. All calculations were performed with SPSS software (version 19.0, SPSS Inc., Chicago, IL, United States).
One hundred and forty-seven (89.1%) patients were included in the final modified intention-to-treat analysis. Of the patients excluded from the final analysis, 3 did not take medication, 7 experienced adverse events (1 severe flu-like syndrome, 3 bone marrow suppression, 1 loss of appetite, and 2 abnormal liver function), and 7 were lost to follow-up. At 52 wk, 135 (91.8%) patients completed treatment and 12 (8.2%) did not [5 because of adverse events (3 alopecia, 2 psychiatry), 4 because of poor efficacy of IFN-α treatment and addition of nucleotide analogue treatment, and 3 lost to follow-up].
We stratified the patients according to whether or not they experienced HBeAg seroconversion after IFN-α therapy (Table 1). By the end of treatment, 47 (32.0%) patients experienced HBeAg seroconversion and 2 (1.4%) patients were HBeAg-/anti-HBe-. Patients with HBeAg seroconversion had lower HBeAg than those without HBeAg seroconversion (2.30 ± 0.13 vs 2.67 ± 0.07, P = 0.016). There were no significant differences between the two groups with regard to sex, age, HBV genotype, ALT, HBV DNA, HBsAg, or anti-HBc (P > 0.05).
| Characteristic | Total (n = 147) | RS (n = 47) | NRS (n = 100) |
| Sex | |||
| Male | 106 (72.1) | 33 (70.2) | 73 (73.0) |
| Female | 41 (27.9) | 14 (29.8) | 27 (27.0) |
| Age (yr) | 25.02 ± 056 | 26.40 ± 1.19 | 24.37 ± 0.59 |
| ALT (U/L) | 214.25 ± 17.38 | 226.23 ± 19.19 | 208.62 ± 23.94 |
| HBV DNA | |||
| (log10 copies/mL) | 7.16 ± 0.07 | 6.93 ± 0.14 | 7.27 ± 0.09 |
| HBsAg | |||
| (log10 IU/mL) | 4.11 ± 0.07 | 3.93 ± 0.14 | 4.20 ± 0.07 |
| HBeAg | |||
| (log10 S/CO) | 2.55 ± 0.07 | 2.30 ± 0.13a | 2.67 ± 0.07a |
| Anti-HBc (S/CO) | 11.13 ± 0.15 | 11.54 ± 0.27 | 10.94 ± 0.17 |
| Genotype | |||
| B | 85 (57.8) | 26 (55.3) | 59 (59.0) |
| C | 55 (37.4) | 21 (44.7) | 34 (34.0) |
| Other | 7 (4.8) | 0 (0.0) | 7 (7.0) |
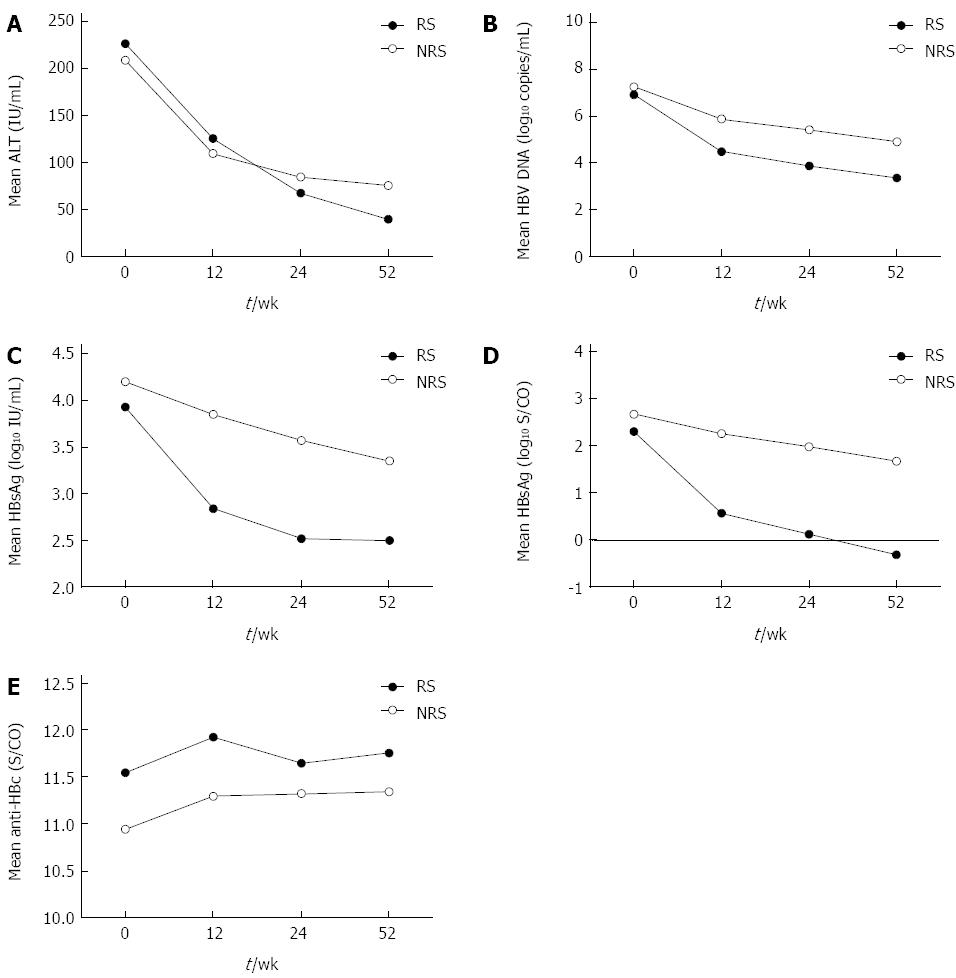
To develop models to predict response to IFN-α therapy for CHB patients, changes in ALT, HBsAg, HBeAg, HBV DNA, and anti-HBc levels were analyzed in patients who had undergone HBeAg seroconversion (RS group), compared to those who had not (NRS group) (Figure 1). While ALT, HBV DNA, HBsAg, and anti-HBc levels at baseline, ALT levels at 12 wk and 24 wk, and anti-HBc levels at 24 wk were not statistically significantly (P > 0.05), all other variables were significantly different between the two groups (P < 0.05). The decline in ALT and anti-HBc levels was not statistically significant (P > 0.05), but the decline in all other variables was statistically significant (P < 0.05).
To investigate whether response to IFN-α could be predicted from the start of treatment, ROC curves were derived from clinical characteristics and biological markers. The areas under the ROC curves for age, sex, HBV genotype, serum ALT, HBV DNA, HBsAg, HBeAg, and anti-HBc were 0.57, 0.51, 0.50, 0.63, 0.60, 0.59, 0.64, and 0.64, respectively. Thus, the predictive value of each single factor was not high.
As shown in Table 2, in the univariate logistic regression analysis, sex, age, HBV genotype, and serum HBV DNA were not statistically different between those who responded to INF-αvs those who did not (P > 0.05). The incidence of HBeAg seroconversion was significantly higher among patients with ALT > 4 × ULN, HBeAg ≤ 500 S/CO, anti-HBc > 11.4 S/CO, and HBsAg ≤ 15000 IU/mL (P < 0.05). A total of 55 patients had an HBeAg value ≤ 500 S/CO, and at 52 wk, 26 (47.3%) of these patients had undergone HBeAg seroconversion. A total of 74 patients had an ALT value > 4 × ULN, and at 52 wk, 33 (44.6%) of these patients had undergone HBeAg seroconversion. A total of 72 patients had anti-HBc value > 11.4 S/CO, and at 52 wk, 33 (45.8%) of these patients had undergone HBeAg seroconversion.
| Parameter | RS | Univariate analysis | P value | Multivariate analysis | P value |
| OR (95%CI) | OR (95%CI) | ||||
| Female gender | 14 (34.1) | 1.15 (0.53-2.47) | 0.725 | ||
| Age > 24 yr | 26 (40.0) | 1.94 (0.96-3.91) | 0.065 | ||
| ALT > 4 × ULN | 33 (44.6) | 3.39 (1.62-7.12) | 0.001 | 2.44 (1.10-5.42) | 0.028 |
| HBV DNA ≤ 7 | |||||
| log10 copies/mL | 22 (39.3) | 1.71 (0.84-3.46) | 0.138 | ||
| HBsAg ≤ 15000 IU/mL | 27 (40.3) | 2.03 (1.00-4.09) | 0.049 | ||
| HBeAg ≤ 500 S/CO | 26 (47.3) | 3.03 (1.48-6.22) | 0.003 | 2.12 (0.97-4.64) | 0.060 |
| anti-HBc > 11.4 S/CO | 33 (45.8) | 3.69 (1.75-7.75) | 0.001 | 3.02 (1.39-6.57) | 0.005 |
| Genotype B | 26 (30.6) | 0.91 (0.50-1.65) | 0.749 |
In the multivariate logistic regression analysis, ALT level > 4 × ULN (OR = 2.44, 95%CI: 1.10-5.42, P = 0.028), HBeAg ≤ 500 S/CO (OR = 2.12, 95%CI: 0.97-4.64, P = 0.060), and anti-HBc > 11.4 S/CO (OR = 3.02, 95%CI: 1.39-6.57, P = 0.005) were independent predictors of HBeAg seroconversion. A model was constructed to predict IFN-α response from the start of therapy based on 3 variables: ALT, HBeAg, and anti-HBc. Each variable was assigned a score of 1. A score of 0 was given if patients did not have any of the 3 variables.
After applying the model, 26 patients had a score of 3 and 15 of those (57.7%) exhibited HBeAg seroconversion. A score of 0 was observed in 34 patients and only 1 (2.9%) exhibited HBeAg seroconversion (Figure 2A).
The levels of ALT, HBV DNA, HBsAg, HBeAg, and anti-HBc, and the decline in ALT, HBV DNA, HBsAg, HBeAg, and anti-HBc levels at 12 wk after initiating IFN-α therapy were comparable in those who responded vs those who did not. In univariate analysis, patients who achieved HBeAg seroconversion after IFN-α therapy had lower serum HBV DNA, HBsAg, and HBeAg levels, higher anti-HBc levels, and a greater decline in HBV DNA, HBsAg, and HBeAg than those without HBeAg seroconversion. ALT and decline in ALT and anti-HBc levels were not significantly different between patients who had undergone HBeAg seroconversion and those who had not. A total of 51 patients showed a decline in HBeAg values > 1 log10 copies/mL, and at 52 wk, 34 (66.7%) of these patients had undergone HBeAg seroconversion. A total of 79 patients had an HBeAg value ≤ 250 S/CO, and at 52 wk, 44 (55.7%) of these patients had undergone HBeAg seroconversion. A total of 48 patients had anti-HBc value > 11.8 S/CO, and at 52 wk, 23 (47.9%) of these patients had undergone HBeAg seroconversion.
In the multivariate stepwise logistic regression analyses, HBeAg level ≤ 250 S/CO (OR = 10.90, 95%CI: 2.63-45.27, P = 0.001), decline in HBeAg > 1 log10 S/CO (OR = 4.53, 95%CI: 1.59-12.91, P = 0.005), and anti-HBc > 11.8 S/CO (OR = 3.72, 95%CI: 1.41-9.84, P = 0.008) were predictive of HBeAg seroconversion (Table 3). Each variable was assigned a score of 1, and a score of 0 was given if patients did not have any of the 3 variables.
| Parameter | RS | Univariate analysis | P value | Multivariate analysis | P value |
| OR (95%CI) | OR (95%CI) | ||||
| ALT > 7 × ULN | 8 (61.5) | 3.90 (1.20-12.66) | 0.024 | ||
| HBV DNA ≤ 5 log10 copies/mL | 31 (53.4) | 5.24 (2.48-11.06) | < 0.001 | ||
| HBsAg ≤ 10000 IU/mL | 41 (48.2) | 8.70 (3.39-22.34) | < 0.001 | ||
| HBeAg ≤ 250 S/CO | 44 (55.7) | 27.24 (7.89-94.09) | < 0.001 | 10.90 (2.63-45.27) | 0.001 |
| anti-HBc > 11.8 S/CO | 23 (47.9) | 2.88 (1.39-5.96) | 0.005 | 3.72 (1.41-9.84) | 0.008 |
| Decline ALT > 3 × ULN | 20 (40.0) | 1.73 (0.84-3.55) | 0.136 | ||
| Decline HBV DNA > 1.5 log10 copies/mL | 34 (48.6) | 4.65 (2.18-9.93) | < 0.001 | ||
| Decline HBsAg > 0.5 log10 IU/mL | 29 (55.8) | 5.39 (2.55-11.42) | < 0.001 | ||
| Decline HBeAg > 1 log10 S/CO | 34 (66.7) | 12.77 (5.60-29.14) | < 0.001 | 4.53 (1.59-12.91) | 0.005 |
| Decline anti-HBc > 0.5 S/CO | 13 (40.6) | 1.64 (0.53-5.07) | 0.393 |
After applying the model, 18 patients had a score of 3, 15 of whom (83.3%) responded to IFN-α therapy by exhibiting HBeAg seroconversion. When the score was 0, the incidence of HBeAg seroconversion was 0.0% (0/50) (Figure 2B).
At 24 wk, all parameters were significantly different between those who responded to IFN-α therapy vs those who did not (Table 4). A total of 63 patients had an HBeAg value ≤ 5 S/CO, and at 52 wk, 41 (65.1%) of these patients had undergone HBeAg seroconversion. A total of 67 patients had an anti-HBc value > 11.4 S/CO, and at 52 wk, 30 (44.8%) of these patients had undergone HBeAg seroconversion. A total of 45 patients showed a decline in HBeAg values ≥ 2 log10, and at 52 wk, 30 (66.7%) of these patients had undergone HBeAg seroconversion. In the multivariate stepwise logistic regression analyses, HBeAg level ≤ 5 S/CO and anti-HBc level > 11.4 S/CO were independent factors predictive of HBeAg seroconversion (Table 4). Based on clinical considerations, we included a decline in HBeAg > 2 log10 S/CO in the construction of the scoring model. Each variable was assigned a score of 1, and a score of 0 was given if patients did not have any of the 3 variables.
| Parameter | RS | Univariate analysis | P value | Multivariate analysis | P value |
| OR (95%CI) | OR (95%CI) | ||||
| ALT ≤ 1 × ULN | 25 (45.5) | 2.65 (1.30-5.42) | 0.008 | ||
| HBV DNA ≤ 4 log10 copies/mL | 32 (50.8) | 4.48 (2.25-10.01) | < 0.001 | ||
| HBsAg ≤ 2000 IU/mL | 33 (52.4) | 5.50 (2.58-11.73) | < 0.001 | ||
| HBeAg ≤ 5 S/CO | 41 (65.1) | 24.23 (9.11-64.47) | < 0.001 | 16.82 (5.07-55.79) | < 0.001 |
| anti-HBc > 11.4 S/CO | 30 (44.8) | 3.01 (1.46-6.18) | 0.003 | 3.01 (1.20-7.56) | 0.019 |
| Decline ALT > 2 × ULN | 35 (42.2) | 3.16 (1.47-6.78) | 0.003 | ||
| Decline HBV DNA > 2 log10 copies/mL | 36 (46.8) | 4.71 (2.15-10.32) | < 0.001 | ||
| Decline HBsAg > 1 log10 IU/mL | 26 (49.1) | 3.35 (1.62-6.91) | 0.001 | ||
| Decline HBeAg > 2 log10 S/CO | 30 (66.7) | 10.00 (4.45-22.47) | < 0.001 | 1.85 (0.62-5.49) | 0.269 |
| Decline anti-HBc > 0.5 S/CO | 19 (47.5) | 2.55 (1.20-5.43) | 0.015 |
After applying the model, 25 patients had a score of 3 and 21 (84.0%) responded to IFN-α therapy by exhibiting HBeAg seroconversion. A score of 0 was observed in 48 patients, only 1 (2.1%) of which exhibited HBeAg seroconversion (Figure 2C).
The study showed that CHB patients with a score of 0 at baseline, 12 wk, or 24 wk of IFN-α therapy have a high NPV independent of their score at other time points. Only 2 of 68 CHB patients with a score of 0 at any time point responded to IFN-α therapy by 52 wk. Therefore, patients with a score of 0 should not continue IFN-α therapy and should pursue other treatment options. However, 60 patients had a score of 2 or 3 at the different time points and 65.0% (39/60) of these patients responded to IFN-α therapy by 52 wk. Therefore, a score of 2 or 3 correlates well with IFN-α response as determined by HBeAg seroconversion.
Monitoring responses to IFN-α treatment requires evaluating sustained suppression of HBV replication, biochemical remission, histological improvement, and HBeAg/HBsAg loss or seroconversion in HBeAg-positive patients[29-31]. HBeAg seroconversion usually predicts long-lasting suppression of HBV, reduced infectivity, and improved clinical prognosis[32]. Because many patients in our study did not achieve HBeAg seroconversion and may have therefore continued treatment using nucleotide analogues, we chose to end the study after 52 wk[32-34].
Our study showed that 32.0% of the patients achieved HBeAg seroconversion by the end of treatment, consistent with other reports[4]. The CHB patients in this study mostly came from the Anhui Province in China where HBV genotypes B and C are prevalent. Our results are similar to those of other studies in the region, and HBV genotype did not affect IFN-α efficacy.
Our data indicate that ALT, HBV DNA, HBsAg, HBeAg, and anti-HBc levels at baseline and 12 and 24 wk are predictive of response to IFN-α therapy in HBeAg-positive CHB patients, consistent with previous reports[9,11-19]. Some studies suggested that HBsAg levels correlate with response to IFN therapy[9,11,13,18]. However, other studies have shown no significant difference[35]. In our study, HBsAg levels showed predictive trends between responders vs non-responders, but the results did not reach statistical significance (P = 0.068).
If efficacy of IFN-α treatment could be predicted prior to and during treatment, many medical resources would be saved and complications of therapy would be avoided. Previous models have some drawbacks. First, some did not use multivariate analyses, hence reducing the accuracy of their studies[26]. They also did not use anti-HBc, which is a predictor for IFN-α response in our study. Some models did not use more than one time point; therefore, their results were not conducive to guiding therapy based on response over time[20]. Some studies used complicated mathematical models not conducive to clinical use[24]. Our study overcame these problems.
ALT, HBeAg, and anti-HBc levels are commonly tested for application in a clinical setting and could be used to build predictive models by multivariate step-wise logistic regression analysis at baseline. HBeAg, decline in HBeAg, and anti-HBc levels could be used to build predictive models by multivariate step-wise logistic regression analysis at 12 and 24 wk. At the 3 time points, the PPVs were 57.7%, 83.3%, and 84.0%, respectively, when the score was 3. In addition, the NPVs were 97.1%, 100%, and 97.9%, respectively, at the 3 time points when the score was 0. For example, In the study by Lau et a[21], the PPV was 61% and NPV was 76% at baseline. van der Eijk et al[26] reported a PPV of 46% and NPV of 100% at 12 wk. In the study by Fried et al[12], the NPV was 96% at 24 wk. Therefore, our model has a higher PPV or NPV compared with other models.
In this study, the best single factor to predict IFN-α response was HBeAg level. However, its NPVs were only 66.3%, 86.7% and 79.5% at baseline, 12 and 24 wk, respectively. In comparison, the model has a better NPV (97.1%, 100%, and 97.9%, respectively). Only 2 (2.9%) CHB patients with a score of 0 at any time point (baseline, 12 or 24 wk) responded to IFN-α therapy at 52 wk. Therefore, we recommend that patients with a score of 0 at baseline, 12, or 24 wk may stop IFN-α therapy and pursue other treatment options to reduce unnecessary costs. While patients scoring 2 or 3 at the different time points have a higher chance of responding to IFN-α therapy and should continue to treat with IFN-α.
Our study had some limitations. The endpoint of HBeAg seroconversion was evaluated at 52 wk rather than 24 wk after initiating treatment. Since the study was open, most patients began using nucleotide analogs if they did not achieve HBeAg seroconversion and so were not still taking IFN-α by 52 wk.
In conclusion, we developed models for predicting responses to IFN-α therapy in CHB patients. The models have a good NPV and PPV and are relatively simple, which is beneficial for clinical application. Future research should explore whether these models also hold for prolonged therapy or PegIFN-α therapy.
It is very difficult to predict whether interferon (IFN)-α treatment will be successful before initiating therapy for an individual chronic hepatitis B (CHB) patient.
Recent research has been carried out to develop models to assess the efficacy of IFN-α. However, previous studies used relatively few variables and did not have good positive and/or negative predictive values.
In this study, the authors (1) showed that the presence of antibody to hepatitis B core antigen may be an independent predictor of IFN-α response in CHB patients; and (2) developed prediction models for hepatitis B e antigen (HBeAg) seroconversion in response to IFN-α therapy to help guide treatment at baseline and 12 and 24 wk after initiating therapy.
It is crucial to identify which CHB patients are responding to IFN-α treatment in order to determine whether to continue therapy or change the course of treatment. Using the predictive models developed in this study, practitioners would be able to monitor IFN-α response and to individualize treatments.
HBeAg is a soluble protein found in hepatitis B virus core particles. It can cause severe liver cell damage and more infectivity. HBeAg seroconversion improves the long-term prognosis of chronic hepatitis B patients and is an important indication of when antiviral therapy is no longer required.
In this study, the authors have developed scoring systems which can be used for predicting HBeAg seroconversion after IFN-α treatment. The models demonstrated high negative and positive predictive values and will benefit the patients who undergo anti-hepatitis B virus therapy.
| 1. | Mohamed R, Ng CJ, Tong WT, Abidin SZ, Wong LP, Low WY. Knowledge, attitudes and practices among people with chronic hepatitis B attending a hepatology clinic in Malaysia: a cross sectional study. BMC Public Health. 2012;12:601. [PubMed] |
| 2. | You CR, Lee SW, Jang JW, Yoon SK. Update on hepatitis B virus infection. World J Gastroenterol. 2014;20:13293-13305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 3. | Tujios SR, Lee WM. Update in the management of chronic hepatitis B. Curr Opin Gastroenterol. 2013;29:250-256. [PubMed] |
| 4. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1203] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 5. | Yu S, Zhou Q, Zhao XM, Yuan M, Wang CT, Cheng XG, Zhang ZH, Li X. Comparison of the antiviral effects of different nucleos(t)ide analogues in chinese patients with chronic hepatitis B: a head-to-head study. Saudi J Gastroenterol. 2014;20:350-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140-1149.e3; quiz e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 7. | Perrillo RP. Therapy of hepatitis B -- viral suppression or eradication? Hepatology. 2006;43:S182-S193. [PubMed] |
| 8. | Tseng TC, Kao JH, Chen DS. Peginterferon α in the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2014;14:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 362] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 10. | Jia JD. Diagnosis and treatment of hepatitis B on public health. The 9th National Conference of Liver Disease in China. O1. 2014;. |
| 11. | Chen EQ, Wang TT, Bai L, Tao CM, Liang T, Liu C, Liao J, Tang H. Quantitative hepatitis B surface antigen titres in Chinese chronic hepatitis B patients over 4 years of entecavir treatment. Antivir Ther. 2013;18:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, Paik SW, Liaw YF, Button P. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 13. | Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Marinos G, Smith HM, Naoumov NV, Williams R. Quantitative assessment of serum IgM anti-HBc in the natural course and during interferon treatment of chronic hepatitis B virus infection. Hepatology. 1994;19:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kong LN, Qin B, Ma Q, Li L, Yao Y. Relationship between hepatitis B virus genotype B and C and response to interferon therapy in HBeAg positive chronic hepatitis B patients: A meta-analysis. J Gastroenterol Hepatol. 2014;29:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Luo K, He H, Liu Z, Zhu Y, Mao Q, Liang W. No significant differences in histology and response to interferon treatment in hepatitis B carriers of genotypes C and recombinant B. J Viral Hepat. 2007;14:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Tangkijvanich P, Komolmit P, Mahachai V, Sa-Nguanmoo P, Theamboonlers A, Poovorawan Y. Comparison between quantitative hepatitis B surface antigen, hepatitis B e-antigen and hepatitis B virus DNA levels for predicting virological response to pegylated interferon-alpha-2b therapy in hepatitis B e-antigen-positive chronic hepatitis B. Hepatol Res. 2010;40:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sonneveld MJ, Zoutendijk R, Janssen HL. Hepatitis B surface antigen monitoring and management of chronic hepatitis B. J Viral Hepat. 2011;18:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Yuan Q, Song LW, Liu CJ, Li Z, Liu PG, Huang CH, Yan Y, Ge SX, Wang YB, Peng CY. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut. 2013;62:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Lau DT, Comanor L, Minor JM, Everhart JE, Wuestehube LJ, Hoofnagle JH. Statistical models for predicting a beneficial response to interferon-alpha in patients with chronic hepatitis B. J Viral Hepat. 1998;5:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mao QG, Pan JS, Fang KN, Zhang RM, Hong QY, Song MN, Zhu JP, Huang WQ, Chen LM, Hong MZ. Precise prediction model and simplified scoring system for sustained combined response to interferon-alpha. World J Gastroenterol. 2010;16:3465-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Hansen BE, Buster EH, Steyerberg EW, Lesaffre E, Janssen HL. Prediction of the response to peg-interferon-alfa in patients with HBeAg positive chronic hepatitis B using decline of HBV DNA during treatment. J Med Virol. 2010;82:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | ter Borg MJ, Hansen BE, Herrmann E, Zeuzem S, Cakaloglu Y, Karayalcin S, Flisiak R, van’ t Veen A, de Man RA, Schalm SW, Janssen HL, Haagmans BL, HBV 99-01 Study Group. Modelling of early viral kinetics and pegylated interferon-alpha2b pharmacokinetics in patients with HBeag-positive chronic hepatitis B. Antivir Ther. 2007;12:1285-1294. [PubMed] |
| 25. | Zhu X, Gong Q, Yu D, Zhang D, Gu L, Han Y, Chen J, Zhang Y, Zhang X. Early serum hepatitis B virus large surface protein level: a stronger predictor of virological response to peginterferon alfa-2a than that to entecavir in HBeAg-positive patients with chronic hepatitis B. J Clin Virol. 2013;57:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | van der Eijk AA, Niesters HG, Hansen BE, Heijtink RA, Janssen HL, Schalm SW, de Man RA. Quantitative HBV DNA levels as an early predictor of nonresponse in chronic HBe-antigen positive hepatitis B patients treated with interferon-alpha. J Viral Hepat. 2006;13:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW, HBV 99-01 Study Group. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 910] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 28. | Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, Qiao FY, Campbell F, Chang CN. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 30. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S, Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 31. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (2)] |
| 32. | Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48 Suppl 1:S2-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Reijnders JG, Rijckborst V, Sonneveld MJ, Scherbeijn SM, Boucher CA, Hansen BE, Janssen HL. Kinetics of hepatitis B surface antigen differ between treatment with peginterferon and entecavir. J Hepatol. 2011;54:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Ma Q, Qin B, Gong X, Lu X. Prediction of response to interferon α-1b in HBeAg-positive chronic hepatitis B: a clue from HBsAg levels. Eur J Gastroenterol Hepatol. 2013;25:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Su TH, Liu CJ, Yang HC, Jeng YM, Cheng HR, Liu CH, Tseng TC, Ling TY, Chen PJ, Chen DS. Clinical significance and evolution of hepatic HBsAg expression in HBeAg-positive patients receiving interferon therapy. J Gastroenterol. 2014;49:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ahmed Said ZN, Kanizaj TF, Tu H S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM