Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5654
Peer-review started: November 17, 2014
First decision: December 11, 2014
Revised: December 31, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: May 14, 2015
Processing time: 183 Days and 18.2 Hours
AIM: To assess how ascites and alpha-fetoprotein (AFP) added to the Barcelona Clinic Liver Cancer (BCLC) staging predict hepatocellular carcinoma survival.
METHODS: The presence of underlying cirrhosis, ascites and encephalopathy, Child-Turcotte-Pugh (CTP) score, the number of nodules, and the maximum diameter of the largest nodule were determined at diagnosis for 1060 patients with hepatocellular carcinoma at a tertiary referral center for liver disease in Egypt. Demographic information, etiology of liver disease, and biochemical data (including serum bilirubin, albumin, international normalized ratio, alanine and aspartate aminotransferases, and AFP) were evaluated. Staging of the tumor was determined at the time of diagnosis using the BCLC staging system; 496 patients were stage A and 564 patients were stage B. Patients with mild ascites on initial ultrasound, computed tomography, or clinical examination, and who had a CTP score ≤ 9 were included in this analysis. All patients received therapy according to the recommended treatment based on the BCLC stage, and were monitored from the time of diagnosis to the date of death or date of data collection. The effect of the presence of ascites and AFP level on survival was analyzed.
RESULTS: At the time the data were censored, 123/496 (24.8%) and 218/564 (38.6%) patients with BCLC stages A and B, respectively, had died. Overall mean survival of the BCLC A and B patients during a three-year follow-up period was 31 mo [95% confidence interval (95%CI): 29.7-32.3] and 22.7 mo (95%CI: 20.7-24.8), respectively. The presence of ascites, multiple focal lesions, large tumor size, AFP level and CTP score were independent predictors of survival for the included patients on multivariate analysis (P < 0.001). Among stage A patients, 18% had ascites, 33% had AFP ≥ 200 ng/mL, and 8% had both. Their median survival in the presence of ascites was shorter if AFP was ≥ 200 ng/mL (19 mo vs 24 mo), and in the absence of ascites, patients with AFP ≥ 200 ng/mL had a shorter survival (28 mo vs 39 mo). For stage B patients, survival for the corresponding groups was 12, 18, 19 and 22 mo. The one-, two-, and three-year survival rates for stage A patients without ascites and AFP < 200 ng/mL were 94%, 77%, and 71%, respectively, and for patients with ascites and AFP ≥ 200 ng/mL were 83%, 24%, and 22%, respectively (P < 0.001). Adding ascites and AFP ≥ 200 ng/mL improved the discriminatory ability for predicting prognosis (area under the curve, 0.618 vs 0.579 for BCLC, P < 0.001).
CONCLUSION: Adding AFP and ascites to the BCLC staging classification can improve prognosis prediction for early and intermediate stages of hepatocellular carcinoma.
Core tip: Although the Barcelona Clinic Liver Cancer (BCLC) system contains Child-Turcotte-Pugh classification as a main variable, a patient within BCLC stage A or B may have ascites, which can interfere with the recommended treatment. An elevated alpha-fetoprotein level, which is an integral part of other staging systems, may also impact prognosis and influence treatment decision. In this study, we evaluated the utility of adding ascites and the alpha-fetoprotein level to the BCLC system for predicting prognosis and survival in a large cohort of early and intermediate hepatocellular carcinoma patients at a tertiary referral center for liver disease in Egypt.
- Citation: Gomaa AI, Al-Khatib A, Abdel-Razek W, Hashim MS, Waked I. Ascites and alpha-fetoprotein improve prognostic performance of Barcelona Clinic Liver Cancer staging. World J Gastroenterol 2015; 21(18): 5654-5662
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5654
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death worldwide, and its incidence is increasing[1]. Patient prognosis is determined by tumor characteristics of HCC and by the presence of chronic liver disease and cirrhosis, which impact treatment modality and patient survival[2,3]. Moreover, tumor staging at the time of diagnosis is essential for selecting the most appropriate therapy and to predict survival[3].
The Barcelona Clinic Liver Cancer (BCLC) system is the most widely adopted method for staging HCC[4], has been validated in clinical and therapeutic studies[5-8], and is recommended in the practice guidelines of the European Association for the Study of the Liver (EASL)[9] and the American Association for the Study of Liver Diseases (AASLD)[10]. However, patients with early (BCLC stage A) and intermediate (BCLC stage B) HCC have varying disease severity, provided that there is no vascular invasion, extrahepatic spread, or compromised performance status. Stage A or B patients can have a Child-Turcotte-Pugh (CTP) score of 5 (class A)[11], or can have ascites or encephalopathy and a CTP score of 9 (class B). A meta-analysis of 30 randomized controlled trials of palliative treatment in HCC found that ascites was highly correlated with poor outcome in intermediate and advanced BCLC stages[12]. One-year and two-year survival rates in these patients varied widely among studies included in the meta-analysis, ranging from 0% to 75% and 0% to 50%, respectively.
Elevated alpha-fetoprotein (AFP) level, which is an integral part of several staging systems[5-8], may also impact prognosis and influence treatment decision. A high AFP level predicts recurrence after resection and poor outcome after liver transplantation, even for patients fulfilling the Milan criteria[13]. In a previous report, we found that the BCLC staging system provided the best prognostic stratification, and showed that the presence of ascites and AFP above 200 ng/mL (occasionally encountered in stage A and B patients) were independent predictors of survival[14]. Other identified independent predictors of survival (portal vein invasion and extra-hepatic spread) are used to assign patients to advanced (stage C) and terminal (stage D) stages, where the Cancer of the Liver Italian Program (CLIP) score was found to have the highest stratification ability. The present study evaluated the impact of elevated AFP level and presence of ascites independently on predicting the prognosis of patients with BCLC stages A and B HCC.
This prospective study was conducted between January 2010 and December 2013 and included stages A and B HCC patients at the National Liver Institute, a tertiary referral center for liver disease in Egypt. The study conformed to the ethical guidelines of the Declaration of Helsinki (1964, as revised in 2004), and was approved by the Institutional Review Board (IRB00003413). Written informed consent was obtained from each patient. The diagnosis of HCC was mainly non-histologic, according to the AASLD criteria of 2005[15].
All patients diagnosed with BCLC stages A and B in our center were invited to participate in this observational study. Of the 2250 eligible patients, 1060 consented to participate, and were the subjects of this analysis (these patients’ data were part of a previous publication comparing different staging systems in HCC patients[14]). For all patients, demographic information, etiology of liver disease, and biochemical data [including serum bilirubin, albumin, international normalized ratio (INR), alanine and aspartate aminotransferases, and AFP] were evaluated. Presence of underlying cirrhosis, ascites and encephalopathy, CTP score, the number of nodules, and the maximum diameter of the largest nodule were recorded.
The presence of ascites was assessed at the initial evaluation. Patients who had clinically detectable, large-volume ascites were considered to have advanced liver disease (CTP class C), and were excluded from analysis. Patients with newly diagnosed, mild ascites on initial ultrasound, computed tomography (CT) or clinical examination, or those who were already being treated for ascites who had a CTP score ≤ 9 were included in this analysis. A committee determined the therapy for patients based on the BCLC recommendation, and patients were monitored from the time of diagnosis to the date of death or date of data collection at the end of the study period.
Overall survival was the single end point used to assess the performance of the staging system. The length of survival was calculated from the date of HCC diagnosis to the date of death or, in the case of survivors, the date of the last follow-up visit. Patients who were alive at the end of the study or lost to follow-up were censored.
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, United States). The Kaplan-Meier method was used to compare overall survival with respect to patient age, sex, presence of ascites, albumin level, total bilirubin, prothrombin time, CTP class, serum AFP level, AFP above or below 200 ng/mL, greatest tumor dimension > 5 cm, and presence of more than one focal lesion. A univariate analysis was performed to identify predictors of survival at the time of HCC diagnosis by the log-rank test[16]. The overall predictive power of the staging system for survival was evaluated by the linear trend χ2 test using a Cox regression model[17]. The accuracy of prediction of death at one, two and three years was evaluated by calculating the area under the receiver operating characteristic curve (AUROC) for each score, which is equivalent to the concordance statistic[18]. To perform this test, patients censored before one, two and three years were excluded from the analysis. Continuous data are expressed as the mean ± standard deviation. P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Sameera Ezzat, M.D., Associate Professor of Epidemiology, National Liver Institute.
The study included a total of 1060 patients, with 866 men and 194 women with a mean age of 56 ± 8.4 years. The baseline patient characteristics and CTP classification are shown in Table 1. In both groups, > 90% of patients had cirrhosis. All patients received an intervention according to the BCLC stage and recommended therapies[4]. Trans-arterial chemoembolization (TACE) was recommended for all stage B patients, and 34/496 (6.9%) stage A patients received TACE because their lesion was considered difficult for local ablation under ultrasound guidance or was > 5 cm and not suitable for resection. Only 7/496 (1.4%) stage A patients received a living donor liver transplant. The presence of ascites did not influence referral or administration of recommended treatment, and patients with AFP levels ≥ 1000 ng/mL were not considered for liver transplantation or resection.
| BCLC A | BCLC B | |
| Number | n = 496 | n = 564 |
| Age (yr), mean ± SD | 56.2 ± 8.3 | 56.6 ± 8.7 |
| Male | 414 (83.5) | 452 (80.3) |
| Cirrhosis | 448 (90.5) | 510 (90.4) |
| Ascites | 91 (18.3) | 151 (26.8) |
| Etiology of liver disease | ||
| HCV | 986 (93) | 973 (91.8) |
| HBV | 43 (4) | 52 (4.9) |
| Non-HCV, non-HBV | 31 (3) | 35 (3.3) |
| AFP (ng/mL), mean (range) | 365 (20-12, 660) | 890 (20-26, 400) |
| AFP ≥ 200 (ng/mL) | 166 (33.5) | 242 (42.9) |
| Number of tumor nodule | ||
| One | 404 (81.5) | 0 |
| Two | 53 (10.7) | 204 (36.2) |
| Three | 39 (7.9) | 187 (33.1) |
| More than three | 0 | 173 (30.7) |
| Size of nodule, mean ± SD | 3.9 ± 1.8 | 7.4 ± 2.7 |
| Child-Pugh | ||
| A | 268 (54) | 261 (46.3) |
| B | 228 (46) | 303 (53.7) |
| Treatment modality | ||
| Resection | 113 (22.8) | 0 |
| Transplantation | 7 (1.4) | 0 |
| PEI | 62 (12.5) | 0 |
| RFA | 262 (52.8) | 0 |
| Microwave ablation | 18 (3.6) | 0 |
| TACE | 34 (6.9) | 564 (100) |
| Biochemical data, mean ± SD | ||
| Serum total bilirubin (mg/dL) | 1.5 ± 1.3 | 1.5 ± 1.1 |
| Serum albumin (g/dL) | 3.3 ± 0.6 | 3.3 ± 0.6 |
| International normalized ratio | 1.2 ± 0.4 | 1.2 ± 0.5 |
| ALT (IU/mL) | 63 ± 45 | 62 ± 58 |
| AST (IU/mL) | 82 ± 61 | 83 ± 80 |
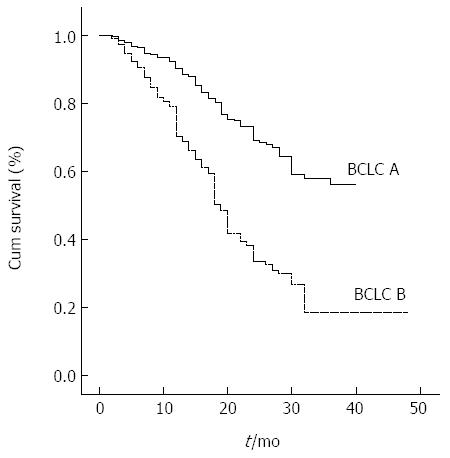
At the time the data were censored during an average follow-up of 18 mo, 123/496 (24.8%) and 218/564 (38.6%) patients had died in BCLC stage A and B groups, respectively. Overall survival according to BCLC stage, AFP level, and presence of ascites is presented in Table 2. The overall median survival after a three-year follow-up period was 37 mo [95% confidence interval (95%CI): 35-40 mo] for stage A patients and 19 mo (95%CI: 18-20 mo) for stage B patients.
| 1-yr survival | 2-yr survival | 3-yr survival | Median survival (mo) | P value | ||
| BCLC | A (n = 496) | 90% | 69% | 56% | 37 | < 0.001 |
| B (n = 564) | 69% | 33% | 18% | 19 | ||
| AFP | < 200 ng/mL (n = 652) | 85% | 59% | 50% | 33 | < 0.001 |
| ≥ 200 ng/mL (n = 408) | 71% | 41% | 22% | 21 | ||
| Ascites | Absent (n = 818) | 83% | 58% | 44% | 31 | < 0.001 |
| Present (n = 242) | 69% | 29% | 23% | 19 | ||
Univariate analysis showed that presence of ascites, ≥ 2 neoplastic nodules, maximum tumor diameter > 5 cm, AFP ≥ 200 ng/mL, and CTP class B were all significantly associated with poor survival (P < 0.001 for all) (Table 3). Multivariate analysis showed that the presence of ascites, multiple focal lesions, large tumor size, AFP level and CTP score were independent predictors of survival for the included patients (P < 0.001) (Table 4).
| Variable | BCLC A | BCLC B | |||||
| Number of patients | Median survival (mo) | P value | Number of patients | Median survival (mo) | P value | ||
| Age | < 56 | 250 | 39 | 0.590 | 259 | 19 | 0.230 |
| ≥ 56 | 246 | 37 | 305 | 18 | |||
| Sex | Male | 414 | 37 | 0.550 | 452 | 18 | 0.080 |
| Female | 82 | 39 | 112 | 23 | |||
| Ascites | Absent | 405 | 37 | < 0.001 | 413 | 2014 | < 0.001 |
| Present | 91 | 22 | 151 | ||||
| Number of tumor nodules | One | 404 | 37 | 0.003 | 0 | - | - |
| > 1 | 92 | 28 | 564 | 18 | |||
| Maximum tumor diameter | ≤ 5 cm | 454 | 37 | 0.700 | 119 | 25 | < 0.001 |
| > 5 cm | 42 | 34 | 445 | 18 | |||
| AFP (ng/mL) | < 200 | 330 | 37 | < 0.001 | 322 | 20 | < 0.001 |
| ≥ 200 | 166 | 28 | 242 | 17 | |||
| CTP class | A | 268 | 39 | < 0.001 | 261 | 24 | < 0.001 |
| B | 228 | 24 | 303 | 15 | |||
| Variable | BCLC A | BCLC B | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Number of tumor nodules > 1 | 1.82 (1.21-2.75) | 0.004 | - | - |
| Maximum tumor diameter > 5 cm | 1.02 (0.49-2.13) | 0.942 | 2.95 (1.87-4.6) | < 0.001 |
| Elevated Child score | 3.2 (2.12-4.87) | < 0.001 | 1.75 (1.27-2.42) | 0.001 |
| AFP ≥ 200 ng/mL | 1.6 (1.11-2.32) | 0.012 | 1.3 (1.01-1.74) | 0.040 |
| Presence of ascites | 1.7 (1.16-2.66) | 0.007 | 1.44 (1.03-2.04) | 0.030 |
Figure 1 illustrates the survival curves of HCC patients stratified according to the BCLC classification. The BCLC system had a χ2 of 87.3 using multivariate analysis, and the AUROC for BCLC stages A and B was 0.579 (95%CI: 0.543-0.616).
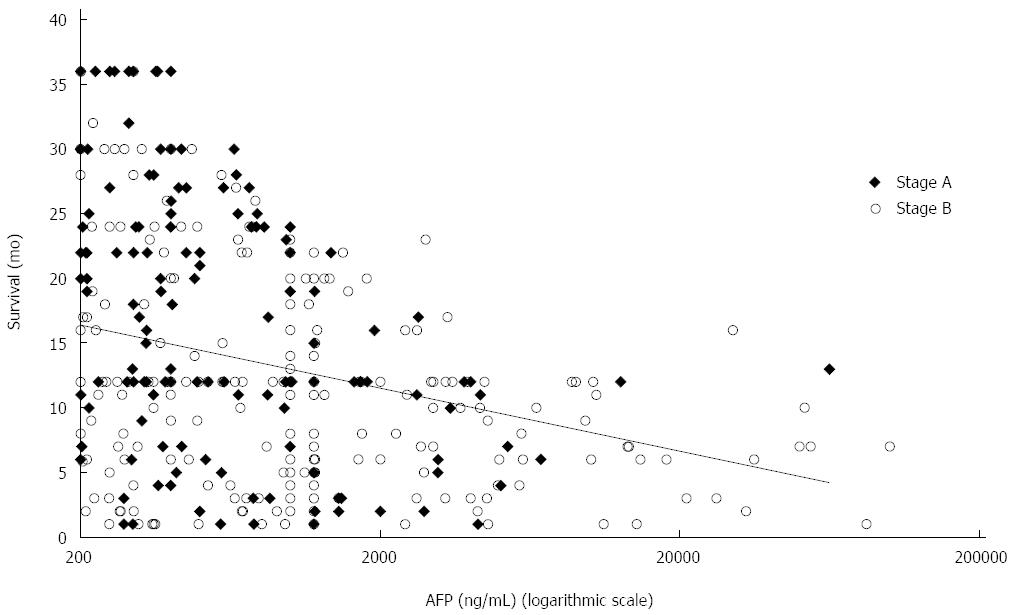
For patients with an AFP level ≥ 200 ng/mL, AFP was inversely correlated with survival (r = -0.142; P < 0.001) (Figure 2). The risk of death increased with a hazard ratio of 1.6 (95%CI: 1.11-2.32) and 1.3 (95%CI: 1.01-1.74) for BCLC A and B patients, respectively, compared to those with AFP < 200 ng/mL. For BCLC A and B patients with ascites, the risk of death increased with a hazard ratio of 1.7 (95%CI: 1.16-2.66) and 1.44 (95%CI: 1.03-2.04), respectively.
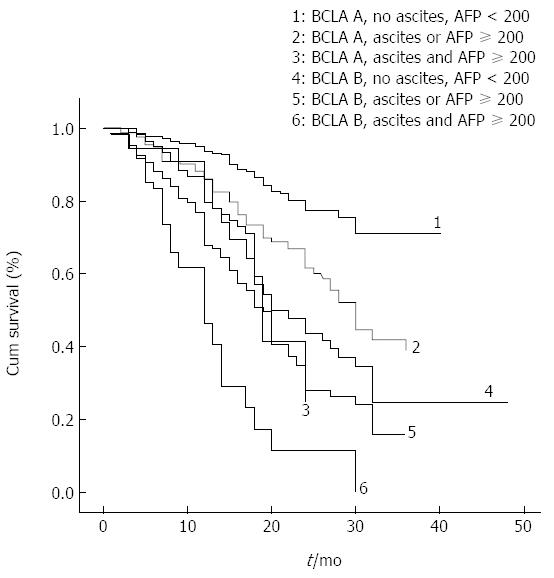
Among stage A patients, the 280 patients without ascites and with an AFP level < 200 ng/mL had a median survival of 39 mo. The 41 patients with ascites and AFP ≥ 200 ng/mL had a median survival of 19 mo. The 125 patients without ascites and with AFP ≥ 200 ng/mL had a median survival of 28 mo, and the 50 patients with ascites and AFP < 200 ng/mL had a median survival of 24 mo. The survival curves for the last two subgroups were nearly identical, and the median survival estimates were very close. The one-, two-, and three-year rates of survival for BCLC stage A patients without ascites and AFP < 200 ng/mL were 94%, 77% and 71%, and for patients with ascites and AFP ≥ 200 ng/mL were 83%, 24%, and 23%, respectively (P < 0.001).
The median survival for stage B patients with either AFP ≥ 200 ng/mL, or ascites, or both, was 19, 18, and 12 mo, respectively, compared to 22 mo in the absence of both ascites and AFP ≥ 200 ng/mL. The one-, two-, and three-year rates of survival for stage B patients without ascites and AFP < 200 ng/mL were 79%, 43% and 24%, respectively, and for those with both ascites and AFP ≥ 200 ng/mL were 46%, 15% and 7%, respectively.
Patient survival according to a modified BCLC staging system, inclusive of ascites and AFP factors, is presented in Figure 3 and Table 5. When entered into a Cox regression model, ascites and AFP added to the BCLC showed better performance in predicting overall survival compared to the BCLC, with higher homogeneity (χ2, 130 vs 87), and a greater AUROC (0.618, 95%CI: 0.583-0.654 vs 0.579, 95%CI: 0.543-0.616).
| 1-yr survival | 2-yr survival | 3-yr survival | Median survival (mo) | P value | ||
| BCLC A | No ascites, AFP < 200 ng/mL (n = 280) | 93% | 77% | 70% | 39 | < 0.001 |
| Ascites or AFP ≥ 200 ng/mL (n = 175) | 84% | 59% | 36% | 30 | ||
| Ascites, AFP ≥ 200 ng/mL (n = 41) | 83% | 23% | 23% | 19 | ||
| BCLC B | No ascites, AFP < 200 ng/mL (n = 237) | 77% | 40% | 21% | 22 | |
| Ascites or AFP ≥ 200 ng/mL (n = 261) | 67% | 27% | 15% | 18 | ||
| Ascites, AFP ≥ 200 ng/mL (n = 66) | 46% | 15% | 7% | 12 | ||
The lack of consensus concerning staging systems[19-23], and the recommendation of the EASL-EORTC (European Organisation for Research and Treatment of Cancer) for clinical or biomarker refinement of the BCLC staging system[9], necessitates improvements in classifying HCC patients to achieve better prognostic utility. Some modifications to various prognostic scores have been made, which appeared to improve prognostic stratification of the Tumor-Node-Metastasis (TNM), Japan Integrated Stage (JIS), and CLIP scores[24-26]. A simplified BCLC staging system with the inclusion of AFP was found to be more useful for stratifying 232 early HCC patients and allocating them for hepatic resection[27].
Although the presence of ascites or elevated AFP in this study did not prevent or delay referral for therapeutic interventions, the survival was affected. Only a small number of patients in this study received liver transplants, as liver transplants are only performed from living donors in Egypt, and are only partially reimbursed. Only patients younger than 60 years of age, with HCC within the Milan criteria, AFP < 1000 ng/mL, and who had a willing related donor were referred to the transplant unit for evaluation, where the donor acceptance rate is very low.
Uncontrolled or refractory ascites modifies the Child-Pugh score, and advances BCLC staging to stage D[4,11]. However, the presence of mild or minimal ascites detected clinically or radiologically changes the CTP score by only one point, and may not change the BCLC stage. Although the grading of ascites is subjective, large-volume ascites can be easily demarcated based on clinical judgment, and these patients were assigned to CTP class C and not included in these analyses. Thus, only patients who were within Child-Pugh A or B and BCLC stages A and B were investigated in this study. The results show that the presence of even minimal ascites was significantly associated with a shorter survival compared to patients with the same BCLC stage without ascites, which is consistent with previous reports[28,29].
Disadvantages of the BCLC system are that there is substantial variation in prognosis among patients within the same stage, and that it encompasses a very heterogeneous population in the intermediate stage B[30-32]. Stage B includes patients with a tumor burden that varies from four small tumors to near complete replacement of the liver. Thus, this group of patients is treated by a variety of methods, such as TACE, radioembolization, and surgical resection[33]. Recently, a sub-staging of stage B patients (from B1 to B4) has been proposed, which incorporates the tumor burden (the “up-to-seven” criteria developed for liver transplantation) and Child-Pugh score (A5 to B9)[34]. Using this strategy, the median survival was poor for patients with a higher B sub-stage with 34, 24, 15, and 12 mo for sub-stages B1, B2, B3, and B4, respectively[35].
The correlation between serum AFP and the severity of HCC has been investigated in several studies[5,28,29,36]. Our earlier study showed that an AFP ≥ 200 ng/mL was associated with shorter survival across all BCLC stages[14]. The elevated AFP level may be an indication of vascular invasion and HCC progression, and can help identify subsets of HCC patients with an increased risk for early recurrence and poor prognosis after hepatectomy[36] or transplant[37,38]. The AFP level can also be used to predict the antitumor response to radiofrequency ablation[39] and sorafenib therapy[40]. The results of the present study show that patients with early (stage A) or intermediate (stage B) HCC and AFP ≥ 200 ng/mL have shorter survival. Further analyses indicate that the addition of ascites as a factor provided better stratification of survival across different stages.
Patients with HCC and ascites have a high risk of death due to the high rate of ascites-related complications, such as spontaneous bacterial peritonitis, hyponatremia and hepatorenal syndrome. Although ascites is a parameter of the Child-Pugh classification of liver disease and is part of the BCLC staging system, patients with earlier stage HCC can have ascites. This study shows that including both the presence of ascites and AFP to the BCLC staging system increases its prediction of survival and prognostic determination ability. In addition, this modification may facilitate treatment decisions. A patient with early stage HCC with both ascites and high AFP has an expected survival similar to an intermediate stage HCC patient with either ascites or high AFP. This knowledge could help modify the management of these patients and aid in the selection of the best possible treatment. Furthermore, this addition to the BCLC staging system may aid in the proper stratification of HCC patients in controlled trials, particularly for patients with early and intermediate HCC.
Although the study included a large number of patients prospectively, no patients were in the very early stage of HCC, a result of the absence of a national screening program for early detection of HCC in patients with cirrhosis. The low accessibility to liver transplantation and the high cost of sorafenib, which is currently recommended in combination with TACE, may explain the low survival in this cohort. Furthermore, this study was conducted from patients within a single center. Although our hospital is a tertiary referral center, a referral bias cannot be ruled out.
In conclusion, our results confirm that the prognostic determination ability of the BCLC staging classification for early and intermediate HCC needs improvement, and that AFP and ascites further distinguish patients into subclasses with significantly different prognoses, who might need different classification and management. The large cohort of > 1000 patients and the rigorous follow-up validate the results, which can be generalized to patients with HCC elsewhere. Nevertheless, external validation is needed.
The Barcelona clinic liver cancer (BCLC) staging classification for hepatocellular carcinoma (HCC) is widely applied to predict prognosis and allocate treatment. However, BCLC stages A and B patients have a wide range of tumor burden and liver disease severity with Child-Turcotte-Pugh scores ≤ 9 with encephalopathy or ascites.
A current area of intense research is to assess whether the inclusion of ascites and alpha-fetoprotein (AFP) to BCLC can improve prediction of survival in stages A and B patients.
BCLC is a widely accepted staging system for the prediction of prognosis. AFP level is an independent predictor of prognosis among various populations of HCC patients, and ascites is an important prognostic determinant for mortality. In this study, the inclusion of ascites and AFP level prior to treatment (which are not individual components of the original BCLC system) to variables included in BCLC staging increases its prognostic significance and further discriminates patients within early and intermediate stages with very different prognoses.
The study results help to describe a subgroup of patients who might require different classification and management.
BCLC is the recommended staging system in the practice guidelines of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases.
This is an interesting study in which the authors demonstrate that adding ascites and AFP to BCLC can improve prediction of survival and prognosis in stages A and B HCC patients.
| 1. | WHO: International Agency for Research on Cancer: PRESS RELEASE N° 224. 2014 Feb 3; Lyon/London. Available from: http://www.iarc.fr/en/media-centre/pr/2014/pdfs/pr224_E.pdf. Accessed August 5, 2014. |
| 2. | Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010;15 Suppl 4:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 3. | Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 4. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2916] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 5. | Chen TW, Chu CM, Yu JC, Chen CJ, Chan DC, Liu YC, Hsieh CB. Comparison of clinical staging systems in predicting survival of hepatocellular carcinoma patients receiving major or minor hepatectomy. Eur J Surg Oncol. 2007;33:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 6. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 334] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 7. | Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D’Amico F, Ciarleglio FA, Boccagni P, Brolese A. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Guglielmi A, Ruzzenente A, Pachera S, Valdegamberi A, Sandri M, D’Onofrio M, Iacono C. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol. 2008;103:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 10. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6630] [Article Influence: 442.0] [Reference Citation Analysis (1)] |
| 11. | Child CG, Turcotte JG. Surgery and portal hypertension. Edited by CG Child. Philadelphia: Saunders 1964; 50-64. |
| 12. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Dumitra TC, Dumitra S, Metrakos PP, Barkun JS, Chaudhury P, Deschênes M, Paraskevas S, Hassanain M, Tchervenkov JI. Pretransplantation α-fetoprotein slope and milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation. 2013;95:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 14. | Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One. 2014;9:e90929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4519] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 16. | Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31472] [Article Influence: 462.8] [Reference Citation Analysis (0)] |
| 17. | Cox DR. Regression models and life tables. J R Stat Soc. 1974;34:187-220. |
| 18. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13773] [Cited by in RCA: 12584] [Article Influence: 286.0] [Reference Citation Analysis (1)] |
| 19. | Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L, Barbare JC. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY, Park YO, Kim WT, Byun JH. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, Minami Y, Ueshima K, Fukunaga T, Matsunaga T. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | op den Winkel M, Nagel D, Sappl J, op den Winkel P, Lamerz R, Zech CJ, Straub G, Nickel T, Rentsch M, Stieber P. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS One. 2012;7:e45066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, Hokotate H, Inoue H, Baba Y, Imamura Y. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395-403. [PubMed] |
| 26. | Huo TI, Huang YH, Lin HC, Wu JC, Chiang JH, Lee PC, Chang FY, Lee SD. Proposal of a modified Cancer of the Liver Italian Program staging system based on the model for end-stage liver disease for patients with hepatocellular carcinoma undergoing loco-regional therapy. Am J Gastroenterol. 2006;101:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Santambrogio R, Salceda J, Costa M, Kluger MD, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. External validation of a simplified BCLC staging system for early hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Liem MS, Poon RT, Lo CM, Tso WK, Fan ST. Outcome of transarterial chemoembolization in patients with inoperable hepatocellular carcinoma eligible for radiofrequency ablation. World J Gastroenterol. 2005;11:4465-4471. [PubMed] |
| 30. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 641] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 31. | Vitale A, Navaglia F, Ramírez Morales R, Frigo AC, Basso D, D’Amico F, Zanus G, Bonsignore P, Farinati F, Burra P. Molecular refinement of clinical staging in hepatocellular carcinoma patients evaluated for potentially curative therapies. PLoS One. 2011;6:e23093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, Larson JJ, Pedersen RA, Therneau TM, Gores GJ. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Radu P, Groza I, Iancu C, Al Hajjar N, Andreica V, Sparchez Z. Treatment of hepatocellular carcinoma in a tertiary Romanian center. Deviations from BCLC recommendations and influence on survival rate. J Gastrointestin Liver Dis. 2013;22:291-297. [PubMed] |
| 34. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 35. | Piscaglia F, Pecorelli A, VenerandiL , Farinati F, Del Poggio P, RapacciniG . Clinical validation of a sub-staging proposal of patients with intermediate HCC (BCLC-B). J Hepatol. 2013;58:S45-S61. |
| 36. | Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50. [PubMed] |
| 37. | Ravaioli M, Ercolani G, Cescon M, Vetrone G, Voci C, Grigioni WF, D’Errico A, Ballardini G, Cavallari A, Grazi GL. Liver transplantation for hepatocellular carcinoma: further considerations on selection criteria. Liver Transpl. 2004;10:1195-1202. [PubMed] |
| 38. | Yaprak O, Akyildiz M, Dayangac M, Demirbas BT, Guler N, Dogusoy GB, Yuzer Y, Tokat Y. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:256-261. [PubMed] |
| 39. | Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, Wu JC, Huo TI, Huang YH, Wu WC, Lin HC. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy. Clin Radiol. 2012;67:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, Tamaki S, Kato T, Yasui Y, Hosokawa T. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ling CQ, Shen SQ S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM