Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5482
Peer-review started: November 26, 2014
First decision: December 26, 2014
Revised: January 22, 2015
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: May 14, 2015
Processing time: 173 Days and 15.2 Hours
AIM: To explore hemodynamics and vasoactive substance levels during renal vein congestion that occurs in the anhepatic phase of liver transplantation.
METHODS: New Zealand rabbits received ligation of the hepatic pedicle, supra-hepatic vena cava and infra-hepatic vena cava [anhepatic phase group (APH); n = 8], the renal veins (RVL; n = 8), renal veins and hepatic pedicle [with the inferior vena cava left open) (RVHP; n = 8)], or a sham operation (SOP; n = 8). Hemodynamic parameters (systolic, diastolic, and mean arterial blood pressures) and the levels of serum bradykinin (BK) and angiotensin II (ANGII) were measured at baseline (0 min), and 10 min, 20 min, 30 min, and 45 min after the surgery. Correlation analyses were performed to evaluate the associations between hemodynamic parameters and levels of vasoactive substances.
RESULTS: All experimental groups (APH, RVL, and RVHP) showed significant decreases in hemodynamic parameters (systolic, diastolic, and mean arterial blood pressures) compared to baseline levels, as well as compared to the SOP controls (P < 0.05 for all). In contrast, BK levels were significantly increased compared to baseline in the APH, RVL, and RVHP groups at all time points measured (P < 0.05 for all), whereas no change was observed in the SOP controls. There were no significant differences among the experimental groups for any measure at any time point. Further analyses revealed that systolic, diastolic, and mean arterial blood pressures were all negatively correlated with BK levels, and positively correlated with ANGII levels in the APH, RVL, and RVHP groups (P < 0.05 for all).
CONCLUSION: In the anhepatic phase of orthotopic liver transplantation, renal vein congestion significantly impacts hemodynamic parameters, which correlate with serum BK and ANGII levels.
Core tip: Hemodynamic disorders remain a focus of concern in the anhepatic phase of orthotopic liver transplantation as they contribute to procedural morbidity and mortality. This study shows that various procedures that cause renal vein congestion significantly reduce hemodynamic measures, which correlate with reduced angiotensin II and increased bradykinin levels in serum.
- Citation: Li ZX, Wang MC, Zhang YC, Mao J, Chen M, Ni R, Wei FX, Wang GN, Zhang LY. Hemodynamics and vasoactive substance levels during renal congestion that occurs in the anhepatic phase of liver transplantation. World J Gastroenterol 2015; 21(18): 5482-5487
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5482
Although orthotopic liver transplantation (OLT) has become the optimal treatment for end-stage liver diseases[1], this procedure is associated with substantial morbidity and mortality[2]. Of primary concern is the development of hemodynamic disorders, which can determine the success of OLT[3]. Many factors can contribute to this hemodynamic instability, including acidosis, and electrolyte and coagulation abnormalities[4], as well as blood loss and ischemia/reperfusion syndrome[5]. Massive liquid infusion does not maintain hemodynamic stability[6], but may increase the incidence of heart failure[7] and kidney injury[2]. Intraoperative hemodialysis can safely be performed to reduce ischemia/reperfusion syndrome and improve the outcomes of OLT, though it does not improve hemodynamics, and complications still occur[8,9].
As a key organ for the regulation of systemic circulation, the kidney may play an important role in hemodynamic stabilization during OLT. Interruption of the inferior vena cava and portal vein during the procedure may lead to renal vein congestion and contribute to the hypodynamic state. Furthermore, peripheral vascular resistance, which typically rises when blood volume is reduced, does not increase during the anhepatic phase of OLT[10], indicating that additional elements are likely involved. Therefore, vasoactive substances, such as bradykinin (BK)[11] and angiotensin II (ANGII)[12], which can control the relaxation and constriction activity of blood vessels, may be important factors contributing to the observed hemodynamic instability. To investigate this, hemodynamic parameters and serum BK and ANGII concentrations were measured during renal vein congestion which occurs in the anhepatic phase of OLT.
Thirty-two male New Zealand rabbits (2.5 ± 0.2 kg, 10 wk) were obtained from the animal center of Lanzhou University, Gansu, China, and individually housed under a 12-h light-dark cycle at least one week prior to the study. Animals were fasted for 12 h and deprived of water 4 h prior to the operation. The experiment was carried out in accordance with the ARRIVE guidelines for animal studies[13], and was approved by the Ethics Committee of the Lanzhou University Second Hospital. At the end of the study, all the animals were sacrificed using diethyl ether.
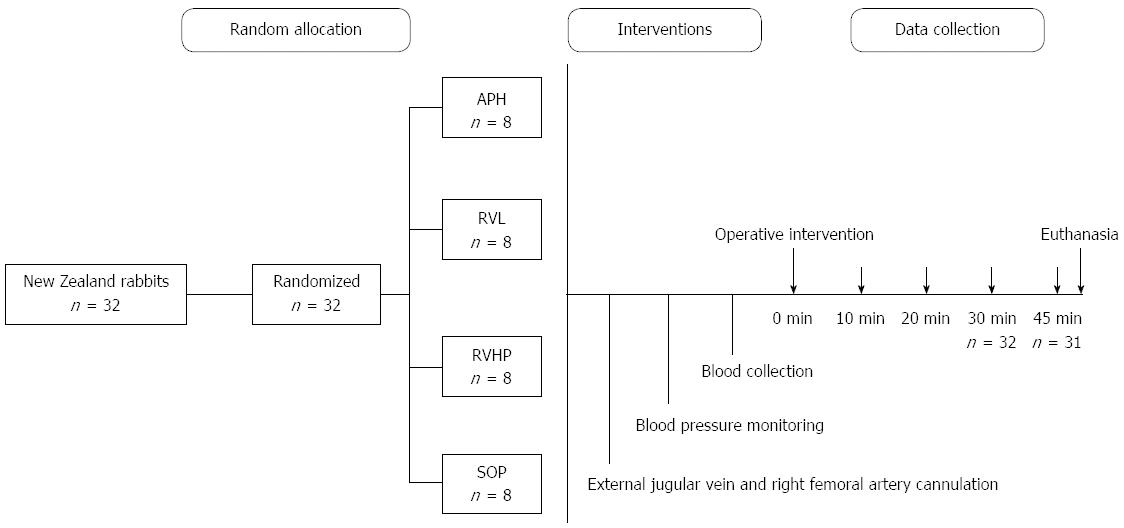
Animals were randomly allocated into four groups (n = 8 each): an anhepatic phase group (APH); a renal vein ligation group (RVL); a renal vein and hepatic pedicle ligation group (RVHP); and a sham operation (SOP) group. All four procedures were performed on a given day, in a random sequence, to eliminate any effect of training by the experimenters.
The rabbits were anesthetized with chloral hydrate (0.2 g/kg) by an i.v. injection in the ear, and catheters were inserted through the external jugular vein to collect blood samples and the right femoral artery for blood pressure monitoring. A large median laparotomy was performed after disinfecting the area with 0.5% iodine. For animals in the APH group, the hepatic pedicle, supra-hepatic vena cava, and infra-hepatic vena cava were dissected and ligated[14-16]. Animals in the RVHP group received dissection and ligation of the hepatic pedicle, supra-hepatic vena cava and renal veins, leaving the inferior vena cava open. Only the renal veins were ligated in the RVL group. The hepatic pedicles, and supra- and infra-hepatic vena cavae of rabbits were all dissected but not ligated in the SOP group. Data were collected at five time points during the procedures (0 min, 10 min, 20 min, 30 min, and 45 min) (Figure 1).
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MABP) were monitored continuously with a multichannel physiologic recorder (RM 6000; Datex-Ohmeda, Madison, WI, United State) through an arterial cannula in the right femoral artery[17,18].
Blood samples collected from the jugular vein were centrifuged, and the serum was stored at -80 °C. Serum concentrations of BK and ANGII were determined using enzyme-linked immunosorbent assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Groups were compared using two-way analyses of variance followed by a Bonferroni-Dunn or Tukey’s post-hoc test. Associations between hemodynamic parameters and serum vasoactive substance concentrations were analyzed by Spearman correlation analysis. Data are shown as mean ± SD, and P < 0.05 was considered statistically significant. All of the analyses were performed using SPSS software, version 18 (SPSS Inc., Chicago, IL, United States). The statistical methods of this study were reviewed by Hu XB from Lanzhou University School of Public Health.
The surgical operations were successfully carried out in all animals. The mean survival time was 50.7 ± 4.6 min, and only one animal in the RVHP group died prior to completion of the study. The baseline hemodynamic parameters and vasoactive substance concentrations did not differ among the groups (Table 1).
| Characteristic | APH | RVL | RVHP | SOP |
| SBP, mmHg | 112.9 ± 2.2 | 109.7 ± 1.9 | 112.6 ± 2.5 | 112.9 ± 2.1 |
| DBP, mmHg | 86.9 ± 1.9 | 83.6 ± 1.7 | 87.5 ± 2.2 | 87.3 ± 2.4 |
| MABP, mmHg | 95.4 ± 2.3 | 91.4 ± 1.5 | 95.7 ± 2.4 | 95.8 ± 2.4 |
| BK, mg/L | 11.92 ± 4.06 | 12.90 ± 4.84 | 14.07 ± 3.7 | 14.95 ± 2.48 |
| ANGII, mg/L | 399.38 ± 132.43 | 416.86 ± 81.59 | 460.63 ± 111.46 | 435.00 ± 71.26 |
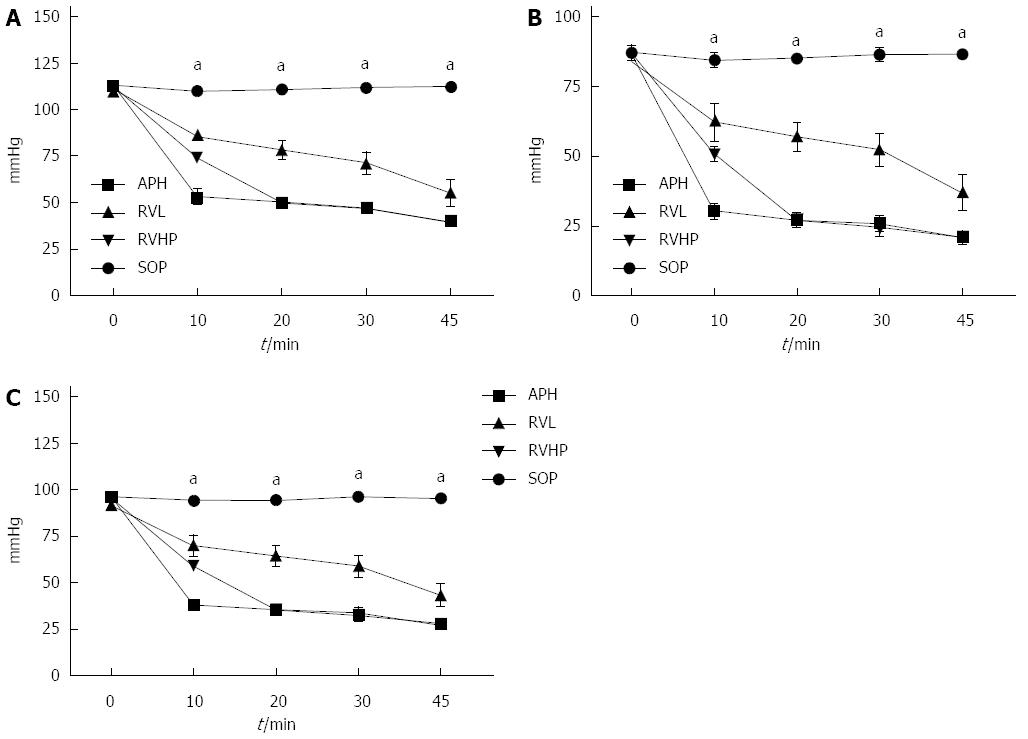
The changes in hemodynamic parameters (SBP, DBP, and MABP) are shown in Figure 2. There were no changes in any blood pressure measures in the SOP group throughout the procedure. However, all hemodynamic parameters were significantly decreased in the APH, RVL, and RVHP groups compared with baseline (0 min) (P < 0.05 for all). Moreover, SBP, DBP, and MABP values were significantly different from the SOP group at 10 min, 20 min, 30 min, and 45 min (P < 0.05 for all). Although values in the RVL group were higher than those in the APH and RVHP groups, the differences were not significant.
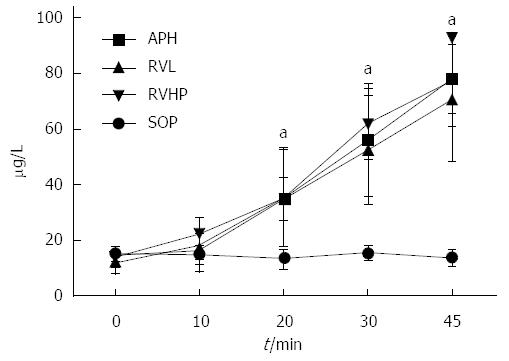
Serum BK levels did not change over the course of the procedure in the SOP group (Figure 3). In contrast, serum BK levels at 10 min, 20 min, 30 min, and 45 min were significantly higher in the APH, RVL, and RVHP groups compared to baseline value (P < 0.05 for all). Furthermore, these BK levels were significantly increased compared to SOP controls at 20 min, 30 min, and 45 min (P < 0.05 for all).
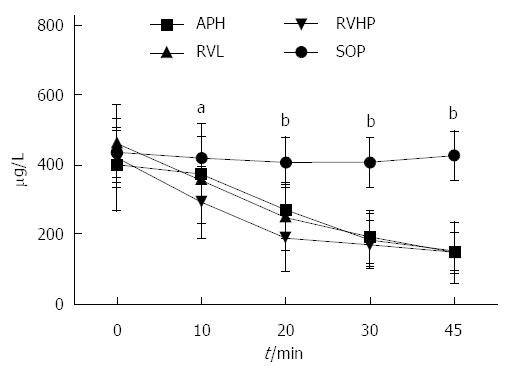
Serum ANGII levels did not change over the course of the procedure in the SOP group (Figure 4). In contrast, serum ANGII levels at 10 min, 20 min, 30 min, and 45 min were significantly lower in the APH, RVL, and RVHP groups compared to baseline value (P < 0.05 for all). Furthermore, these ANGII levels were significantly decreased compared to SOP controls at all postoperative time points (P < 0.05 for all).
Spearman correlation analyses revealed that BK levels were negatively correlated with SBP, DBP, and MABP in all experimental groups (P < 0.05 for all) (Table 2). In contrast, ANGII levels in the APH, RVL, and RVHP groups were positively correlated with all three hemodynamic measures (P < 0.05 for all).
| Blood pressure | BK, r | ANGII, r | ||||||
| 10 min | 20 min | 30 min | 45 min | 10 min | 20 min | 30 min | 45 min | |
| SBP | -0.74 | -0.72 | -0.69 | -0.72 | 0.73 | 0.69 | 0.70 | 0.52 |
| DBP | -0.70 | -0.68 | -0.71 | -0.71 | 0.78 | 0.74 | 0.75 | 0.70 |
| MABP | -0.72 | -0.61 | -0.70 | -0.69 | 0.76 | 0.68 | 0.59 | 0.69 |
The renin-angiotensin-aldosterone system is an important regulator of blood pressure. ANGII is a member of this system that can constrict vascular smooth muscle and can increase blood pressure by activating the angiotensin receptor[12]. Renin, an acidic protein synthesized and secreted by renal cells near the glomerulus and released through the renal vein into systemic circulation[19], converts angiotensinogen synthesized and released by the liver cells into ANGI[20]. ANGI is subsequently converted to ANGII by angiotensin-converting enzyme, which simultaneously inactivates BK, a vasodilator of the kinin family of polypeptides. BK can relax vascular smooth muscle and decrease blood pressure by enhancing the synthesis and release of prostacyclin, nitrogen monoxide, and endothelium source hyperpolarization factor[10]. The results of this study show that renal congestion affects these vasoactive peptides in a way that correlates with hemodynamic changes.
The kidney is an important contributor to these vasoregulatory systems[21]. In the anhepatic phase of orthotopic liver transplantation, renal vein congestion via interruption of the inferior vena cava induces high blood pressure. At the same time, the reduced concentration of angiotensinogen and lack of renin secretion prevent synthesis of ANGI, which can lead to the reduction in systemic ANGII. However, the renin-angiotensin system is also active in myocardial and coronary arterial endothelial cells[22]. Therefore, ANGII may not necessarily be completely depleted from systemic circulation during the anhepatic phase of OLT.
Concentrations of serum BK are typically very low under normal conditions[23]. However, even low amounts of BK can appreciably relax systemic small arteries and reduce peripheral vascular resistance, while increasing vascular permeability and reducing blood pressure[24]. In the present study, a large amount of BK was released with hypotension as a result of renal vein congestion. The release of BK could also promote the vasodilation by breaking the K/Ca2+ channel[25,26].
The results from this study indicate that balance of the kallikrein-kinin and renin-angiotensin-aldosterone systems was destroyed in all experimental groups. Thus, the renal vein congestion produced by the ligation of inferior cavae or renal veins had the same effect. Although the volume of venous blood returning to the heart in the RVL group was more than that in the RVHP group, there were no differences in blood pressure or the levels of vasoactive peptides (BK and ANGII). Thus, we surmise that the hemodynamic disorders in the anhepatic phase of OLT are not solely due to a decrease in blood returning to the heart, but also results from the renal vein congestion.
In conclusion, the present study shows that renal vein congestion significantly impacts hemodynamic stability, and likely contributes to hemodynamic disorders in the anhepatic phase of OLT. The mechanisms may be closely related to the changes in serum BK and ANGII levels.
Although orthotopic liver transplantation (OLT) has become the optimal treatment for end-stage liver diseases, it is still associated with substantial morbidity and mortality. Hemodynamic disorders are of primary concern, as they can determine the success of OLT.
Many factors can contribute to hemodynamic instability in OLT. Blood loss and ischemia/reperfusion syndrome are the most common factors, though major fluid shifts, acidosis, and electrolyte and coagulation abnormalities also contribute. Despite efforts to address these factors, hemodynamics has not been improved and complications remain high.
Although the reduction of blood volume typically increases peripheral vascular resistance, this is not observed during the anhepatic phase of OLT. Therefore, additional elements, such as vasoactive substances, may be involved in the pathogenesis of hemodynamic disorders in these patients. This study shows that renal vein congestion significant impacts hemodynamics, possibly through mechanism involving changes in serum levels of bradykinin (BK) and angiotensin II (ANGII).
Hemodynamic disorders in the anhepatic phase of OLT are not solely due to a decrease in vein blood volume returning to the heart, but may involve renal vein congestion. Serum BK and ANGII levels may be indicators of postoperative complications in OLT.
The kallikrein-kinin and renin-angiotensin-aldosterone systems play a vital role in regulating body blood pressure, and BK and ANGII are the key regulating factors in the two systems. BK is a potent vasodilator, and ANGII is a vasoconstrictor, which have an antagonistic effect on each other. When angiotensin-converting enzyme transforms ANGI into ANGII, it simultaneously inactivates BK.
This is an experimental study in 32 rabbits regarding the effect of renal vein congestion on hemodynamics and vasoactive substances in the anhepatic phase of OLT. The authors found significant hemodynamic differences in the OLT models compared with the sham group due to alterations of BK and ANGII. It provides a new avenue for the prevention of postoperative complications in OLT.
| 1. | Feng AC, Fan HL, Chen TW, Hsieh CB. Hepatic hemodynamic changes during liver transplantation: a review. World J Gastroenterol. 2014;20:11131-11141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 2. | Klaus F, Keitel da Silva C, Meinerz G, Carvalho LM, Goldani JC, Cantisani G, Zanotelli ML, Duro Garcia V, Keitel E. Acute kidney injury after liver transplantation: incidence and mortality. Transplant Proc. 2014;46:1819-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Ogura Y, Hori T, El Moghazy WM, Yoshizawa A, Oike F, Mori A, Kaido T, Takada Y, Uemoto S. Portal pressure & lt; 15 mm Hg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl. 2010;16:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Capela T, Tavares I, Pereira P, Vigia E, Perdigoto R, Barroso E, Marcelino P. Is there a relationship between intraoperative hemodynamic instability and calcineurin inhibitor-related toxicity, early after liver transplantation? A single-center observational study. Transplant Proc. 2014;46:1789-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Glauser FL. Systemic hemodynamic and cardiac function changes in patients undergoing orthotopic liver transplantation. Chest. 1990;98:1210-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 6. | Massicotte L, Perrault MA, Denault AY, Klinck JR, Beaulieu D, Roy JD, Thibeault L, Roy A, McCormack M, Karakiewicz P. Effects of phlebotomy and phenylephrine infusion on portal venous pressure and systemic hemodynamics during liver transplantation. Transplantation. 2010;89:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Qureshi W, Mittal C, Ahmad U, Alirhayim Z, Hassan S, Qureshi S, Khalid F. Clinical predictors of post-liver transplant new-onset heart failure. Liver Transpl. 2013;19:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Nadim MK, Annanthapanyasut W, Matsuoka L, Appachu K, Boyajian M, Ji L, Sedra A, Genyk YS. Intraoperative hemodialysis during liver transplantation: a decade of experience. Liver Transpl. 2014;20:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Nicolau-Raducu R, Gitman M, Ganier D, Loss GE, Cohen AJ, Patel H, Girgrah N, Sekar K, Nossaman B. Adverse cardiac events after orthotopic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transpl. 2015;21:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Li SR, Shen N, Yi HM, Gan XL, Hei ZQ. [Changes of systemic and pulmonary hemodynamics and plasma levels of inducible nitric oxide synthase and endothelin-1 in patients with hepatopulmonary syndrome]. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:2030-2032. [PubMed] |
| 11. | Sharma JN. Basic and clinical aspects of bradykinin receptor antagonists. Prog Drug Res. 2014;69:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Faria-Costa G, Leite-Moreira A, Henriques-Coelho T. Cardiovascular effects of the angiotensin type 2 receptor. Rev Port Cardiol. 2014;33:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Nagai K, Yagi S, Uemoto S, Tolba RH. Surgical procedures for a rat model of partial orthotopic liver transplantation with hepatic arterial reconstruction. J Vis Exp. 2013;e4376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Hori T, Gardner LB, Chen F, Baine AM, Hata T, Herdt AR, Uemoto S, Eckman CB, Nguyen JH. Hepatic arterial reconstruction for orthotopic liver transplantation in the rat. J Surg Res. 2012;178:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Hori T, Nguyen JH, Zhao X, Ogura Y, Hata T, Yagi S, Chen F, Baine AM, Ohashi N, Eckman CB. Comprehensive and innovative techniques for liver transplantation in rats: a surgical guide. World J Gastroenterol. 2010;16:3120-3132. [PubMed] |
| 17. | Shin BS, Kim GS, Ko JS, Gwak MS, Yang M, Kim CS, Hahm TS, Lee SK. Comparison of femoral arterial blood pressure with radial arterial blood pressure and noninvasive upper arm blood pressure in the reperfusion period during liver transplantation. Transplant Proc. 2007;39:1326-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Shin YH, Kim HY, Kim YR, Yoon JS, Ko JS, Gwak MS, Kim GS, Lee SK. The comparison of femoral and radial arterial blood pressures during pediatric liver transplantation. Transplant Proc. 2013;45:1924-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Stefańska A, Péault B, Mullins JJ. Renal pericytes: multifunctional cells of the kidneys. Pflugers Arch. 2013;465:767-773. [PubMed] |
| 20. | Erdös EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension. 2010;55:214-220. [PubMed] |
| 21. | Biggi A, Musiari L, Iori M, De Iaco G, Magnani G, Pelloni I, Pinelli S, Pelà GM, Novarini A, Cabassi A. Contribution of bradykinin B2 receptors to the inhibition by valsartan of systemic and renal effects of exogenous angiotensin II in salt-repleted humans. J Pharmacol Exp Ther. 2010;334:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Castro-Chaves P, Cerqueira R, Pintalhao M, Leite-Moreira AF. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Expert Opin Ther Targets. 2010;14:485-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost. 2013;110:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Golias Ch, Charalabopoulos A, Stagikas D, Charalabopoulos K, Batistatou A. The kinin system--bradykinin: biological effects and clinical implications. Multiple role of the kinin system--bradykinin. Hippokratia. 2007;11:124-128. [PubMed] |
| 25. | Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ Res. 1998;83:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Quilley J, Qiu Y. K(+)-induced vasodilation in the rat kidney is dependent on the endothelium and activation of K+ channels. Eur J Pharmacol. 2005;508:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fourtounas C S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S