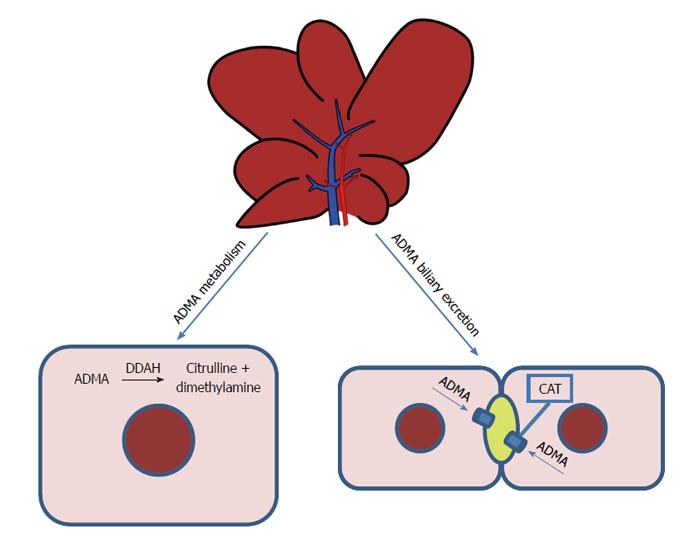
SYNTHESIS, METABOLISM AND EXCRETION OF ADMA
In the early 1990s, Vallance and colleagues, showed that nitric oxide (NO) synthesis could be inhibited by the endogenous circulating amino-acid asymmetric dimethylarginine (ADMA) by inhibition of NO-synthase (NOS)[1]. ADMA competes with L-arginine for each of the three isoforms of NOS, endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) NOS[2]. ADMA is considered to be an important marker of endothelial dysfunction because of its inhibiting role in NO synthesis. In addition, ADMA is also able to inhibit NO synthesis by competing with arginine and symmetric dimethylarginine (SDMA) for cellular transport across cationic amino-acid transporters (CATs). Interestingly, the liver expresses CATs abundantly, especially CAT-2A and CAT-2B, suggesting a higher uptake of ADMA in this organ as compared with the heart, lungs and kidneys[3]. The CAT-2B are low-capacity transporters that have a high affinity for cationic amino acids and in particular present high affinity for ADMA[4]. In contrast, CAT-2A, an alternate splice variant of CAT-2B, possesses low affinity but high transport capacity.
The first step in the synthesis of methylarginines, is the methylation of protein arginine residues by intracellular enzymes termed protein methyltransferases (PRMTs). The second step relates to the proteolytic degradation of the methylated protein which produces free ADMA and SDMA, the latter not biologically active[5]. Protein synthesis and proteolysis are anabolic and catabolic counterparts of protein turnover, respectively[6].
Intracellular ADMA is metabolized to citrulline and dimethylamine, a reaction catalyzed by dimethylarginine dimethylaminohydrolase (DDAH)[7] (Figure 1). The liver and kidneys represent the main sites of ADMA metabolism: DDAH is widely distributed in rats and human subjects, in particular, in the liver, kidney and pancreas[8,9]. This enzyme is very sensitive to oxidative stress because its active site contains a critical sulfhydryl group required for its catalytic activity[10]. Two isoforms of DDAH, Types 1 and 2, have emerged as critical regulators of NO bioavailability[11]. Studies of gene silencing or deletion in rodents have led to the conclusion that plasma levels of ADMA are regulated by DDAH-1, whereas the significance of DDAH-2 lies in preserving the endothelial function[11]. Nijveldt et al[12] provide a detailed insight into the liver’s handling of ADMA, demonstrating that it plays a crucial role in ADMA metabolism, with DDAH taking up a large amount of this dimethylarginine from the circulatory system.
Figure 1 Metabolism and excretion of asymmetric dimethylarginine in the liver.
Intracellular asymmetric dimethylarginine (ADMA) is metabolized to citrulline and dimethylamine, a reaction catalyzed by dimethylarginine dimethylaminohydrolase (DDAH). The liver is responsible for the biliary excretion of ADMA. CAT: Cationic amino-acid transporters.
The kidney plays an important role in ADMA excretion from the body, since ADMA is found in human urine[13]. Recently, we demonstrated that the liver, too, is responsible for the biliary excretion of ADMA (Figure 1): for the first time, this methylarginine was found in bile and a time-dependent increase in biliary excretion was shown to occur during I/R injury[14].
Understanding the mechanisms involved in ADMA synthesis, metabolism and excretion increases the possibility of understanding its modulation, which is crucial in several pathological conditions[15-17].
ADMA AND LIVER DISEASES
Although ADMA has been shown to correlate with cardiovascular risk factors[17], its plasma concentration also increases in patients suffering from end-stage kidney disease[1] and hepatic dysfunction[15]. In particular, a significant correlation has been demonstrated between plasma ADMA levels and the degree of hepatic dysfunction in patients suffering from liver diseases with varying aetiologies[18-20]. Notably, plasma ADMA levels are increased in patients with liver cirrhosis[20], alcoholic hepatitis[21] and acute liver failure[15]. Patients with decompensated cirrhosis have higher ADMA levels compared to compensated cirrhosis; these levels increase further with evolving liver failure[21]. An increase in ADMA plasma levels also occurs in patients with alcoholic cirrhosis associated with an increase in nitrate-nitrite concentrations. This event was probably caused by impaired hepatic removal and, in the cirrhotic liver, may be a prominent factor determining high intrahepatic vascular resistance and the progression of the disease[20].
The high portal pressures observed in alcoholic hepatitis patients were associated with the increased ADMA, which may result from both decreased breakdown (decreased hepatic DDAH) and/or increased production[21]. The mechanism by which liver dysfunction results in raised ADMA concentrations is probably due to impaired DDAH activity, which is highly expressed in normal livers: local processes such as severe inflammation, oxidative stress, and direct damage to DDAH protein may underlie a significant deterioration in DDAH activity in critically ill patients with hepatic dysfunction leading to elevation of ADMA concentrations[22]. High ADMA plasma concentrations may serve as important biological markers of adverse outcomes in alcoholic hepatitis[21].
In support of a crucial role for the liver as an ADMA eliminating organ, its concentration, elevated during hepatic failure, undergoes significant decline on the first postoperative day after liver transplantation, suggesting that DDAH activity is preserved during the transplantation procedure[23]. The hepatic function contributes to ADMA regulation as evidenced by an initial small increase in ADMA during the anhepatic phase of the transplant operation, consistent with complete absence of hepatic DDAH activity[15]. Following restoration of portal and hepatic arterial flow with a new graft placement, there is a significant reduction in ADMA levels, in an environment with reduced inflammatory drive[15]. Notably, in the 85% of patients who rejected the liver graft, a clear increase in ADMA concentrations preceded the onset of the first episode of rejection[23]. Correlation between methylarginine derivates and liver function and survival after liver transplantation was also observed[24].
Furthermore, Mookerjee et al[15] have reported that patients with acute liver failure showed elevated ADMA levels also related to the severity of inflammation supporting the hypothesis that proinflammatory cytokines may regulate ADMA metabolism. Recent results have demonstrated that plasma ADMA evaluation appears to be an early predictor for survival in patients with sepsis associated to acute liver failure[25].
The hepatic I/R injury induced changes on the ADMA/DDAH pathway; consequently, this pathway should be considered as a point of interest potentially capable of reducing the effects of I/R. In particular, the decrease in DDAH-1 activity observed after hepatic I/R is associated with a reduction in mRNA and protein expression and an increase in serum ADMA levels in the early reperfusion period (1 h)[14]. Recent data in rat liver subjected to I/R has confirmed a decrease in DDAH activity[26]. Lanteri et al[27] observed an increase in DDAH-1 expression when the tissue DDAH-1 was evaluated after three hours’ reperfusion.
The serum concentration of ADMA is negatively associated with DDAH activity in the liver[28]. In addition, we observed a decrease in intracellular ADMA levels during reperfusion, together with ADMA release both in the circulation and bile[14].
Increases in serum ADMA levels were also detected two weeks after experimental BDL[29,30], while on the contrary in a period close to BDL-induced damage, no increase in serum ADMA levels was found, even though a tissue increase in ADMA occurred in the days immediately after BDL[31]. In addition, ADMA is significantly higher in the right and median lobes as compared with the left lobe; this heterogeneity is probably associated with a decrease in CAT-2 transporters, particularly evident in RL and ML when compared with the respective sham-operated group[31]. In addition, a decrease in CAT-2 transporters associated with an increase in tissue ADMA was also described 2 wk after BDL[29].
The mechanism by which ADMA causes increased risk of adverse outcome in critically ill patients is probably by inhibition of endothelial nitric oxide elaboration. High ADMA concentrations block NOS[1] and inhibit endothelium-dependent vasodilation in both animals[32] and human beings[33]. The crucial role of the liver can be explained by the high expression of the ADMA-degrading enzyme DDAH, which makes the liver a prime organ in the clearing of ADMA[22]. Interestingly, liver abundantly also express CATs, especially CAT-2A and CAT-2B, and the extensive hepatic expression of CAT-2A mRNA suggests a higher uptake of ADMA in this organ as compared with the heart, lungs and kidneys[3]. Accordingly, the occurrence of liver dysfunction in critical illness places other organs at risk, especially organs that are strongly dependent on basal nitric oxide production[22].
MULTIPLE ORGAN FAILURE AND ADMA
Multi organ failure (MOF) is the concurrent dysfunction of several organs. It is considered the most challenging problem in Intensive Care Unit (ICU) patients: in patients with MOF, the mortality becomes higher, ranging from 30%-80% depending on the number of failed organs[34]. Sepsis and severe trauma are considered the main predisposing factors for the development of MOF. A pivotal role is traditionally attributed to the kidneys and liver in MOF development; however a unifying mechanism has been recently proposed by Nijveldt et al[18], currently known as the ADMA-MOF hypothesis. The hypothesis came into being after a Phase III trial, when the unspecific NOS inhibitor NG-monomethylarginine was shown to increase mortality rates in patients with septic shock[35]. In this trial, the use of the NOS inhibitor was mainly intended to reduce the production of excessive amounts of NO: due to the physiopathological roles of this molecule, NO was assumed to have a role in the deterioration of septic patients, raising the suggestion that NOS inhibitors may have a therapeutic potential.
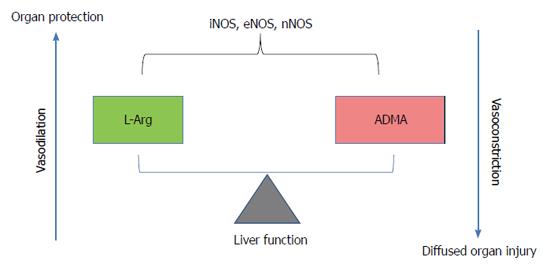
The three known isoforms of NOS are iNOS, nNOS and eNOS. iNOS expression can be induced by bacterial lipopolysaccharide, cytokines, and other agents. When induced in macrophages, iNOS produces large amounts of NO, which represents these cells’ major weapon due to its cytostatic and cytotoxic effects against parasitic micro-organisms and certain tumours[36]. The overproduction of NO in response to septic shock may harm healthy cells when NO is released in the wrong site. This is because cell and tissue damage is attributable to the NO radical itself or to the peroxynitrite ONOO-, whose production arises from the interaction between NO and O2-•[36]. nNOS is constitutively expressed in central and peripheral neurons; its functions include central regulation of blood pressure, smooth muscle relaxation and vasodilatation via peripheral nitrergic nerves[37] (Figure 2). eNOS, due to its localization, is a homeostatic regulator of blood pressure and blood flow, vascular smooth-muscle proliferation, platelet aggregation and leukocyte adhesion[38].
Figure 2 Role of arginine/asymmetric dimethylarginine ratio.
Asymmetric dimethylarginine accumulation blocks nitric oxide synthase (NOS) and induces consequent endothelial dysfunction in the vasculature. On the contrary, high arginine levels as substrate for NOS induces vasodilatation. ADMA: Asymmetric dimethylarginine; iNOS: Inducible nitric oxide-synthase; eNOS: Endothelial nitric oxide-synthase; nNOS: Neuronal nitric oxide-synthase.
As has been already clarified, ADMA inhibits NO synthesis by competing with L-arginine for cellular transport across CATs, and for binding with the three isoforms of NOS. Already in 1995, Huang et al[39] had observed that the pharmacological blockade of NO production with arginine analogues, could simultaneously affect multiple isoforms of NOS; more importantly, they showed that mutant mice lacking the eNOS gene were hypertensive due to the absence of endothelium-derived relaxing factor activity, a mechanism independent of non-endothelial isoforms of NOS. In another ex vivo experiment on isolated rat hearts from endotoxin-treated animals, coronary flow was elevated compared with control hearts; the addition of an NO-synthesis inhibitor to the perfusion medium decreased coronary flow, but local ischemic events still occurred. This local ischemia was reverted by the infusion of L-arginine, so increasing the arginine/ADMA ratio[40].
These results suggest that, in critical illness, the regulation of organ perfusion by NO is of vital importance. Not surprisingly, worsening conditions in critically ill patients are often associated with increased serum ADMA. Nijveldt et al[22] showed that critically ill patients are exposed to elevated ADMA plasma levels, and, importantly, that plasma ADMA concentration was independently related to the presence of hepatic failure. Furthermore, plasma ADMA ranked as the first and strongest predictor for outcome, with an increased risk for patients who were in the highest quartile for ADMA. Recently, it has been shown that the arginine/ADMA ratio is a more powerful predictor of organ failure with respect to ADMA alone. The arginine/ADMA ratio in ICU patients is associated with circulatory failure, organ failure and mortality in septic patients[41,42].
WHAT MIGHT BE THE MECHANISM BEHIND RAISED ADMA LEVELS IN CRITICALLY ILL PATIENTS?
Up-regulation of iNOS occurring in sepsis leads to the release of huge amounts of oxidants. Kupffer cells play a major role in this process as they can release large amounts of inflammatory mediators; the leukocytes activated by the Kupffer cells are an important local source of free radicals, which cause oxidative damage to DNA, membrane lipids and proteins. DDAH, the enzyme responsible for ADMA degradation, is a potential target of reactive species: S-nitrosylation of DDAH-2 has been shown to reduce this enzyme’s activity, leading to the accumulation of ADMA[43]. Many cardiovascular risk factors lead to oxidative stress too, contributing to eNOS uncoupling, ADMA accumulation with consequent endothelial dysfunction in the vasculature[37]. This local increase in ADMA could be the initial cause of liver malperfusion, leading to compromised liver function. Due to the main role of liver in ADMA metabolization, a worsening of the liver function causes a further increase in ADMA in serum, further compromising the perfusion flow of the liver and other organs. For this reason, the liver is considered as playing a crucial role in MOF[44]. Alternatively, pre-existent liver failure or a change causing reduced clearance capability for serum ADMA, could be a decisive contributing factor in MOF development. In septic patients with an acute liver failure, plasma levels of ADMA were significantly increased with respect to patients with an intact hepatic function[25]. Furthermore, it has been shown that defective DDAH, along with the removal of liver tissue and prolonged hepatic injury, influences the liver’s capacity to eliminate ADMA, resulting in higher systemic levels of ADMA and a lower arginine/ADMA ratio[45,46]. In a prospective study, a positive correlation between raised ADMA levels and severity of organ failure, inflammation and presence of early shock in severe sepsis was observed. Furthermore, higher ADMA levels were associated to the occurrence of a genetic polymorphism in the DDAH-2 gene, so a correlation between a defective DDAH-2 gene and the extent of MOF has been proposed by the authors[47]. These studies confirm the pivotal role of the liver, and more specifically, of hepatic DDAH enzymatic activity, in the insurgence of MOF.
The increased circulatory ADMA and MOF have been recently reviewed in a model of cholestasis: the ADMA and NO dysregulation were particularly evaluated into extrahepatic organs such as kidney, brain and heart[48]. The understanding of the role and regulation of ADMA could have clinical implications to treat not only cholestatic liver disease but also ADMA-related disorders.
Other studies suggest that, secondarily to the liver, the kidney, too, plays a role in ADMA accumulation. Patients suffering from renal failure exhibit impaired urinary excretion, reduced arginine synthesis and impaired DDAH activity, all factors leading to increased serum ADMA concentrations[49]. Increased serum levels of ADMA predict the progression to dialysis and death in patients with chronic kidney disease. A randomized, double-blind, placebo-controlled study on cardiovascular and renal outcomes in 2102 renal transplant recipients found ADMA to be a significant risk factor for graft failure, major cardiac events, cerebrovascular events, and all-cause mortality[50]. Other factors contributing to an imbalance in the arginine/ADMA ratio and potentially with a role in MOF are: a higher amount of protein methylation and an increased rate of protein turnover[45]. In a recent study, critically ill patients and healthy volunteers were given isotopically labelled amino acids in order to assess whole protein turnover. In this study, critically ill patients’ whole body protein turnover was significantly higher than that of healthy volunteers during parenteral nutrition delivery[51]. The mechanism by which protein turnover increases in critically ill patients has yet to be elucidated.
CONCLUSION
MOF is the concurrent dysfunction of several organs. Higher morbidity and mortality has been observed in patients with higher serum ADMA[22,41,42]; currently ADMA is considered to be not merely an asymptomatic index but, additionally, a risk factor. The ADMA-MOF hypothesis holds that the liver plays a pivotal role. Liver is the main organ devoted to ADMA clearance; this hepatic capability can be compromised by various factors, such as: severe organ injury, free radical release compromising DDAH activity, genetic polymorphism for hepatic DDAH[25,45-47]. These conditions, with the exception of congenital polymorphism, occur especially in sepsis, but other forms of trauma, as tumours or ischemic injury, can also produce the same result. In all these cases, the loss of liver function causes an imbalance in the arginine/ADMA ratio and the subsequent inhibition of eNOS, resulting in a significant reduction in NO synthesis and leading to malperfusion in various organs, eventually culminating in multi-organ failure. It has been observed that the arginine/ADMA ratio is a better predictor of morbidity and mortality than ADMA alone, suggesting that the restoration of this ratio, for example by means of the administration of L-arginine, should be considered a suitable option when attempting to improve a patient’s condition[45].
ACKNOWLEDGMENTS
We thank Professor Anthony Baldry for revising the English and Mrs. Nicoletta Breda for editing assistance.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dirchwolf M, Eid NAS, Elalfy H S- Editor: Qi Y L- Editor: A E- Editor: Ma S