Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.5090
Peer-review started: October 7, 2014
First decision: October 29, 2014
Revised: November 11, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 28, 2015
Processing time: 203 Days and 18.1 Hours
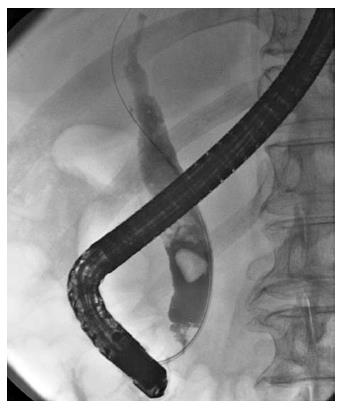
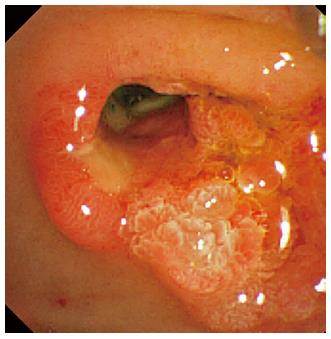
A 78-year-old male was admitted to our hospital because of choledocholithiasis. ERC demonstrated choledocholithiases with a maximum diameter of 13 mm, and we performed endoscopic papillary large balloon dilation (EPLBD) with a size of 15 mm. Immediately following the balloon deflation, spurting hemorrhage occurred from the orifice of the duodenal papilla. Although we performed endoscopic hemostasis by compressing the bleeding point with the large balloon catheter, we could not achieve hemostasis. Therefore, we placed a 10 mm fully covered self-expandable metallic stent (SEMS) across the duodenal papilla, and the hemorrhage stopped immediately. After 1 wk of SEMS placement, duodenal endoscopy revealed ulcerative lesions in both the orifice of the duodenal papilla and the lower bile duct. A direct peroral cholangioscopy using an ultra-slim upper endoscope revealed a visible vessel with a longitudinal mucosal tear in the ulceration of the lower bile duct. We believe that the mucosal tear and subsequent ruptured vessel were caused by the EPLBD procedure.
Core tip: We present a case study of arterial hemorrhage after endoscopic papillary large balloon dilation (EPLBD) that was treated using a covered self-expandable metallic stent (SEMS). After 1 wk of SEMS placement, a direct peroral cholangioscopy revealed a visible vessel with a longitudinal mucosal tear in the ulceration of the lower bile duct. This image is important for understanding the mechanism of hemorrhage after EPLBD.
- Citation: Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Kondo H, Nishi Y, Umemura S, Hori Y, Kato A, Ohara H, Joh T. Case of arterial hemorrhage after endoscopic papillary large balloon dilation for choledocholithiases using a covered self-expandable metallic stent. World J Gastroenterol 2015; 21(16): 5090-5095
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/5090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.5090
Arterial hemorrhagic complications following therapeutic endoscopic retrograde cholangiopancreatography (ERCP) are difficult to manage endoscopically because of the difficulty of observing the bleeding point and maintaining clear endoscopic vision despite the bleeding. Surgical operations or interventional radiology are usually performed to control hemorrhage after the failure of endoscopic hemostasis. However, these procedures are invasive, particularly in patients of advanced age and those with serious underlying diseases.
Endoscopic papillary large balloon dilation (EPLBD) is used for difficult extractions of large choledocholithiases because of the easy removal. The efficacy and safety of EPLBD has been reported[1,2]; however, the complications and long-term outcomes of EPLBD have not been clarified. Post EPLBD hemorrhage is one of the possible ERCP-related complications. Although there have been some reports of endoscopic hemostasis with covered self-expandable metallic stents (SEMS) against post endoscopic sphincterotomy (EST) bleeding[3-7], no post EPLBD hemorrhage cases treated with covered SEMS have been reported. Furthermore, there have been no direct cholangioscopic images to clarify the mucosal tear of the bile duct after EPLBD, which is useful for understanding the mechanism of post EPLBD hemorrhage.
In this paper, we describe a case of endoscopic hemostasis using a fully covered SEMS for a spurting hemorrhage from the lower bile duct following an EPLBD procedure with a notable cholangioscopic image of a mucosal tear in the bile duct.
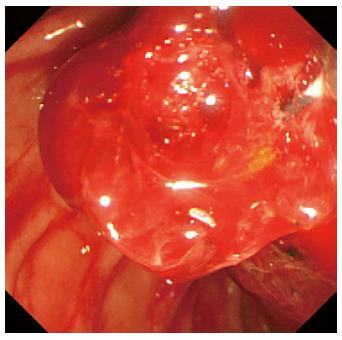
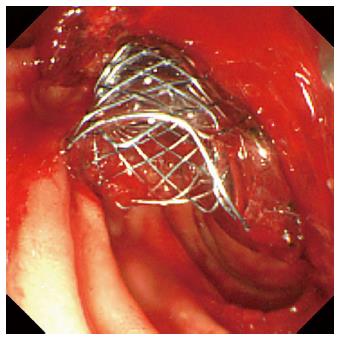
A 78-year-old male was admitted to our hospital in July 2014 because of a fever elevation. He had undergone EST with a small incision in 2011 and had experienced endoscopic removal of choledocholithiasis several times. Laboratory findings on this admission revealed elevated serum levels of total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and γ-glutamyl transpeptidase to 5.6 mg/dL (normal range, 0.3-1.2 mg/dL), 368 U/L (normal range, 13-33 U/L), 210 U/L (normal range, 6-30 U/L), 1583 U/L (normal range, 100-340 U/L), and 336 U/L (normal range, 10-47 U/L), respectively. The serum level of C-reactive protein was 0.77 mg/dL (normal range, ≤ 0.3 mg/dL), and the white blood cell count was 9600/mm3 (normal range, 3600-9600/mm3). Computed tomography (CT) showed the presence of high-density lesions in the common bile duct and gallbladder with a slightly dilated biliary tract. Therefore, we diagnosed acute cholangitis because of the choledocholithiases and performed an emergency endoscopic biliary drainage. The patient did not take any anticoagulants or antiplatelet medicines. After choledocholithiases with a maximum diameter of 13 mm were identified on the cholangiogram (Figure 1), we decided to attempt EPLBD. Because the outer diameter of the therapeutic duodenoscope (TJF-260V; Olympus, Tokyo, Japan) was 13.5 mm, the diameter of the lower bile duct was estimated at 15 mm. Accordingly, we performed EPLBD using a Giga® wire-guided balloon dilator (Century Medical, Inc., Japan) with a maximum size of 15 mm that was placed across the papilla of Vater. The balloon was gradually inflated to an adequate size for stone removal with a contrast medium under endoscopic and fluoroscopic imaging until we confirmed the disappearance of the notch at the papilla of Vater. Immediately following the balloon deflation, spurting hemorrhage occurred from the orifice of the papilla of Vater. After extraction of the choledocholithiases, we performed endoscopic hemostasis by immediately compressing the bleeding point with a large balloon catheter several times; however, we could not detect the bleeding point, and hemostasis could not be achieved. We were unable to control the hemorrhage, and the endoscopic image of the papilla of Vater was gradually worsening because of the adhesion and the significant amount of coagulation (Figure 2). We wanted to adopt a minimally invasive treatment to control hemorrhage because of serious dementia symptoms in this patient’s background; therefore, we decided to place a covered SEMS for the purpose of astriction. We placed a fully covered Wallflex® SEMS (Microvasive Endoscopy, Boston Scientific Corporation, Natick, MA, United States) that measured 10 mm with an 8-cm diameter across the papilla of Vater, and hemorrhage stopped immediately (Figure 3). The hemoglobin level dropped from 11 g/dL to 7.5 g/dL; therefore, a blood transfusion of 2 units of packed red cell concentrate was performed. However, re-bleeding and early complications with respect to the SEMS placement, such as cholecystitis and pancreatitis, did not occur after endoscopic hemostasis with the SEMS placement. Thus, we could avoid surgical operation or interventional radiology (IVR) to manage the hemorrhage. In addition, acute cholangitis on admission improved after the therapeutic ERCP procedure.
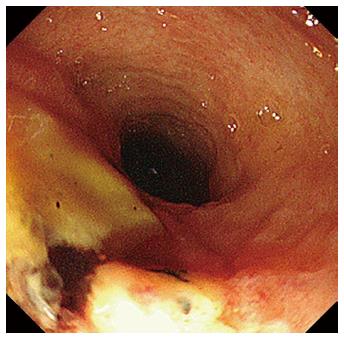
After 1 wk of covered SEMS placement, although the SEMS had migrated slightly to the duodenal site, hemorrhage was not observed in the endoscopic findings. Re-bleeding was not observed after the SEMS removal, and an ulcerative lesion appeared from the orifice of the papilla of Vater to the lower bile duct (Figure 4). Subsequently, we performed a direct peroral cholangioscopy using an ultra-slim upper endoscope (GIF-XP290N; Olympus, Tokyo, Japan). The cholangioscopy revealed an exposed vessel with a laceration in the lower bile duct ulcerative area (Figure 5), and we believe that this lacerative lesion was caused by mucosal damage of the lower bile duct during the EPLBD procedure.
Because re-bleeding was not observed after consuming a meal, the patient was safely discharged and is currently undergoing outpatient follow-up.
EPLBD has increasingly been performed as therapeutic ERCP against large common bile duct stones. The rates of hemorrhagic adverse events with EPLBD were reportedly 0%-6.7%[8-11]. There are no significant differences of hemorrhagic complication rates between EPLBD and EST in some reports[11,12], and the rate of hemorrhage was lower in EPLBD than EST in other studies[13]. In general, the rate of hemorrhage after EPLBD is considered low. However, fatal hemorrhage with EPLBD has also been reported[14]. Accordingly, we should keep in mind the possibility of life-threatening massive bleeding with EPLBD. Furthermore, we should understand how to manage hemorrhagic complications.
Endoscopic interventions for bleeding complications include conventional injection therapy using hypertonic saline epinephrine, balloon tamponade, the application of coagulation current during a sphincterotomy, endoclip placement, etc. When complete endoscopic hemostasis cannot be achieved in spite of these procedures, invasive interventions such as surgical operations or IVR are required. There are several reports of endoscopic hemostasis using covered SEMS for post-EST bleeding in recent years[3-7] (Table 1). The authors placed a covered SEMS that was 10 mm in diameter across the papilla of Vater and removed it endoscopically 1 wk to 2 mo after successful hemostasis. According to these reported cases, complete hemostasis was achieved in all the patients. Therefore, covered SEMS placement is considered useful in selected patients with uncontrolled post-EST bleeding. However, there have been no reported cases of endoscopic hemostasis using covered SEMS after hemorrhage during an EPLBD procedure. In our case, we performed EPLBD with a maximum size of 15 mm, and the size of the SEMS was 10 mm in diameter. Therefore, theoretically, the bleeding point was not fully compressed by a 10 mm covered SEMS. Nevertheless, we could manage the bleeding, and the SEMS had not migrated at the time of placement. Presumably, even if we performed EPLBD with a maximum size of 15 mm, the dilated bile duct would become slightly smaller after deflation of the balloon dilation, and blood clots might occur between the bleeding point and the covered SEMS. Hence, we succeeded in endoscopic hemostasis with a covered SEMS 10 mm in size. The covered SEMS was removed 1 wk after the confirmation of controlled hemorrhage, as in the reported cases[3,5].
| Ref. | No. of Patients | Previous attempted treatments | SEMS type, diameter × length (mm) | Rate of complete hemostasis | The mean duration of SEMS placement |
| Canena et al[3] (2013) | 4 | Endoclips: 1 case | Fully covered type Hanarostent1 or Niti-S2 or Wallflex3, | 100% | 7.5 d |
| Epinephrine injection: 4 cases | |||||
| Balloon tamponade: 2 cases | 10 × 40: 1 case | ||||
| 10 × 60: 3 cases | |||||
| Itoi et al[5] (2011) | 11 | Endoclips: 7 cases | Covered type | 100% | 8.2 d |
| Epinephrine injection: 9 cases | Wallstent4: 8 cases | ||||
| Balloon tamponade: 11 cases | Wallflex: 2 cases | ||||
| Niti-S: 1 case | |||||
| 10 × 60 | |||||
| Shah et al[6] (2010) | 5 | Endoclips: 1 case | Fully covered Wallflex, | 100% | 4-5 wk |
| Epinephrine injection: 2 cases | 10 × 40: 2 cases | ||||
| Thermal coagulation: 1 case | 10 × 60: 2 cases | ||||
| IVR embolization: 1 case | 8 × 60: 1 case | ||||
| Balloon tamponade: 1case | |||||
| Di Pisa et al[7] (2010) | 2 | Endoclips: 1 case | Partially covered type Wallstent, | 100% | 3 wk |
| Epinephrine injection: 2 cases | 10 × 40 | ||||
| Balloon tamponade: 2 cases |
EPLBD is the procedure used to dilate the lower bile duct by randomly cutting off the sphincter of ampulla. We performed a direct peroral cholangioscopy after SEMS removal and confirmed a laceration with an exposed vessel in the ulcerative lesion of the lower bile duct. A direct cholangioscopic image after EPLBD has not been previously reported; however, there is literature on a linear mucosal tear after balloon dilation in the esophagus[15]. Adams et al[15] reported that 15% of treated achalasia cases were abnormal after balloon dilation, with complications such as complete rupture or incomplete tears of the esophageal wall and localized outpouch or diverticulum. Moreover, the patient had undergone EST in 2011, and we did not perform EST before EPLBD during this admission. Thus, we believe that the mucosal tear and subsequent ruptured vessel were caused by the EPLBD procedure. There is a possibility that a mucosal tear of the duodenal papilla or lower bile duct often occurs after EPLBD, similar to in the esophagus. This image is important for understanding the mechanism of hemorrhage after EPLBD. Arterial hemorrhage after EST usually occurs from the duodenal papilla. However, arterial hemorrhage after EPLBD might occur not only from the duodenal papilla but also from the lower or middle bile duct. We should consider the possibility of hemorrhage from the bile duct when we encounter hemorrhage after EPLBD. It is difficult to manage hemorrhage from the lower bile duct by endoscopic hemostasis, such as through conventional injection therapy, application of coagulation current, or endoclip placement. In the present case, we finally obtained hemostasis using a covered SEMS. Endoscopic hemostasis using a covered SEMS is one of the optional procedures for post EPLBD hemorrhage after the failure of other endoscopic therapies. We believe the cholangioscopic image of laceration with an exposed vessel in an ulcerative lesion of the lower bile duct is beneficial and educational for endoscopists performing EPLBD because we can investigate the mechanism of hemorrhage after EPLBD.
In summary, we have described a case of endoscopic hemostasis using a fully covered SEMS for arterial hemorrhage from the lower bile duct following EPLBD. We must consider the possibility of hemorrhage from the bile duct when we encounter post EPLBD bleeding. Endoscopic hemostasis using a covered SEMS might be a useful option.
A 78-year-old male with choledocholithiasis.
Arterial hemorrhage from the orifice of the duodenal papilla after endoscopic papillary large balloon dilation (EPLBD).
Hemorrhage occurred from the duodenal papilla or the lower bile duct.
The hemoglobin level dropped from 11 g/dL to 7.5 g/dL; therefore, a blood transfusion of 2 units of packed red cell concentrate was performed.
The cholangioscopy revealed an exposed vessel with a laceration in the lower bile duct ulcerative area, which was caused by mucosal damage during the EPLBD procedure.
The authors did not perform a pathological examinations in this case study.
The authors placed a 10 mm fully covered self-expandable metallic stent across The authors duodenal papilla after the failure of hemostasis by compressing the bleeding point with a large balloon catheter.
There have been several reports of endoscopic hemostasis using a covered SEMS for post- endoscopic sphincterotomy bleeding in recent years. However, there have been no reported cases of endoscopic hemostasis using a covered SEMS after hemorrhage during an EPLBD procedure.
EPLBD is the procedure used to dilate the lower bile duct by randomly cutting off the sphincter of ampulla. EPLBD has increasingly been performed as therapeutic ERCP against large common bile duct stones.
The authors should keep in mind the possibility of life-threatening massive bleeding with EPLBD. Arterial hemorrhage after EPLBD might occur from both the duodenal papilla and the lower or middle bile duct.
This is a straight forward case report whose main point is the cholangioscopic image demonstrating a bile duct tear following endoscopic balloon dilation for removal of a bile duct stone. The figures are adequate. The paper is likely of interest, not because of as the authors state “understanding the mechanism post EPLBD hemorrhage” but rather highlighting the use of fully covered stents in this situation (i.e., for bleeding).
| 1. | Tonozuka R, Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J. Efficacy and safety of endoscopic papillary large balloon dilation for large bile duct stones in elderly patients. Dig Dis Sci. 2014;59:2299-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Kogure H, Tsujino T, Isayama H, Takahara N, Uchino R, Hamada T, Miyabayashi K, Mizuno S, Mohri D, Yashima Y. Short- and long-term outcomes of endoscopic papillary large balloon dilation with or without sphincterotomy for removal of large bile duct stones. Scand J Gastroenterol. 2014;49:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Canena J, Liberato M, Horta D, Romão C, Coutinho A. Short-term stenting using fully covered self-expandable metal stents for treatment of refractory biliary leaks, postsphincterotomy bleeding, and perforations. Surg Endosc. 2013;27:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Mavrogenis G, Coumaros D. Use of covered self-expandable metallic stents in post-endoscopic sphincterotomy bleeding. Endoscopy. 2011;43:1112; author reply 1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Itoi T, Yasuda I, Doi S, Mukai T, Kurihara T, Sofuni A. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011;43:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Shah JN, Marson F, Binmoeller KF. Temporary self-expandable metal stent placement for treatment of post-sphincterotomy bleeding. Gastrointest Endosc. 2010;72:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Di Pisa M, Tarantino I, Barresi L, Cintorino D, Traina M. Placement of covered self-expandable metal biliary stent for the treatment of severe postsphincterotomy bleeding: outcomes of two cases. Gastroenterol Res Pract. 2010;2010:138748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Meine GC, Baron TH. Endoscopic papillary large-balloon dilation combined with endoscopic biliary sphincterotomy for the removal of bile duct stones (with video). Gastrointest Endosc. 2011;74:1119-1126; quiz 1115.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Youn YH, Lim HC, Jahng JH, Jang SI, You JH, Park JS, Lee SJ, Lee DK. The increase in balloon size to over 15 mm does not affect the development of pancreatitis after endoscopic papillary large balloon dilatation for bile duct stone removal. Dig Dis Sci. 2011;56:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Kim HW, Kang DH, Choi CW, Park JH, Lee JH, Kim MD, Kim ID, Yoon KT, Cho M, Jeon UB. Limited endoscopic sphincterotomy plus large balloon dilation for choledocholithiasis with periampullary diverticula. World J Gastroenterol. 2010;16:4335-4340. [PubMed] |
| 11. | Jin PP, Cheng JF, Liu D, Mei M, Xu ZQ, Sun LM. Endoscopic papillary large balloon dilation vs endoscopic sphincterotomy for retrieval of common bile duct stones: a meta-analysis. World J Gastroenterol. 2014;20:5548-5556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Oh MJ, Kim TN. Prospective comparative study of endoscopic papillary large balloon dilation and endoscopic sphincterotomy for removal of large bile duct stones in patients above 45 years of age. Scand J Gastroenterol. 2012;47:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Feng Y, Zhu H, Chen X, Xu S, Cheng W, Ni J, Shi R. Comparison of endoscopic papillary large balloon dilation and endoscopic sphincterotomy for retrieval of choledocholithiasis: a meta-analysis of randomized controlled trials. J Gastroenterol. 2012;47:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Park SJ, Kim JH, Hwang JC, Kim HG, Lee DH, Jeong S, Cha SW, Cho YD, Kim HJ, Kim JH. Factors predictive of adverse events following endoscopic papillary large balloon dilation: results from a multicenter series. Dig Dis Sci. 2013;58:1100-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Adams H, Roberts GM, Smith PM. Oesophageal tears during pneumatic balloon dilatation for the treatment of achalasia. Clin Radiol. 1989;40:53-57. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Wilcox CM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S