Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4986
Peer-review started: November 15, 2014
First decision: December 2, 2014
Revised: December 9, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: April 28, 2015
Processing time: 165 Days and 5.5 Hours
AIM: To investigate the effect of herb-partitioned moxibustion combined with acupuncture on the expression of intestinal epithelial tight junction (TJ) proteins.
METHODS: Sixty patients diagnosed with mild to moderate Crohn’s disease (CD) were allocated into the herb-partitioned moxibustion combined with acupuncture (HMA) group (n = 30) or the mesalazine (MESA) group (n = 30) using a parallel control method. There were 2 sets of acupoints used alternately for HMA treatment. The following points were included in Set A: ST25 (Tianshu), RN6 (Qihai), and RN9 (Shuifen) for herb-partitioned moxibustion and ST36 (Zusanli), ST37 (Shangjuxu), LI11 (Quchi), and LI4 (Hegu) for acupuncture. The points for Set B included BL23 (Shenshu) and BL25 (Dachangshu) for herb-partitioned moxibustion and EX-B2 of T6-T1 (Jiajixue) for acupuncture. The patients received the same treatment 6 times a week for 12 consecutive weeks. The MESA group received 1 g of mesalazine enteric coated tablets 4 times daily for 12 consecutive weeks. Intestinal tissues were stained and examined to compare the morphological and ultrastructural changes before and after the treatment session. Immunohistochemistry and in situ hybridization assays were used to detect the expression of intestinal epithelial TJ proteins zonula occludens-1 (ZO-1), occludin, and claudin-1. The mRNA levels were also evaluated.
RESULTS: After the treatment, both herb-partitioned moxibustion combined with acupuncture and mesalazine improved intestinal morphology and ultrastructure of CD patients; the patients treated with HMA showed better improvement. HMA significantly increased the expression of ZO-1 (P = 0.000), occludin (P = 0.021), and claudin-1 (P = 0.016). MESA significantly increased the expression of ZO-1 (P = 0.016) and occludin (P = 0.026). However, there was no significant increase in the expression of claudin-1 (P = 0.935). There was no statistically significant difference between the two groups for the expression of occludin and claudin-1 (P > 0.05). The HMA group showed a significant improvement in ZO-1 expression compared to the MESA group (2333.34 ± 352.51 vs 2160.38 ± 307.08, P = 0.047). HMA significantly increased the expression of ZO-1 mRNA (P = 0.000), occludin mRNA (P = 0.017), and claudin-1 mRNA (P = 0.017). MESA significantly increased the expression of ZO-1 mRNA (P = 0.000), occludin mRNA (P = 0.042), and claudin-1 mRNA (P = 0.041). There was no statistically significant difference between the two groups in the expression of occludin and claudin-1 mRNA (P > 0.05). However, the HMA group showed a significant improvement in ZO-1 mRNA expression compared with the MESA group (2378.17 ± 308.77 vs 2200.56 ± 281.88, P = 0.023).
CONCLUSION: HMA can repair intestinal epithelial barrier lesions and relieve inflammation by upregulating the expression of TJ proteins and their mRNAs.
Core tip: Crohn’s disease (CD) is a chronic relapsing inflammatory condition involving all layers of the gastrointestinal tract. Although its etiopathogenesis remains unclear, increased permeability of the intestinal epithelial barrier is one of the crucial factors in CD onset. Tight junctions (TJs) within intestinal epithelial cells form the structural basis of the intestinal epithelial barrier, and reduced expression of TJ proteins is positively correlated with CD severity. This study investigated the therapeutic effect of herb-partitioned moxibustion combined with acupuncture on CD. We found that this treatment upregulated the expression of intestinal epithelial TJ proteins and their mRNAs.
- Citation: Shang HX, Wang AQ, Bao CH, Wu HG, Chen WF, Wu LY, Ji R, Zhao JM, Shi Y. Moxibustion combined with acupuncture increases tight junction protein expression in Crohn’s disease patients. World J Gastroenterol 2015; 21(16): 4986-4996
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4986
Crohn’s disease (CD) is a chronic relapsing inflammatory condition involving all layers of the gastrointestinal (GI) tract and can affect any part of the GI tract from the mouth to anus[1]. Clinical epidemiological studies have shown the prevalence of CD in mainland China is 1.4 cases per 100000 person-years, and the incidence has increased steadily and rapidly in recent years[2].
The clinical management of CD involves conventional medical treatments including non-steroidal anti-inflammatory drugs, such as 5-aminosalicylate (5-ASA), corticosteroids and immunosuppressive drugs, such as thiopurines[3]. However, the European Crohn’s and Colitis Organization has recognized the efficiency of 5-ASA as “limited”[4] in improving patients’ CD Activity Index (CDAI). The inappropriate use of large amounts of corticosteroids and insufficient amounts of thiopurines is common among Asian physicians[5] and results in drug resistance and adverse effects. One new treatment approach in Western countries is biologic therapy, such as anti-tumor necrosis factor (anti-TNF) agents or infliximab[6]. However, in Asia, the use of biologics is limited by economic burdens due to the strict coverage range of medical insurance.
Complementary and alternative medicines (CAMs), such as Chinese medicine, flourish in Asia and especially in East Asia. In China, more than half of CD patients receive concomitant traditional Chinese therapies[7] including Chinese materia medica decoction, acupuncture and moxibustion. The efficacy and safety of these therapies have been frequently questioned because of the indiscriminate use of CAMs in patients with CD. As a result, many attempts have been made to obtain concrete evidence of their therapeutic effect and to investigate their possible mechanisms. Recent studies[8] have shown that apart from the undeniable placebo effect, acupuncture and moxibustion offer additional therapeutic benefits in patients with mild to moderately active CD. Compared with the conventional 5-ASA treatment, moxibustion and acupuncture have a significant advantage in improving quality-of-life ratings and CDAI scores of patients with mild to moderate CD[9]. In further clinical studies, we found that herb-partitioned moxibustion combined with acupuncture can inhibit intestinal epithelial cell apoptosis by decreasing the overexpression of intestinal mucosa tumor necrosis factor alpha (TNF-α), tumor necrosis factor 1 (TNFR1), and tumor necrosis factor 2 (TNFR2)[10]. The treatments also reduce intestinal inflammation by increasing hemoglobin (HGB) counts and by decreasing C-reactive protein levels and erythrocyte sedimentation rates in CD patients[11].
Previous studies have suggested that increased intestinal permeability may appear precede clinical manifestations[12] and could be used to predict clinical relapses[13] of CD. Animal experiments have been performed to understand the pathological basis underlying the occurrence of CD and to investigate the cause of increased intestinal permeability from two aspects. One aspect is excessive epithelial cell apoptosis[14,15]. The other aspect is intestinal mucosal epithelial barrier dysfunction[16]. The main function of the intestinal epithelial barrier is to maintain intestinal permeability, and the barrier depends on the dynamically changing formation of tight junctions (TJs) reacting to varied extracellular stimuli[17]. Through protein-protein interactions, the cytoplasmic protein zonula occludens-1 (ZO-1) and the transmembrane proteins claudin-1 and occludin are able to modulate and associate with different forms of multimolecular complexes to regulate the formation of TJs[18]. In previous study, we observed decreased intestinal permeability and reduced expression of TJ proteins ZO-1, claudin-1 and occludin in a TNBS-induced CD rat model. However, treatment with herb-partitioned moxibustion and acupuncture increased the expression of TJ proteins ZO-1, claudin-1 and occludin. Additionally, the inflammatory reaction in the intestinal mucosa was improved based on histological observation[19].
Therefore, we hypothesized that herb-partitioned moxibustion combined with acupuncture could increase intestinal permeability by upregulating the expression of TJ proteins ZO-1, claudin-1 and occludin in CD patients. In this study, we examine the mechanism of herb-partitioned moxibustion combined with acupuncture in CD patients and try to verify the feasibility of acupuncture and moxibustion methods for treating mild to moderate CD.
The study was performed from July 2009 through March 2010 in the CD clinic of the Shanghai Research Institute of Acupuncture within the Moxibustion and Meridian Endoscopy Center of Zhongshan Hospital affiliated to Fudan University. Ethics approval was obtained from the Chinese Clinical Trial Register Center (registration number: ChiCTR-TRC-10000950). All patients provided written informed consent prior to the beginning of the trial. Patients with CD were recruited in accordance with Jinan diagnostic criteria (revised by the National Conference on Inflammatory Bowel Disease in 2007[20]). Patients with mild to moderate disease and CDAI scores between 150 and 450 were included. The patients were not treated with other relevant pharmacological therapies and signed an informed consent. The exclusion criteria included the following: pregnant or lactating patients, psychotic patients, and patients with severe heart, brain, liver, kidney, or hematopoietic system diseases and other severe diseases. This study was conducted as a controlled trial with 2 parallel treatment groups. Patients from the Shanghai Research Institute of Acupuncture-Moxibustion and Meridian were enrolled in the herb-partitioned moxibustion combined with acupuncture group (HMA group, n = 30). The patients from the endoscopy center of Zhongshan Hospital affiliated with Fudan University were included in the mesalazine group (MESA group, n = 30).
The patients in the HMA group received herb-partitioned moxibustion combined with acupuncture. There were 2 sets of acupoints used for treatment. The points in Set A included the following: ST25 (Tianshu), RN6 (Qihai), and RN9 (Shuifen) for herb-partitioned moxibustion and ST36 (Zusanli), ST37 (Shangjuxu), LI11 (Quchi), and LI4 (Hegu) for acupuncture. The points in Set B were BL23 (Shenshu) and BL25 (Dachangshu) for herb-partitioned moxibustion and EX-B2 of T6 - T1 (Jiajixue) for acupuncture. These acupoints were located based on the national GB-12346-90 acupoint standard. The procedure used involved placing moxa cones (1.7 cm in height and 1.8 g in weight; Hanyi, Henan, China) on a herbal cake (2.3 cm in diameter and 0.5 cm in length). The herbal cake consisted of 3 g of Shaoxing wine and 2.5 g of herbal powder [medicinal formula: Aconite preparata (radix), Cinnamomi (cortex), etc.]. Four sets of moxa cones and herbal cakes were used for each treatment. The skin was cleaned with a tincture of iodine and alcohol, and then sterile single-use acupuncture needles (Φ 0.30 mm × 40 mm specification, Huatuo, Suzhou, China) were inserted between 20 mm and 40 mm into the acupoints. Acupuncture was performed by the same qualified and skilled physician. When two moxa cones burned out, the moxa cones, herbal cake and needles were removed. The treatment was applied once per day, 6 times per week for 12 consecutive weeks. The MESA group received mesalazine enteric coated tablets four times a day for 12 consecutive weeks.
On a voluntary basis and after signing an informed consent form, intestinal mucosa tissue samples from 10 patients of each group were removed via painless enteroscopy before and after the treatment session.
Pieces of intestinal mucosa tissues (0.5 cm3 for each) were collected from CD patients. The intestinal mucosa tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin and then sectioned into 4-μm-thick tissue sections. The tissue sections were then stained with hematoxylin and eosin, dehydrated in 95%, 90% and 70% ethanol and cleared in xylene. The stained sections were mounted in Permount or Histoclad and observed using an Olympus DP73 microscope (Olympus, Tokyo, Japan).
The intestinal mucosa tissues were cut into 1-mm3 strips, fixed for 4 h at 4 °C in 5% glutaraldehyde and then washed 3 times in 0.1 mol/L phosphate buffered saline (PBS). The tissues were then postfixed for 2 h at 4 °C in 2% osmium tetroxide and dehydrated in a graded ethanol series. The tissues were embedded in Epon 812 and then cut into ultrathin sections (75 nm) and stained with uranyl acetate and lead citrate. The sections were viewed in a HITACHI H-600 electron microscope at 80 kV (HITACHI, Tokyo, Japan).
The expression levels of occludin, claudin-1, and ZO-1 were detected by immunohistochemical assays. The sections were dewaxed, hydrated, and then pretreated in a microwave (antigen retrieval). The endogenous peroxidase activity was inhibited with 0.3% H2O2. The nonspecific binding was blocked with 20% normal goat serum. All sections were incubated with ZO-1 (Rabbit anti-ZO-1 polyclonal antibody 1:50, Invitrogen, New York, United States), occludin (Rabbit anti-Occludin polyclonal antibody 1:100, Invitrogen, New York, United States) and claudin-1 antibodies (Mouse anti-Claudin-1 monoclonal antibody 1:100, Invitrogen, New York, United States) for 2 h at 37 °C. The samples were washed and then incubated for 30 min at room temperature with appropriate preabsorbed biotinylated secondary antibody. The antigen was visualized using the streptavidin-peroxidase method (JRDUN Biotechnology Co., Ltd., Shanghai, China), and 3,3-diaminobenzidine (DAB) (Liquid DAB-Plus Substrate Kit, JRDUN Biotechnology Co., Ltd., Shanghai, China) was used as a chromogen. The slides were washed in distilled water and counterstained with Mayer’s hematoxylin before they were dehydrated and mounted. The primary antibody was replaced with PBS for a negative control. A semi-quantitative analysis of the staining results was conducted using the IMS medical image quantitative analysis system (JRDUN Biotechnology Co., Ltd., Shanghai, China). Positive results for ZO-1, occludin and claudin-1 were brown or yellow particles stained among intestinal epithelium. The positive area and the optical density (OD) values in 3 high-power optical fields (× 200) of every slice were measured. The immune positive area index (positive area/total area × OD) values of ZO-1, occludin and claudin-1 were calculated in every high-power optical field.
The expression levels of ZO-1, occludin and claudin-1 mRNAs were detected by in situ hybridization. Digoxigenin-labelled RNA probes were generated with a DIG RNA labeling kit (Boehringer Mannheim, Mannheim, Germany) and a relevant plasmid (provided by JRDUN Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s protocol. The tissues were dewaxed and hydrated. The sections were heated at 98 °C for 20 min in EDTA solution (0.01 mol/L, pH 8.0) and pre-treated with the following solutions: 2 μg/mL proteinase K in TE at 37 °C for 8 min; 0.1 mol/L glycine in PBS for 10 min; a graded ethanol series (each concentration for 1 min); and 0.2 × SSC and 50% formamide to pre-hybridize at 37 °C for 30 min. Pre-treated sections were covered with 20-30 mL of hybridization buffer (1 μg/mL RNA probe) and incubated at 48 °C for 8-12 h under a coverslip. The slides were washed twice at 45 °C for 5 min in 2 × SSC and twice at 37 °C for 5 min in 1 ×, 0.5 ×, 0.1 × SSC. The samples were then blocked in 10% serum. The sections were then incubated at 37 °C for 30 min in mouse McAb anti-Dig IgG (cat#84-0146, lot#305359, Invitrogen). The slides were washed three times in PBS and further incubated at 37 °C for 40 min with goat anti-Mouse IgG-HRP antibody (cat#84-01450, lot#651053A, Invitrogen). The sections were washed three times in PBS for 3 min each. The staining detection was performed using 0.04% DAB and 0.03% H2O2 according to the manufacturer’s recommendations. The tissues were dehydrated and mounted after color development. We performed semi-quantitative analyses of the results, and the positive expression area index (positive area/total area × OD) values of ZO-1, occludin and claudin-1 mRNA were calculated in 3 high-power optical fields.
All measurement data are presented as the mean ± SD. All statistical analyses were performed using SPSS 16.0 (SPSS Inc. Illinois, United States). The comparison of sex and severity from the baseline data were analyzed using the χ2 test. The changes in age, duration of disease from the baseline data and between group differences after treatment were compared using two independent sample t-tests. A paired-samples t-test was used for within group comparisons. All two-sided P values < 0.05 were considered statistically significant.
Table 1 presents the baseline characteristics of the two groups by age, sex, duration of disease, and severity of disease. There were no differences between the HMA group and the MESA group before the procedure.
| HMA n = 30 | MESA n = 30 | P value | |
| Age (yr) | |||
| Min | 22 | 18 | |
| Max | 56 | 65 | |
| mean ± SD | 31.77 ± 8.77 | 36.93 ± 13.25 | 0.080 |
| Sex (M/F) | 23/7 | 19/11 | 0.260 |
| Duration of disease (yr) | |||
| Min | 1 | 1 | |
| Max | 12 | 18 | |
| mean ± SD | 6.33 ± 3.63 | 5.67 ± 4.08 | 0.507 |
| Severity of disease (mild/moderate) | 22/8 | 18/12 | 0.273 |
Figure 1 shows the morphological observations of intestinal mucosa tissues from both groups before and after treatment. Figure 1A shows that in the HMA group before treatment, the mucosal epithelium was seriously damaged. The intestinal glands were rare, and there were ulcerations and obvious submucosal hyperemia and edema. Tissue damage was observed in the mucosa, submucosa and muscular layer, and there was substantial eosinophil and inflammatory cell infiltration in the intestinal mucosa and submucosa. Figure 1B shows that in the HMA group after treatment, there was only a small amount of hyperemia and inflammatory cell infiltration in the intestinal mucosa and submucosa. The intestinal glands were arranged in an orderly manner. Figure 1C shows that in the MESA group before treatment, the mucosal epithelium was also seriously damaged. There was obvious hyperemia and edema, and eosinophils and inflammatory cells had infiltrated into the intestinal mucosa and submucosa. There was also ulceration and tissue damage in the mucosa, submucosa and muscular layer. Figure 1D shows that in the MESA group after treatment, there was less severe hyperemia and edema in intestinal mucosa and submucosa. Additionally, some of the intestinal glands were restored but some were poorly organized.
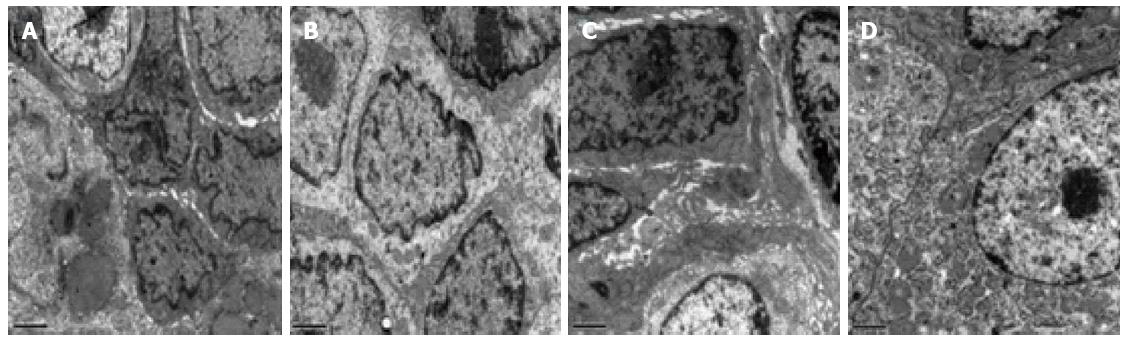
Figure 2 presents ultrastructural images of the intestinal mucosa tissues from both groups before and after treatment. Figure 2A shows that in the HMA group before treatment, the connections between the epithelial cells were loose and that there was a significant broadening of intercellular spaces. Furthermore, the cell membranes were partly injured, and intestinal epithelial cells contained a small number of bubbles inside of the cytoplasm. Figure 2B shows that after HMA treatment the connections between the epithelial cells were relatively tight and that the intercellular spaces between cells were not broadened. A small amount of particle secretion was observed, and a few villi could be observed on the cell surfaces. Figure 2C shows that in the MESA group before treatment, the connections between the epithelial cells were quite loose and that there was significant broadening of intercellular spaces. Particle secretion was detected. Figure 2D shows that after MESA treatment the connections between the epithelial cells were tight. However, there was broadening of some intercellular spaces, and particle secretion was detected.
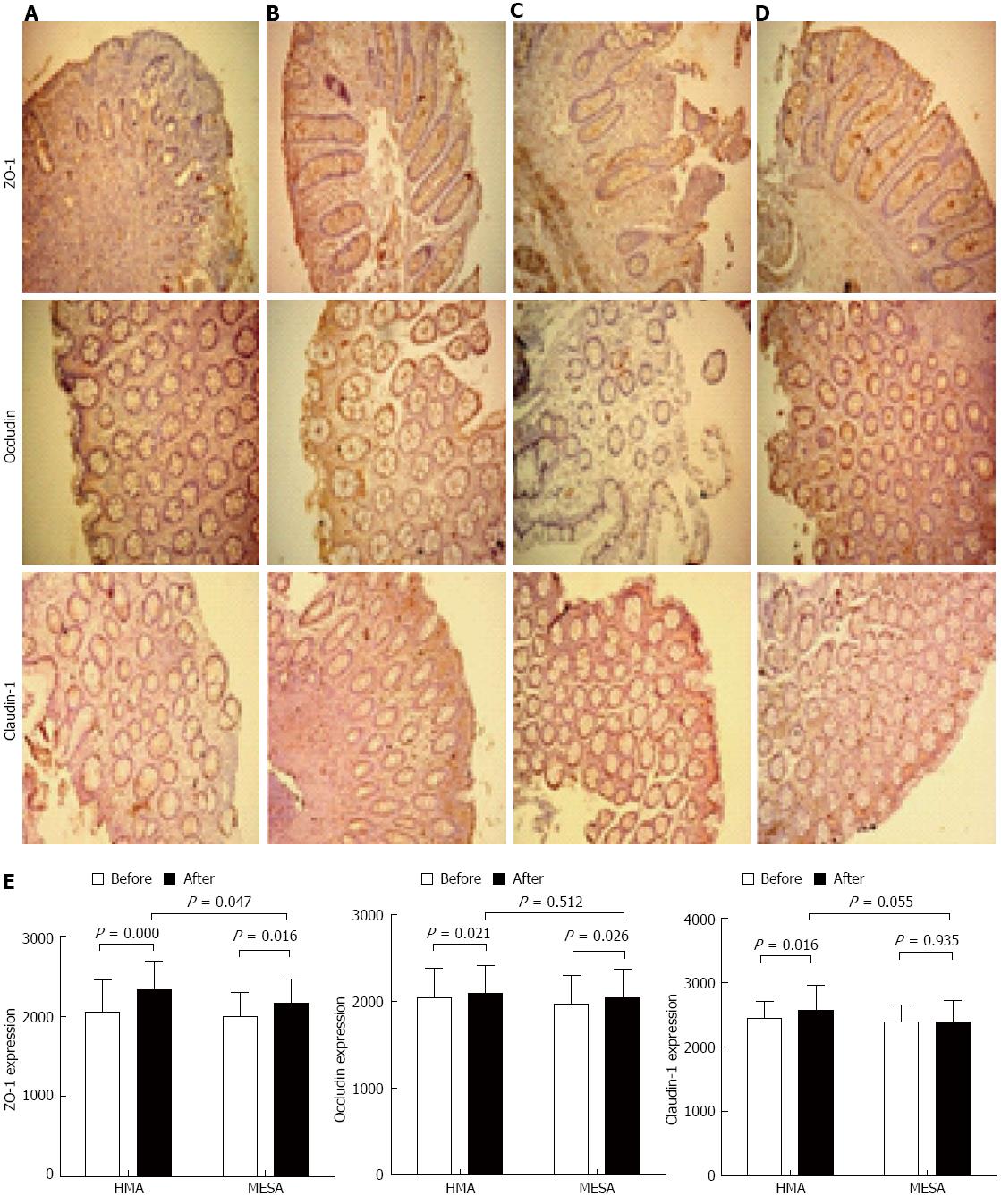
After treatment, the HMA group showed significantly increased expression of occludin (P = 0.021), claudin-1 (P = 0.016), and ZO-1 (P = 0.000) (Figure 3A, B and E). The MESA group showed a significant increase in the expression of occludin (P = 0.026) and ZO-1 (P = 0.016). However, there was no significant increase in the expression of claudin-1 (P = 0.935) (Figure 3C-E). There was no statistical difference for the expression of occludin (P = 0.512) and claudin-1 (P = 0.055) between groups. The HMA group showed a significant improvement in ZO-1 expression compared to the MESA group (2333.34 ± 352.51 vs 2160.38 ± 307.08, P = 0.047) (Figure 3B, D and E).
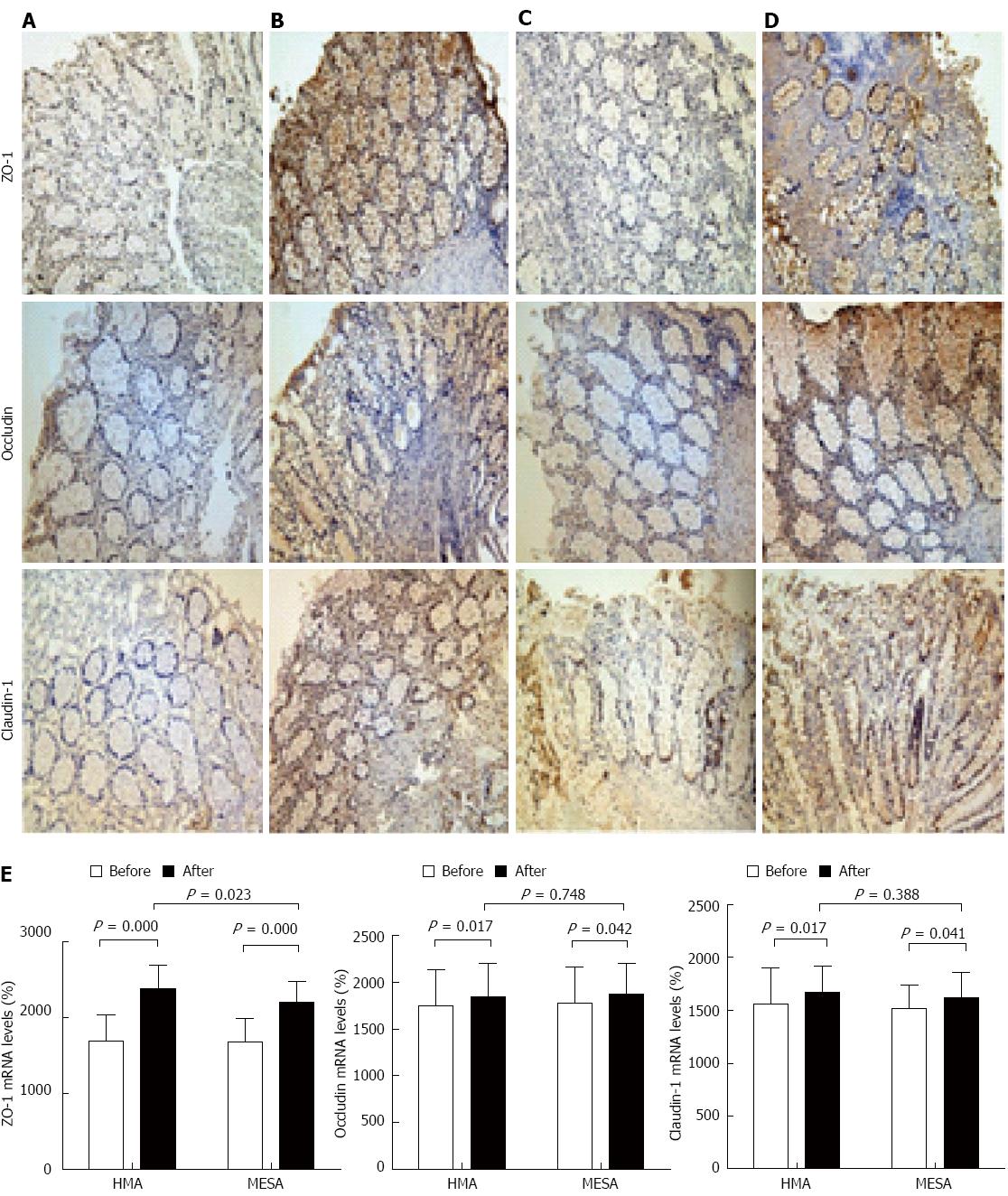
The HMA group showed significant increases in the expression of ZO-1 mRNA (P = 0.000), occludin mRNA (P = 0.017), and claudin-1 mRNA (P = 0.017) (Figure 4A, B and E). The MESA group showed significant increases in the expression of ZO-1 mRNA (P = 0.000), occludin mRNA (P = 0.042), and claudin-1 mRNA (P = 0.041) (Figure 4C-E). There was no difference between groups in the expression of occludin mRNA and claudin-1 mRNA (P = 0.748, P = 0.388). The HMA group showed a significant increase in ZO-1 mRNA expression compared to the MESA group (2378.17 ± 308.77 vs 2200.56 ± 281.88, P = 0.023) (Figure 4B, D and E).
The dominant symptom of CD is “leak-flux diarrhea” due to epithelial barrier dysfunction, which results in increased epithelial permeability and a continuous loss of solutes[21]. Previous studies have shown that increases in intestinal permeability not only act as an etiological factor in CD[22] but also precede clinical relapses in CD and are an indicator of subclinical disease[13,23]. However, increased intestinal permeability has also presented in first-degree relatives of CD patients in the absence of clinical symptoms[24,25]. This result suggests that increased intestinal permeability might be one of several pathogenic factors in CD. In recent decades, new findings have revealed the major factors underlying CD etiopathogenesis. These factors include the following: excessive bacterial translocation caused by intestinal epithelial barrier dysfunction[26], infection resulting in dysfunction of immunotolerance and aggressive immune response to bacteria[27,28], significant loss of complexity in species of the Firmicutes and the Bacteroidetes phyla and increased Enterobacteriaceae, particularly Escherichia coli species[29,30]. The epithelial barrier is the primary defense against exogenous pathogens. Thus, maintaining and repairing the epithelial barrier is crucial for the treatment and prevention of CD.
The epithelial barrier is a single layer of epithelial cells that lines the entire digestive tract. A TJ seals the intercellular space between adjacent epithelial cells[31], thus, serve as the major determinant of epithelial permeability[32]. The crucial feature of TJs is a fibril-like protein structure called TJ strands. The strands are connected with each other to create a continuous network. The TJ strands act as a diffusion barrier to regulate the transport of ions, macromolecules and immune cells in the paracellular pathway[33]. A TJ is a membrane-associated multimolecular complex composed of three transmembrane protein families[34]. The protein families consist of the claudin family[35], the junctional adhesion molecule (JAM) protein family[36] and the TJ-associated Marvel domain proteins (TAMPs) family, which includes occludin, tricellulin and Marvel D3[37]. Claudins are responsible for the charge[38] and size-selectivity[39,40] of the TJ barrier. The JAM proteins and TAMPs are mainly responsible for the stabilization of TJs and the regulation of epithelial permeability[41,42]. ZO-1 is a member of the membrane-associated guanylate kinases family. ZO-1 is a multi-domain scaffolding protein with an important role in the assembly of the TJ barrier and in the maintenance of the cytoskeleton[32] because it establishes a connection between the TJ barrier and perijunctional actomyosin[43].
Previous studies have shown that the expression of TJ proteins occludin, claudin-1, and ZO-1 were significantly decreased in the lamina propria in both active and chronic CD patients. The decrease in ZO-1 leads to increased intestinal epithelial permeability and TJ barrier dysfunction[44,45]. In this study, we evaluated occludin, claudin-1, and ZO-1 as indicators of intestinal epithelial barrier dysfunction during CD pathologic processes. We also examined the impact of HMA therapy on the intestinal epithelium. We chose mesalazine treatment for the control group patients because it is one of the most commonly used 5-ASA therapies used for treating mild to moderate CD. We compared HMA with mesalazine treatment to evaluate the possible application of HMA in CD management.
In this study, we evaluated the morphology and ultrastructure of intestinal mucosa tissue before and after treatment. Before treatment, the morphological observations using light microscopy showed epithelia impairment, intestinal gland loss and inflammatory cellular infiltration. These findings suggested that the basic structure of the epithelial mucosa was damaged and that mucosal inflammation occurred. Furthermore, analysis of the tissue ultrastructure by electron microscopy showed intercellular connection loss and broadened intercellular spaces. These results suggested that TJs were damaged and failed to strengthen the intercellular connections and seal the intercellular spaces. These defects may lead to a continuous loss of solute and compromise resistance against pathogens in the gut lumen. After the treatments, the morphological and ultrastructural observations of the MESA group showed partially recovered but still disorganized intestinal glands. There was also limited inflammatory cellular infiltration and comparatively strengthened intercellular connections with partially narrowed intercellular spaces. These results suggest that mesalazine ameliorate inflammation in the intestinal mucosa and can restore the TJ barrier function. In the HMA group, the morphological and ultrastructural observations revealed regularly arranged intestinal glands, mild and localized inflammatory cellular infiltration, tight intercellular connections and few broadened intercellular spaces. These findings suggest that HMA induces mucosa inflammation remission and repairs the TJ barrier structure. Our comparisons between the MESA group and the HMA group indicate that HMA achieves inflammatory remission similar to mesalazine and surpasses mesalazine in repairing the TJ barrier structure in intestinal epithelial mucosa.
We compared the expression of the TJ proteins and their mRNAs before and after treatment. The data show that the expression of occludin, claudin-1, and ZO-1 and their mRNAs are significantly increased in both the MESA and HMA group after treatment. This result in combination with morphological evidence suggests the mechanism of mesalazine and HMA is to repair the TJ barrier by upregulating the expression of TJ proteins such as occludin, claudin-1, and ZO-1 and their mRNAs. The expression of occludin and claudin-1 and their mRNAs showed no significant differences between the MESA group and the HMA group. However, the HMA group showed a significant increase in ZO-1 (P = 0.047) and ZO-1 mRNA (P = 0.023) expression compared to the MESA group.
ZO-1 mediates the assembly of TJs by organizing components of TJs and linking them to the cortical actin cytoskeleton[46]. We found that there was a significant difference between groups in the expression ZO-1 and its mRNA, which may suggest a different mechanism of HMA and mesalazine in CD patients. However, the specific functional mechanism of HMA still requires further investigation.
This study is only an initial attempt to investigate the effectiveness of HMA treatment and its underlying mechanism. We should examine several questions in future studies. What is the indication for HMA in CD treatment - mild or moderate CD? Is HMA alone effective in controlling CD symptoms, or does HMA only function as a supplement to conventional management? Is there any short-term or long-term adverse reaction to HMA, and what is the corresponding measurement?
In conclusion, HMA improves intestinal epithelial barrier repair and reduces inflammation in CD patients by upregulating the expression of the TJ proteins occludin, claudin-1, and ZO-1 and their mRNAs.
Crohn’s disease (CD) is a chronic, recurrent inflammatory bowel disease. Although its etiopathogenesis remains obscure, compromised permeability of the intestinal epithelial barrier is recognized to play a pivotal role in CD pathology.
Tight junctions (TJs) within intestinal epithelial cells form the structural basis of the intestinal epithelial barrier and are the major determinant of epithelial permeability. Recent studies have confirmed that the decreased expression of TJ proteins is positively correlated with CD severity.
Previous studies by Shang et al have demonstrated the efficiency of acupuncture and herb-partitioned moxibustion in improving CD patients’ clinical symptoms and pathological changes. In the current study, they found that herb-partitioned moxibustion combined with acupuncture (HMA) can improve epithelial barrier repair and reduce inflammation by upregulating the expression of TJ proteins in CD patients.
This study provides initial evidence of the therapeutic effect of HMA on CD by upregulating the expression of intestinal epithelial TJ proteins and their mRNAs. Therefore, HMA might be considered as an alternative option to treat mild to moderate CD.
The intestinal epithelial barrier is a monolayer lining the entire digestive tract. Tight junctions are fibril-like structures connecting adjacent cells, sealing the intercellular spaces to control paracellular transportation of ions, macromolecules and immune cells.
This study evaluated whether herb-partitioned moxibustion combined with acupuncture changes the permeability of the intestinal epithelial barrier by affecting the expression of colonic epithelial TJ-related proteins in mild to moderate CD patients. The question posed by the authors is well defined and the methods are described appropriately. The data and figures in the manuscript appear to be genuine.
| 1. | Hedrick TL, Friel CM. Colonic crohn disease. Clin Colon Rectal Surg. 2013;26:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Ye L, Cao Q, Cheng J. Review of inflammatory bowel disease in China. ScientificWorldJournal. 2013;2013:296470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol. 2010;24:127-133. [PubMed] |
| 4. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1040] [Article Influence: 65.0] [Reference Citation Analysis (1)] |
| 5. | Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20:11525-11537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, Hahn EG, Schuppan D. Acupuncture and moxibustion in the treatment of active Crohn’s disease: a randomized controlled study. Digestion. 2004;69:131-139. [PubMed] |
| 9. | Shi Y, Wu HG. The clinical study on herbs-partitioned moxibustion treatment of Crohn’s disease. JiangXi Zhongyi Xueyuan Xuebao. 2003;38:16-17. |
| 10. | Shi Y, Bao CH, Wu HG, Chen WF, Qin XD, Zhang R, Wu LY. Effect of herbs-partitioned moxibustion combined with acupuncture on the expressions of intestinal mucosa TNF-α, TNFR1, TNFR2 and apoptosis of intestinal epithelial cells in Crohn’s disease patients. Shanghai Zhongyiyao Zazhi. 2011;45:46-50. |
| 11. | Bao CH, Zhao JM, Liu HR, Lu Y, Zhu YF, Shi Y, Weng ZJ, Feng H, Guan X, Li J. Randomized controlled trial: moxibustion and acupuncture for the treatment of Crohn’s disease. World J Gastroenterol. 2014;20:11000-11011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627-1632. [PubMed] |
| 13. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437-1439. [PubMed] |
| 14. | Shi Y, Zhou EH, Wu HG, Zhou CL, Wang QY, Qi L. Moxibustion treatment restoring the intestinal epithelium barrier in rats with Crohn’s disease by down-regulating tumor necrosis factor alpha, tumor necrosis factor receptor 1, and tumor necrosis factor receptor 2. Chin J Integr Med. 2011;17:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Bao CH, Wu LY, Shi Y, Wu HG, Liu HR, Zhang R, Yu LQ, Wang JH. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn’s disease. World J Gastroenterol. 2011;17:4960-4970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851-G857. [PubMed] |
| 18. | Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Bao CH, Wu HG, Ma XP, Yu LQ, Zhang R, Chen WF. [Effect of moxibustion on colonic TNF-alpha content and influence of colonic supernatant of crohn’s disease rats undergoing moxibustion on expression of occludin, claudin-1 and zonula occludens-1 proteins and genes in cultured colonic epithelial cells]. Zhen Ci Yan Jiu. 2011;36:235-241. [PubMed] |
| 20. | The branch of inflammatory bowel disease collaborative group of digestive disease of Chinese Medical Association. Chinese normative consensus on the diagnosis and treatment of Inflammatory bowel disease. Chin J Intern Med. 2008;47:73-79. [DOI] [Full Text] |
| 21. | Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 22. | Hollander D. Crohn’s disease--a permeability disorder of the tight junction? Gut. 1988;29:1621-1624. [PubMed] |
| 23. | Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97:927-931. [PubMed] |
| 24. | Hollander D. Permeability in Crohn’s disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848-1851. [PubMed] |
| 25. | Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802-807. [PubMed] |
| 26. | Carrière J, Darfeuille-Michaud A, Nguyen HT. Infectious etiopathogenesis of Crohn’s disease. World J Gastroenterol. 2014;20:12102-12117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Yamamoto-Furusho JK. Genetic factors associated with the development of inflammatory bowel disease. World J Gastroenterol. 2007;13:5594-5597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 469] [Article Influence: 31.3] [Reference Citation Analysis (3)] |
| 29. | Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis. 2008;14:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3505] [Article Influence: 184.5] [Reference Citation Analysis (1)] |
| 31. | Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 32. | Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1085] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 33. | Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 34. | Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J Cell Sci. 2013;126:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [PubMed] |
| 36. | Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520-20526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 334] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 38. | Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142-C147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 727] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 40. | Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 41. | Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1051] [Cited by in RCA: 999] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 42. | Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930-3940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 44. | Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res. 2004;116:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 45. | Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 1081] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Jang SH, Shi Y S- Editor: Yu J L- Editor: Logan S E- Editor: Zhang DN