Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4975
Peer-review started: December 1, 2014
First decision: December 26, 2014
Revised: January 16, 2015
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: April 28, 2015
Processing time: 150 Days and 6.3 Hours
AIM: To evaluate the impact of metadoxine (MTD) on the 3- and 6-mo survival of patients with severe alcoholic hepatitis (AH).
METHODS: This study was an open-label clinical trial, performed at the “Hospital General de México, Dr. Eduardo Liceaga”. We randomized 135 patients who met the criteria for severe AH into the following groups: 35 patients received prednisone (PDN) 40 mg/d, 35 patients received PDN+MTD 500 mg three times daily, 33 patients received pentoxifylline (PTX) 400 mg three times daily, and 32 patients received PTX+MTD 500 mg three times daily. The duration of the treatment for all of the groups was 30 d.
RESULTS: In the groups treated with the MTD, the survival rate was higher at 3 mo (PTX+MTD 59.4% vs PTX 33.3%, P = 0.04; PDN+MTD 68.6% vs PDN 20%, P = 0.0001) and at 6 mo (PTX+MTD 50% vs PTX 18.2%, P = 0.01; PDN+MTD 48.6% vs PDN 20%, P = 0.003) than in the groups not treated with MTD. A relapse in alcohol intake was the primary independent factor predicting mortality at 6 mo. The patients receiving MTD maintained greater abstinence than those who did not receive it (74.5% vs 59.4%, P = 0.02).
CONCLUSION: MTD improves the 3- and 6-mo survival rates in patients with severe AH. Alcohol abstinence is a key factor for survival in these patients. The patients who received the combination therapy with MTD were more likely to maintain abstinence than those who received monotherapy with either PDN or PTX.
Core tip: Severe alcoholic hepatitis (AH) has a high mortality rate. Oxidative stress and the depletion of mitochondrial glutathione are factors implicated in injury to the liver. Metadoxine (MTD), an antioxidant that participates in the synthesis of glutathione and inhibits hepatic lipid accumulation, appears to be a novel therapeutic agent in patients with severe AH because it improves their 3- and 6-mo survival. The patients who received MTD were also better able to abstain from alcohol use, which is a key factor contributing to the improved survival in patients with severe AH.
- Citation: Tijera FHDL, Servín-Caamaño AI, Serralde-Zúñiga AE, Cruz-Herrera J, Pérez-Torres E, Abdo-Francis JM, Salas-Gordillo F, Pérez-Hernández JL. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol 2015; 21(16): 4975-4985
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4975
Severe alcoholic hepatitis (AH) has a high mortality rate despite its standard therapy[1,2]. Some populations, such as Hispanics, have responded poorly to standard therapy and show a mortality rate similar to those treated with placebos, particularly in patients classified as Age-Bilirubin-International normalized ratio-Creatinine (ABIC) classes B and C[3-5]. Therefore, it is important to search for new therapeutic options that are effective and safe.
Acute and chronic alcohol exposure is associated with high oxidative stress[6] and liver injury mediated by acetaldehyde[7,8]. Reactive oxygen species (ROS) are responsible for activating redox-sensitive transcription factors, such as nuclear factor-kappa B (NF-κB), thereby maintaining a pro-inflammatory profile[7].
Metadoxine (MTD), an antioxidant, participates in the synthesis of glutathione (GSH) and inhibits hepatic steatosis[9]. The preliminary findings in patients with severe AH have demonstrated that MTD in combination with glucocorticoids improves the survival rates at 30 and 90 d as well as the response to steroid therapy, according to the Lille score[10].
The aim of this study was to evaluate the impact of MTD added to standard therapy using prednisone (PDN) or pentoxifylline (PTX) compared with monotherapy on the 3- and 6-mo survival rates of patients with severe AH.
This study was a randomized, open-label clinical trial, performed at the “Hospital General de Mexico, Dr. Eduardo Liceaga”, Mexico City, from April 2010 to December 2013.
We used the Epidat 3.1 (Galicia, Spain 2006) statistical program to calculate the sample size, considering a difference in the survival rate at 3 mo of 30% between the groups receiving MTD and those receiving the standard treatment to be significant. We also considered a two-tailed confidence level of 95%, a potency of 80%, and an additional 20% of subjects as potential losses. There were a total of 35 patients per group.
Patients between 18 and 65 years old who met the clinical and biochemical criteria of severe AH[11,12], as characterized by a history of chronic and heavy alcohol intake (> 80 g/d for the previous 5 years), the rapid onset of jaundice in the absence of a biliary tract obstruction, painful hepatomegaly and ascites, transaminases ≥ two times above the normal value, an aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio ≥ 2, neutrophilia, a total bilirubin > 5 mg/dL, and a Maddrey’s discriminant function > 32 (calculated with the formula [4.6 × (patient prothrombin time (PT)-control PT, in seconds) + total bilirubin in mg/dL]), were included in the study.
Patients with acquired immunodeficiency syndrome; neoplasms; autoimmune diseases; psychiatric disorders other than alcoholism, such as depression and anxiety according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); a history of atopy or asthma; diabetes; obesity; pregnancy; hepatitis B or C virus infection; or tuberculosis were excluded from the study.
Patients with an intake of illicit drugs, herbal products, antioxidant supplements (multivitamins, S-adenosyl-L-methionine, MTD, silymarin), or previous treatment with steroids or PTX within the previous two years were excluded.
Patients without family support or without access to telephone communication were also excluded.
We obtained written informed consent from all of the patients before they were enrolled in the study. On the day of admission, we performed a clinical history and collected peripheral blood samples to determine each patient’s glucose, urea, creatinine, total bilirubin, alkaline phosphatase, gamma glutamyl transpeptidase, ALT, AST, sodium, potassium, albumin, leucocytes, neutrophils, hemoglobin, platelets, PT and international normalized ratio levels. The patients were also tested for HBsAg, anti-HBs, anti-HBc, IgM anti-HBc, with serological screening for the hepatitis C virus and human immunodeficiency virus. The screening for bacterial infections included urine, blood and ascites cultures, as well as chest radiography and a neutrophil count in ascites. A liver ultrasound and endoscopy were performed to determine the presence of varices in all of the patients.
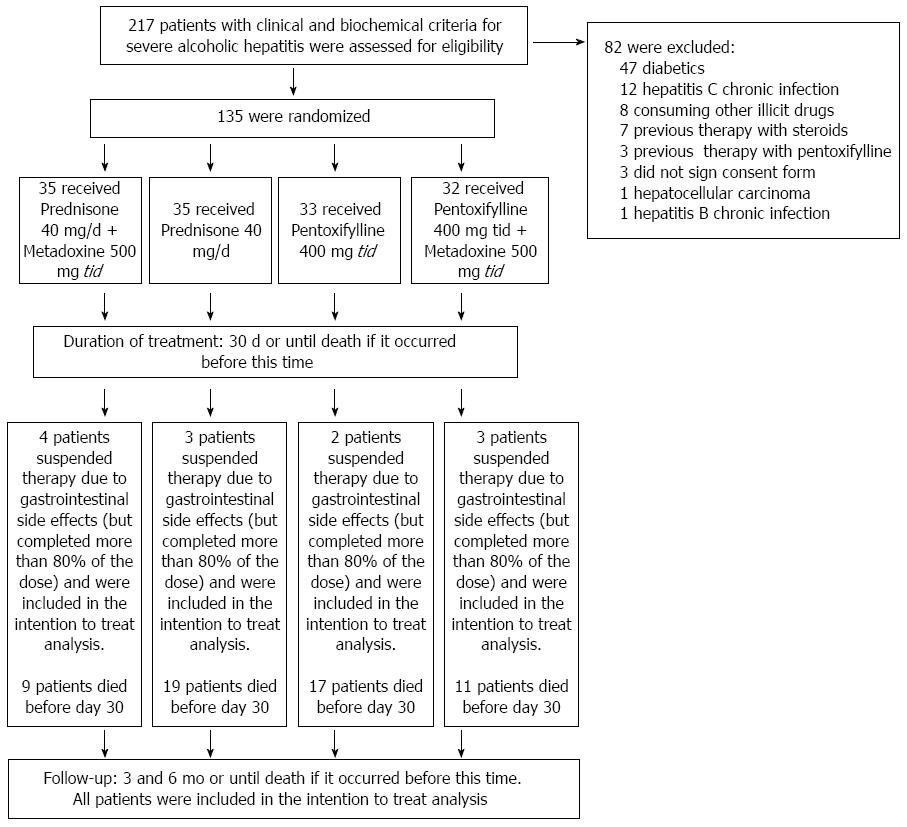
The patients were randomized into four treatment groups. For the randomization, we used the Epidat 3.1 statistical program (Galicia, Spain 2006) to construct a table of random numbers to compose four groups of equal size (Figure 1).
We evaluated 217 patients who met clinical and biochemical criteria for severe AH. However, 82 subjects met one or more exclusion criteria: 47 were diabetics, 12 had hepatitis C chronic infection, 8 consuming illicit drugs, 7 were previously treated with steroids, 3 were previously treated with PTX, 3 did not sign consent form, 1 had also hepatocellular carcinoma, and 1 had also hepatitis B chronic infection. Therefore, 135 subjects who met all eligibility criteria and provided written informed consent were randomly assigned to one of four groups of treatment: A group receiving PDN (at a dose of 40 mg once daily); a group receiving PDN (at a dose of 40 mg once daily) and MTD (at a dose of 500 mg three times daily); a group receiving PTX (at a dose of 400 mg three times daily); a group receiving PTX (at a dose of 400mg three times daily) and MTD (at a dose of 500 mg three times daily). In all cases, the duration of treatment was 30 d, or until death if it occurs earlier. The 500-mg metadoxine tablets (Abrixone®) were provided by Eurodrug Laboratories as a donation for our institution. The 40-mg prednisone tablets and the 400-mg pentoxifylline tablets provided by our institution. Eurdorug Laboratories provided comments regarding the study design but were not involved in the writing of the protocol, the conduct of the trial, the decision-making with respect to the trial, the analysis of the data, or the preparation of the manuscript. The doses were selected on the basis of previous studies[1,2,13]. The dose for metadoxine was selected on the basis of the study conducted by Caballería et al[13].
The primary endpoints were the 3- and 6-mo survival rates. The secondary endpoints were the development or progression of acute renal failure (ARF), variceal bleeding (VB), hepatic encephalopathy (HE), bacterial or fungal infections, adverse effects and a relapse to alcohol consumption between 30 d and 6 mo of the follow-up. We also performed a sub-analysis stratifying the patients according to their ABIC class.
The patients were monitored weekly during the first month, two times per month during the second and third months, and monthly thereafter until 6 mo. Each visit included a clinical examination and the collection of peripheral blood samples. During the first two weeks, all of the patients were hospitalized; subsequently, each investigator determined the duration of the hospitalization. For the hospitalized patients, the medications were administered under the supervision of physicians and nurses. For outpatients, adherence to treatment was monitored by a family member and reported in a control diary. To increase compliance with the medication regimen, the patients were required to return the empty blisters at each visit.
In patients who were suspected of developing an infection during the study, cultures and chest radiography were performed if necessary. In patients who developed odynophagia or dysphagia, an endoscopic study was performed to identify esophageal candidiasis; when suspicious lesions were observed, brushing and mycological examinations were performed.
ARF: This condition was defined according to the criteria of the Acute Kidney Injury Network as an abrupt reduction (48 h) in renal function characterized by an increase of 0.3 mg/dL in the serum creatinine compared with the baseline value. The patients who had a baseline value of serum creatinine ≥ 1.5 mg/dL at the time of admission were considered to have ARF[14,15]. These patients were treated with intravascular volume expansion using an albumin infusion at 1 g/kg for 48 h. The patients who did not respond to this treatment were evaluated for hepatorenal syndrome (HRS) according to the Ascites International Club criteria and were treated with a vasopressor (terlipressin or norepinephrine) and intravascular volume expansion with albumin[16,17].
HE: This condition was defined clinically by both neuropsychiatric alterations and neuromuscular signs according to the West-Haven criteria[18]. Patients with an HE grade of I or II were treated orally with L-ornithine-L-aspartate. Patients with an HE grade of III or IV were treated intravenously with L-ornithine-L-aspartate. In patients for whom L-ornithine-L-aspartate was contraindicated, oral lactulose for HE grades I or II and lactulose enemas for HE grades III or IV were prescribed.
VB: This condition was defined by the presence of melena or hematemesis associated with gastroesophageal varices as determined by an endoscopy. These patients were treated with terlipressin or octreotide, and fresh frozen plasma and blood were transfused as necessary. An endoscopic band ligation for esophageal varices or cyanoacrylate injection for gastric varices was performed, and antibiotic prophylaxis was prescribed. Subsequently, the patients received a secondary prophylaxis[19].
Spontaneous bacterial peritonitis: This condition was defined and treated according to the most recent guidelines of the European Association for Study of Liver Diseases[17].
Other infections: Urinary tract infections were diagnosed in the patients having urinary symptoms that were associated with abnormal urinary examinations and urinary cultures, including a bacterial count greater than 100000 CFU. An antibiotic therapy was prescribed based on the results of the urinary cultures.
Pneumonia was diagnosed in the patients who developed a cough with expectoration and confirmation on the chest X-ray. Treatment was initiated with ceftriaxone and clarithromycin, after which the antibiotic therapy was adjusted depending on the sputum culture results.
When patients developed odynophagia or dysphagia, esophageal candidiasis was suspected. The diagnosis was confirmed through oral cavity examinations and an endoscopy indicating the presence of compatible lesions; brushing was performed for a mycological examination in all of the cases, and treatment with fluconazole 100 mg twice daily was prescribed.
Patients with diarrhea were evaluated by microscopic examinations of fresh stool and stool cultures. These patients were treated empirically with ciprofloxacin and/or metronidazole.
The statistical methods of this study were reviewed by Fátima Higuera-de la Tijera, MD, MSc. and José L. Pérez Hernández, MD, MSc. From the “Hospital General de México, Dr. Eduardo Liceaga”. The distribution of variables was analyzed; in cases of quantitative variables with a non-normal distribution base, a 10-logarithmic transformation was performed to normalize their distribution for the analysis using parametric tests. Descriptive statistics were used. The quantitative variables were expressed as the mean ± SD, and the qualitative variables were expressed as proportions and percentages. To compare the basal characteristics between the groups, a one-way ANOVA was performed for the quantitative variables. Tukey’s or Tamhane’s T2 tests were used according to the homogeneity of the variance for the post hoc tests, and a χ2 test with Yates correction or Fisher’s exact test were used for the qualitative variables. To compare the primary and secondary endpoints between the groups, an analysis with an intention to treat (ITT) was conducted. The χ2 test with a Yates correction, Fisher’s exact test or Student’s t-test was used when needed, based on the variable type. A survival analysis was performed using Kaplan-Meier curves to evaluate the 3- and 6-mo survival and to evaluate the alcohol intake relapse between 30 d and 6 mo of follow-up and compare it with the log-rank test. To identify the main risk factors associated with 6-mo mortality, a multivariate analysis using a Cox regression was conducted. SPSS version 18.0 (Chicago, IL, United States 2009) and Epidat 3.1 (Galicia, Spain 2006) were used to perform the statistical analyses. A two-sided P value of 0.05 was considered to be statistically significant.
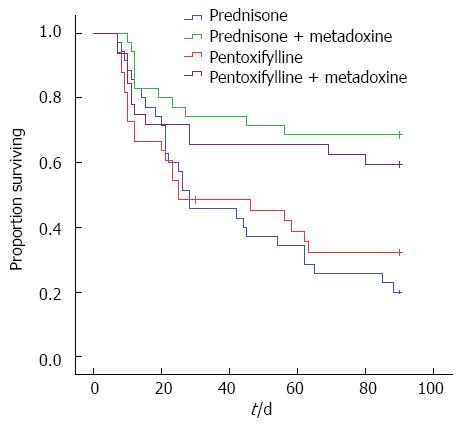
The baseline characteristics of the patients are listed in Table 1. In the groups receiving MTD, the survival rate was significantly higher at 3 mo than in the groups not receiving MTD: PTX+MTD 19/32 (59.4%) vs PTX 11/33 (33.3%), P = 0.04; PDN+MTD 24/35 (68.6%) vs PDN 7/35 (20%), P = 0.0001 (Figure 2).
| Characteristic | PDN (n = 35) | PDN+MTD (n = 35) | PTX (n = 33) | PTX+MTD (n = 32) | P value |
| Male, n (%) | 33 (94.3) | 32 (91.4) | 31 (93.9) | 28 (87.5) | 0.40 |
| Age, yr | 43.1 ± 9.5 | 43.4 ± 9.0 | 43.4 ± 9.9 | 44.4 ± 9.1 | 0.94 |
| Alcohol intake, g/d | 313.7 ± 157.1 | 328.9 ± 142.5 | 365.9 ± 196.2 | 346.5 ± 173.6 | 0.61 |
| Child-Pugh | 12.4 ± 1.0 | 12.4 ± 0.9 | 12.6 ± 1.0 | 12.5 ± 1.0 | 0.82 |
| Maddrey´s modified discriminant function | 70.9 ± 25.6 | 67.3 ± 19.3 | 93.4 ± 84.7 | 78.3 ± 40.2 | 0.14 |
| MELD | 28 ± 4 | 29 ± 6 | 31 ± 9 | 31 ± 7 | 0.19 |
| ABIC | 8.199 ± 1.3 | 8.604 ± 2.9 | 8.675 ± 1.8 | 8,677 ± 1.6 | 0.73 |
| Urea, mg/dL Log10 | 1.60 ± 0.34 | 1.64 ± 0.31 | 1.66 ± 0.32 | 1.64 ± 0.42 | 0.94 |
| Creatinine, mg/dL | 1.5 ± 0.7 | 1.5 ± 0.8 | 1.7 ± 1.1 | 1.9 ± 1.5 | 0.21 |
| Sodium, mEq/L | 131.3 ± 5.8 | 132.7 ± 5.8 | 130.9 ± 5.1 | 131.7 ± 4.7 | 0.57 |
| Albumin, mg/dL | 1.9 ± 0.4 | 1.9 ± 0.5 | 1.8 ± 0.4 | 1.8 ± 0.5 | 0.89 |
| Total bilirubin, mg/dL | 24.4 ± 10.5 | 24.5 ± 10.1 | 23.0 ± 9.5 | 25.9 ± 11.6 | 0.73 |
| Alkaline phosphatase, UI/L Log10 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.4 ± 0.2 | 0.29 |
| Gamma-glutamyltransferase, UI/L Log10 | 2.5 ± 0.3 | 2.4 ± 0.3 | 2.4 ± 0.4 | 2.4 ± 0.3 | 0.73 |
| Aspartate aminotransferase, UI/L Log10 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.3 | 0.39 |
| Alanine aminotransferase, UI/L Log10 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.4 | 0.28 |
| Leucocytes, cell/mm3 | 20.9 ± 8.0 | 18.5 ± 8.0 | 19.5 ± 9.0 | 19.2 ± 10.3 | 0.71 |
| Neutrophils, cell/mm3 | 16.2 ± 7.0 | 15.7 ± 7.5 | 16.6 ± 8.5 | 17.0 ± 9.9 | 0.92 |
| Hemoglobin, g/dL | 11.6 ± 2.7 | 11.8 ± 2.5 | 10.9 ± 3.0 | 11.4 ± 2.4 | 0.52 |
| Platelets, cell/mm3 | 178.1 ± 119.5 | 186.0 ± 113.6 | 182.8 ± 117.7 | 153.0 ± 84.0 | 0.61 |
| Prothrombin time in seconds | 22.0 ± 5.8 | 21.1 ± 3.6 | 27.6 ± 18.5 | 23.2 ± 8.8 | 0.06 |
| INR | 1.9 ± 0.5 | 1.8 ± 0.3 | 2.4 ± 1.9 | 2.0 ± 0.7 | 0.06 |
| Cirrhosis on liver ultrasound, n (%) | 25 (71.4) | 20 (57.1) | 26 (78.8) | 20 (62.5) | 0.24 |
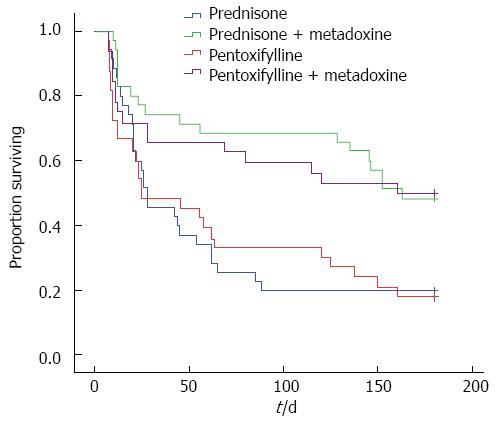
In the groups receiving the MTD, the survival rate was significantly higher at 6 mo than in the groups not receiving MTD: PTX+MTD 16/32 (50%) vs PTX 6/33 (18.2%), P = 0.01; PDN+MTD 17/35 (48.6%) vs PDN 7/35 (20%), P = 0.003; (Figure 3).
There was no difference in the survival rates between the PDN and PTX monotherapy groups at 3 and 6 mo. There was no difference in the survival rate between the PDN+MTD and PTX+MTD groups at 3 and 6 mo.
According to their ABIC class, 13 patients (9.6%) were classified as class A, 82 patients (60.7%) as class B, and 40 patients (29.6%) as class C. The global survival according to the ABIC class was 10 patients (76.9%) for class A, 42 patients (51.2%) for class B, and 9 patients (22.5%) for class C. We performed a sub-analysis stratifying patients according to their ABIC class according to the two different groups of treatment into the group of 67 patients who received concomitant therapy with MTD (MTD Group) and the group of 68 patients who did not receive MTD (the standard therapy or ST Group). The improvement in the survival in the MTD Group was observed primarily in the ABIC class B: the MTD Group 30/38 (78.9%) vs the ST Group 12/44 (27.3%), P = 0.0001. There were no significant differences between the treatment groups in either the ABIC class A or ABIC class C patients: the MTD Group 6/6 (100%) vs ST Group 4/7 (57.1%) P = 0.19, and the MTD Group 7/23 (30.4%) vs ST Group 2/17 (11.8%) P = 0.25, respectively.
Regarding the development of complications at 3 mo of follow-up, there was significantly less development of HE and HRS in the patients who received the concomitant therapy vs the patients who received the PDN alone. There was no difference between the PTX+MTD vs the PTX group. Neither were there any differences among the groups regarding the development of VB or infections (Table 2)
| Complication | PDN+MTD (n = 35) | PDN (n = 35) | PTX+MTD (n = 32) | PTX (n = 33) | P value2 | HR (95%CI)3 | P value4 | HR (95%CI)5 |
| HE | 10 (28.6) | 21 (60.0) | 13 (40.6) | 17 (51.5) | 0.0081 | 0.2 (0.1-0.7) | 0.38 | 0.6 (0.2-1.7) |
| HRS | 11 (31.4) | 19 (54.3) | 11 (34.4) | 16 (48.5) | 0.051 | 0.3 (0.1-1.0) | 0.25 | 0.5 (0.2-1.5) |
| VB | 10 (28.6) | 13 (37.1) | 11 (34.4) | 14 (42.4) | 0.44 | 0.6 (0.2-1.8) | 0.51 | 0.7 (0.2-1.9) |
| Infections | 11 (31.4) | 14 (40.0) | 11 (34.4) | 12 (36.4) | 0.45 | 0.6 (.02-1.8) | 0.87 | 0.9 (0.3-2.5) |
| None | 24 (68.6) | 21 (60) | 21 (65.6) | 21 (63.6) | ||||
| UTI | 0 (0) | 3 (8.6) | 3 (9.4) | 6 (18.25) | ||||
| SBP | 2 (5.7) | 2 (5.7) | 3 (9.4) | 3 (9.15) | ||||
| Pneumonia | 9 (25.7) | 7 (20) | 1 (3.1) | 1 (3.0) | ||||
| EC | 0 (0) | 1 (2.85) | 3 (9.4) | 1 (3.0) | ||||
| Diarrhea | 0 (0) | 1 (2.85) | 1 (3.1) | 1 (3.0) |
The occurrence of adverse effects was similar in all of the groups, principally consisting of epigastric burning, nausea and vomiting, due to which 12 patients dropped out of the study. The patients who dropped out included 4 patients in the PDN and MTD group, 3 patients in the PDN group, 2 patients in the PTX group, and 3 patients in the PTX and MTD group. These patients were included in the ITT analysis because we verified that they had received at least 80% of the treatment. Serious adverse effects were not reported in any of the groups.
Maintenance of abstinence: Seventy-nine of the patients survived after 30 d (the end of therapy), 54 (68.4%) of whom had maintained alcohol abstinence and 25 (31.6%) of whom had relapsed into alcohol intake at 6 mo of follow-up. When we compared the groups, the patients receiving MTD were better able to maintain abstinence than the patients who did not receive MTD; MTD Group 35/47 (74.5%) vs ST Group 19/32 (59.4%), P = 0.02.
In the multivariate analysis, a relapse in alcohol intake was the primary independent factor predicting mortality at 6 mo. Additionally, the coexistence of cirrhosis on the ultrasound was identified as a predictor factor that was associated with mortality at 6 mo. In this study, the quantity of the alcohol intake was not associated with the 6-mo mortality. On the other hand, the treatment with MTD was identified as a protective factor (Table 3).
The mortality rate in our patients was high despite the treatment with PDN or PTX. However, other studies in Mexican population have also shown a high mortality rate and a poor response to steroid therapy. In a cross-sectional study, Ruíz-Zavála A reported a failure to respond to corticosteroids, evidenced by a Lille score greater than 0.45 in 90% of the patients diagnosed with alcoholic hepatitis (a mean Lille score of 0.80 ± 0.18)[20]. Additionally, in a clinical trial in Mexican patients with severe AH that compared treatment with PTX vs treatment with PDN, the mortality rate at 30 d was high, at 46.6% vs 59.9%, respectively, and there was no difference between the groups (P = 0.30)[3]. If we compare the mortality rates according to the ABIC class, Mexican patients have a higher mortality rate than other populations despite the treatment with steroids or PTX. The survival rate in the Mexicans vs the Europeans according to their ABIC class was as follows: an ABIC class of A, 81% vs 100%; an ABIC class of B, 50% vs 70%; and an ABIC class of C, 13% vs 25%, respectively[4,5]. The quantity of the alcohol intake may be an explanation for the higher mortality observed in the Mexican population, as Altamirano et al[5] demonstrated that the consumption of more than 120 g per day of alcohol is associated with greater mortality[5]. In our study, the mean alcohol intake was greater than 300 g per day. Moreover, Mexican-American males have a higher prevalence of alcoholic cirrhosis and a higher mortality rate compared with Caucasians[21].
Controversial results exist concerning whether steroids or PTX is superior for improving the survival of patients with severe AH. In our study, there was no difference in the survival between the PDN and the PTX groups. Neither was there a difference in the survival between the PDN+MTD and PTX+MTD groups. However, in 2009, De et al[22] performed a randomized, double-blind, controlled clinical trial to compare the efficacy of PTX and prednisolone in the treatment of severe AH. In this study, the probability of dying at 3 mo was higher in the prednisolone group compared with the PTX group (35.29% vs 14.71%, P = 0.04; log rank test). In 2013, a systematic review by Parker et al[2] that included ten trials and a total of 884 patients found that PTX appears to be superior to a placebo in the prevention of fatal HRS and thus may be an effective treatment for severe AH when corticosteroids are contraindicated. However, multiple trials have failed to show the superiority of either PTX or steroids. More recently, Park et al[23] found that PTX was not superior to prednisolone for improving the 6-mo survival rate (64.5% vs 72.9%, respectively; P = 0.23). The investigators in the “Steroids or Pentoxifyllline for Alcoholic Hepatitis” (STOPAH) trial[24], recently presented their findings at the Liver Meeting 2014 in Boston, Massachusetts. The STOPAH trial was a multicenter, double-blind, factorial (2 × 2) trial that included 1103 patients who were randomized to one of four groups: prednisolone + placebo, PTX + placebo, prednisolone + PTX, or a double placebo group. The investigators found that prednisolone, but not PTX, was associated with a lower risk of 28-d mortality. In contrast, the mortality rate in the group that received PTX was similar to the mortality rate of those who received the double placebo. Beyond 28 d, neither of the drugs was associated with a survival benefit, and infections were approximately twice as frequent in the prednisolone group.
Our study shows that treatment with MTD may have a protective role, as it improves 3- and 6-mo survival rates. MTD is the ion pair between pyridoxine and pyrrolidone carboxylate, the cyclic amide of glutamic acid that is responsible for the synthesis and catalysis of GSH[25]. Alcohol exposure is associated with high oxidative stress[6]. The oxidative pathway for metabolizing alcohol involves alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase, and both of these enzymatic reactions reduce nicotinamide dinucleotide (NAD) to its reduced form of NADH. An excess of NADH causes several metabolic disorders, including the inhibition of the Krebs cycle and fatty acid oxidation, which favors steatosis and hyperlipidemia[8]. Acetaldehyde participates in alcohol-mediated liver injury by causing cellular damage, inflammation, and fibrogenesis[7]; it promotes cell death by depleting the concentration of reduced GSH, inducing lipoperoxidation, and increasing the toxic effect of the free radicals. The ROS can oxidize and damage the DNA, proteins and unsaturated fatty acids, thereby altering cell function[6].
The oxidation of alcohol also occurs through cytochrome P450’s generation of ROS, such as hydrogen peroxide and superoxide ions. In particular, cytochrome P450 2E1 (CYP2E1) is increased several-fold and contributes to lipoperoxidation and liver injury. CYP2E1 also converts alcohol to acetaldehyde. The ROS are responsible for activating the redox-sensitive transcription factors, such as NF-κB, and maintaining a pro-inflammatory profile[7]. Other cytochromes, such as CYP1A2 and CYP3A4, may also contribute to the metabolism of ethanol[8].
Several studies have demonstrated that MTD increases the metabolism and depuration of ethanol[26,27] and acetaldehyde in the liver and plasma and prevents the damage caused by ethanol and acetaldehyde in both hepatocytes and hepatic stellate cells[28]. MTD also restores the concentrations of NAD, GSH[29] and adenosine triphosphate in the brain and liver[30,31] and acts as an antioxidant because the ion-pair molecule is capable of dissociating into N-oxide, which acts as scavenger to trap the ROS and free radicals[32,33]. MTD inhibits the synthesis of the fatty acid esters in the liver, reduces the hepatic content of the triglycerides and prevents the injuries associated with lipoperoxidation[13,34-36].
The global survival according to the ABIC class in our patients was similar to that reported by Altamirano et al[5] in a previous cohort of Mexican patients. The majority of our patients (60.7%) were categorized as ABIC class B. We believe that the greatest benefit that we observed using MTD therapy in the ABIC class B may have occurred because this group had the largest proportion of the patients compared with the ABIC classes A and C. However, further studies that include more patients who are categorized as ABIC classes A and C are needed to validate this assumption.
In regard to the development of complications, there was significantly less development of HRS in patients who had received PDN+MTD compared with those who received PDN alone at the 3-mo follow-up. Although there was no significant difference between PTX+MTD and PTX alone, 14.1% fewer patients in the group treated with PTX+MTD developed HRS compared with the patients who received PTX alone (34.4% vs 48.5%, respectively). In our study, MTD had a protective effect on renal function. Previous studies have shown that MTD decreases the formation of acetaldehyde macromolecular adducts in all targets of ethanol toxicity, including the brain, liver and kidneys. The effect in the kidneys is due to two mechanisms of action: the inhibition of adduct formation and the increased excretion rate of acetaldehyde[37].
The impaired renal function is closely associated with the elevation of inflammatory markers (tumor necrosis factor-α, interleukin-1β, and interleukin-6), leading to both an increase in markers of oxidative stress and a decreased in antioxidants[38-40]. All of these mechanisms are involved in the pathophysiology of SAH and could be modulated by MTD therapy.
There was less development of HE in the patients receiving therapy with PDN+MTD compared with those who received PDN alone. Moreover, 10.9% fewer patients developed HE in the group treated with PTX+MTD compared with the patients who received PTX alone (51.5% vs 40.6%, respectively). In our study, MTD demonstrated a protective effect over the patients’ mental status. Pyrrolidone carboxylate is an intermediate in the γ-glutamyl cycle, which is an amino acid transport system into the cell through the cell membrane. Unlike glutamic acid, the uptake of pyrrolidone carboxylate by the central nervous system (CNS) is possible because it crosses the hemato-encephalic barrier. In the CNS, it exerts a number of actions on the cognitive and memory functions that are affected by alcohol, and it is important and clinically relevant to restore those superior functions. Once hydrolyzed by oxoprolinase, the open glutamic acid becomes available for important metabolic processes. Its derivative, N-acetyl glutamate, which is released in the subsequent metabolic steps, plays an essential role in maintaining the nitrogen balance because it activates the carbamoyl-synthetase I, a key enzyme in the urea cycle. Furthermore, by reacting with oxaloacetate, an intermediate of the Kreb’s cycle, it participates in the biosynthesis of aspartate, an essential element in the urea cycle, which is therefore activated from two different entry points. In addition, glutamate may react with ammonia to form glutamine, thereby contributing to the elimination of toxic ammonia and to the nitrogen fixation by the organism[25,41-43].
In our study, a relapse in alcohol intake was the primary independent factor predicting mortality at 6 mo. Alcohol abstinence is considered to be the cornerstone of the management of AH. In the results from the STOPAH trial[24], a relapse in alcohol consumption had a deleterious effect; at 1 year, the patients who either did not reduce or who increased their alcohol consumption had a 3-fold risk for death compared with the patients who abstained (OR = 2.99; P < 0.001). The patients who reduced their alcohol consumption but not below a safe level still had a more than a 2-fold risk for death at 1 year compared with the patients who abstained (OR = 2.28; P = 0.032), as did the patients who reduced their alcohol consumption to below a safe level (OR = 2.17; P = 0.031). Wang et al[44] demonstrated that alcohol abstinence ameliorates AH by decreasing the liver enzyme and fibrotic markers and improving hepatic steatosis. In our study, the therapy with MTD helped patients to maintain alcohol abstinence. This finding is similar to those reported by several studies that have demonstrated that MTD is an effective therapy for abstinence[45-49]. Currently, disulfiram, naltrexone and acamprosate are approved for the treatment of alcoholic dependency; however, all of these medications are contraindicated in patients with severe liver disease[44], such as our patients. Patients who have recovered from an episode of severe alcoholic hepatitis must be supported in maintaining alcohol abstinence without risk or compromise to their liver function. Bono et al[49] found that alcoholic patients who received treatment with MTD achieved alcohol abstinence in a greater proportion compared with those who did not receive it. More recently, an interesting retrospective analysis by Leggio et al[45] found that patients with ALD who were treated with MTD had a significant decrease in drinks per week and demonstrated an improvement in the AST/ALT ratio compared with those who did not receive it. The beneficial neurological effects of MTD therapy in patients with attention-deficit/hyperactivity disorder have been demonstrated in several studies conducted by Manor et al[50-52]. In animal models, the effects of MTD on CNS have been studied. Ethanol and acetaldehyde increase the activity of dopamine neurons in the reward areas of the CNS, and these actions are associated with the rewarding and reinforcing properties of the ethanol. MTD may favor abstinence through its ability to metabolize and to clear ethanol and its metabolites from the organism, as well as through its direct effect on neurotransmitters such as gamma-aminobutyric acid, acetylcholine and dopamine, all of which are involved in the neurobiology of alcohol craving[45-52].
In this study, the presence of cirrhosis on the ultrasound was identified as a predictive factor associated with 6-mo mortality. In a study by Altamirano et al[53], the degree of fibrosis, degree of neutrophil infiltration, type of bilirubinostasis, and presence of megamitochondria were independently associated with 90-d mortality.
In conclusion, MTD improves the 3- and 6-mo survival in patients with severe AH, and it has a tendency to improve serious complications, such as HRS and HE, particularly when it is added to PDN. The greatest benefit of MTD therapy was observed in the ABIC class B patients. However, further studies including a greater sample size with a larger number of severe AH patients categorized as ABIC classes A and C are needed to demonstrate whether MTD also improves survival in these groups. This study reaffirms the knowledge that alcohol abstinence is a key factor for survival in severe AH patients and that MTD is a safe therapy that helps to achieve this objective.
Severe alcoholic hepatitis is a disease with a high mortality rate despite the use of standard therapy with steroids or pentoxifylline. Oxidative stress plays a key role in the physiopathology of alcoholic hepatitis and therefore represents a novel therapeutic target that must be investigated.
Previous studies have demonstrated that metadoxine increases the metabolism and depuration of ethanol and acetaldehyde in the liver and the plasma and prevents the damage caused by ethanol and acetaldehyde in the hepatocytes and hepatic stellate cells. Metadoxine also acts as an antioxidant because its ion-pair molecule is capable of dissociating into N-oxide, which acts as scavenger to trap the reactive oxygen species and free radicals. Furthermore, metadoxine can prevent the steatosis and injury associated with lipoperoxidation. Metadoxine is a drug currently indicated for treating acute alcohol intoxication; several studies have also validated its use for treating alcohol dependence. However, until the current study, metadoxine had not been evaluated as a therapy for patients with severe alcoholic hepatitis.
In the current study, the authors found that metadoxine is an effective therapy for severe alcoholic hepatitis; the patients treated with metadoxine had better survival at 3 and at 6 mo compared with those treated with standard therapy with steroids or pentoxifylline. Furthermore, it is well known that alcohol abstinence is an important factor associated with long-term survival in these patients. In this study, the authors found that the patients who received metadoxine were more likely to maintain alcohol abstinence, and their greater abstinence may be related to the improvements in the 6-mo survival in the patients treated with this drug.
The results of this study suggest that metadoxine could be used as an effective therapy for patients with severe alcoholic hepatitis, and the validated results of other previous studies have found that metadoxine is an effective therapy to achieve alcohol abstinence.
Severe alcoholic hepatitis is a condition characterized by a rapid onset of jaundice in the absence of biliary tract obstruction, painful hepatomegaly and ascites, transaminases ≥ two times above the normal values, an aspartate aminotransferase/alanine aminotransferase ratio ≥ 2, neutrophilia, a total bilirubin > 5 mg/dL, and a Maddrey’s discriminant function > 32 (calculated with the formula [4.6 × (patient prothrombin time (PT)-control PT, in seconds) + total bilirubin in mg/dL]), which occurs in patients with a history of chronic and heavy alcohol intake. Metadoxine is the ion pair between pyridoxine and pyrrolidone carboxylate, the cyclic amid of glutamic acid, which is responsible for the synthesis and catalyzation of glutathione.
This is an interesting study, focusing on a therapeutic area with largely unmet needs. This is a single-center open-label clinical trial comparing pentoxifylline, prednisone or metadoxine alone or in combination to assess their efficacy in severe alcoholic hepatitis. The results showed that the groups that received metadoxine achieved better survival. As in other studies, the maintenance of alcohol abstinence was the best predictor of survival. This study found that alcohol abstinence is an independent prognostic factor of the six-month mortality and that patients treated with metadoxine were more likely not to relapse into alcohol consumption. However, intervention did not prevent the complications associated with cirrhosis, which may be because the study was underpowered or that the effect of abstinence itself rather than the intervention was the main predictor for survival.
| 1. | Mathurin P, Mendenhall CL, Carithers RL, Ramond MJ, Maddrey WC, Garstide P, Rueff B, Naveau S, Chaput JC, Poynard T. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Garrido-García JR, Sánchez-Hernández G, Melchor-López A, Elizalde-Barrera CI, Sánchez-Vargas L. Pentoxifilina versus esteroide en la sobrevivencia a corto plazo en hepatitis aguda alcohólica severa [Article in Spanish]. Med Int Mex. 2012;28:227-233. |
| 4. | Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Altamirano J, Higuera-de laTijera F, Duarte-Rojo A, Martínez-Vázquez MA, Abraldes JG, Herrera-Jiménez LE, Michelena J, Zapata L, Perez-Hernández J, Torre A. The amount of alcohol consumption negatively impacts short-term mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol. 2011;106:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Voican CS, Perlemuter G, Naveau S. Mechanisms of the inflammatory reaction implicated in alcoholic hepatitis: 2011 update. Clin Res Hepatol Gastroenterol. 2011;35:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 8. | Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 458] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Váli L, Blázovics A, Fehér J. [The therapeutic effect of metadoxine on alcoholic and non-alcoholic steatohepatitis]. Orv Hetil. 2005;146:2409-2414. [PubMed] |
| 10. | Higuera-de la Tijera F, Servín-Caamaño AI, Cruz-Herrera J, Serralde-Zúñiga AE, Abdo-Francis JM, Gutiérrez-Reyes G, Pérez-Hernández JL. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol. 2014;13:343-352. [PubMed] |
| 11. | Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut. 1982;23:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 698] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 13. | Caballería J, Parés A, Brú C, Mercader J, García Plaza A, Caballería L, Clemente G, Rodrigo L, Rodés J. Metadoxine accelerates fatty liver recovery in alcoholic patients: results of a randomized double-blind, placebo-control trial. Spanish Group for the Study of Alcoholic Fatty Liver. J Hepatol. 1998;28:54-60. [PubMed] |
| 14. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 5067] [Article Influence: 266.7] [Reference Citation Analysis (0)] |
| 15. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 462] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 16. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 17. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 18. | Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1221] [Article Influence: 64.3] [Reference Citation Analysis (2)] |
| 20. | Ruíz-Zavala A, Gil-Rojas N, Higuera-de la Tijera M. Response to prednisone in Mexican patients with alcoholic hepatitis in a period of four years in the Hospital General de Mexico [Abstract]. Ann Hepatol. 2012;11:602. |
| 21. | Flores YN, Yee HF, Leng M, Escarce JJ, Bastani R, Salmerón J, Morales LS. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. 2008;103:2231-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, Sohn JH, Yoon KT, Kim IH, Kim HS. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Forrest E, Mellor J, Stanton L, Bowers M, Ryder P, Austin A, Day C, Gleeson D, O’Grady J, Masson S. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials. 2013;14:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Addolorato G, Ancona C, Capristo E, Gasbarrini G. Metadoxine in the treatment of acute and chronic alcoholism: a review. Int J Immunopathol Pharmacol. 2003;16:207-214. [PubMed] |
| 26. | Calabrese V, Carlino S, Chinnici V, De Bernardis E, Rizza V. Metadoxine modulates the absorption, metabolism and elimination kinetics of ethanol. Riv Ital Alcol. 1986;5:44-49. |
| 27. | Díaz Martínez MC, Díaz Martínez A, Villamil Salcedo V, Cruz Fuentes C. Efficacy of metadoxine in the management of acute alcohol intoxication. J Int Med Res. 2002;30:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Gutiérrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernández E, Gómez-Quiroz LE, Kershenobich D. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Calabrese V, Calderone A, Ragusa N, Rizza V. Effects of Metadoxine on cellular status of glutathione and of enzymatic defence system following acute ethanol intoxication in rats. Drugs Exp Clin Res. 1996;22:17-24. [PubMed] |
| 30. | Felicioli R, Saracchi I, Flagiello AM, Bartoli C. Effects of pyridoxine-pyrrolidon-carboxylate on hepatic and cerebral ATP levels in ethanol treated rats. Int J Clin Pharmacol Ther Toxicol. 1980;18:277-280. [PubMed] |
| 31. | Baldacci M, Catalani R, Bartoli C, Mura U. Effects of pyridoxine-pyrrolidone-carboxylate on hepatic adenosine triphosphate levels in rats. Boll Soc Ital Biol Sper. 1982;58:1643-1649. [PubMed] |
| 32. | Calabrese V, de Bernardis E, Rizza V. [Metadoxine in the control of oxidative stress caused by acute and chronic ethanol poisoning]. Boll Soc Ital Biol Sper. 1986;62:1357-1363. [PubMed] |
| 33. | Calabrese V, Randazzo G, Ragusa N, Rizza V. Long-term ethanol administration enhances age-dependent modulation of redox state in central and peripheral organs of rat: protection by metadoxine. Drugs Exp Clin Res. 1998;24:85-91. [PubMed] |
| 34. | Malhotra PS, Singh BR, Narotam B, Kaur KP. A study of metadoxine in alcoholic liver disease [Abstract]. J Assoc Physicians India. 2005;53:352-353. |
| 35. | Vedrova NN, Gnezdilova NIu. [Metadoxyl in combined treatment of alcohol damage to the liver]. Klin Med (Mosk). 2001;79:56-58. [PubMed] |
| 36. | Fehér J, Váli L, Blázovics A, Lengyel G. The Beneficial effect of metadoxine (pyridoxine-pyrrolidone-carboxylate) in the treatment of fatty liver diseases. CEMED. 2009;3:65-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (5)] |
| 38. | Tbahriti HF, Meknassi D, Moussaoui R, Messaoudi A, Zemour L, Kaddous A, Bouchenak M, Mekki K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J Nephrol. 2013;2:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Malaponte G, Bevelacqua V, Fatuzzo P, Rapisarda F, Emmanuele G, Travali S, Mazzarino MC. IL-1beta, TNF-alpha and IL-6 release from monocytes in haemodialysis patients in relation to dialytic age. Nephrol Dial Transplant. 2002;17:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Higuchi T, Fukuda N, Yamamoto C, Yamazaki T, Oikawa O, Ohnishi Y, Okada K, Soma M, Matsumoto K. The influence of uremic serum on interleukin-1beta and interleukin-1 receptor antagonist production by peripheral blood mononuclear cells. Ther Apher Dial. 2006;10:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci USA. 1970;67:1248-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 354] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Merrill AH, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Garau B, Fadda F, Melis F, Gelso E, Gessa GL. Metadoxine (pyrrolidone carboxylate of pyridoxine) antagonizes the locomotor-stimulatory effect of ethanol in mice. Alcohol Alcohol. 1992;27:501-504. [PubMed] |
| 44. | Wang T, Zhu D, Xu X, Xu Y. The amelioration of AH by abstinence and the attenuation of oxidative stress. Hepatogastroenterology. 2012;59:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Leggio L, Kenna GA, Ferrulli A, Zywiak WH, Caputo F, Swift RM, Addolorato G. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Hum Psychopharmacol. 2011;26:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Guerrini I, Gentili C, Nelli G, Guazzelli M. A follow up study on the efficacy of metadoxine in the treatment of alcohol dependence. Subst Abuse Treat Prev Policy. 2006;1:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Pár A. [Treatment of alcoholic liver diseases. Abstinence, nutritional support, drug therapy, liver transplantation]. Orv Hetil. 2000;141:827-833. [PubMed] |
| 48. | Rizzo A, Breda A, Moretto F, Pace M, Dotta C, Gelso E, Sanzuol F, Tossani C. [Therapeutic use of metadoxine in chronic alcoholism. Double blind study of patients in a department of general medicine]. Clin Ter. 1993;142:243-250. [PubMed] |
| 49. | Bono G, Sinforiani E, Merlo P, Belloni G, Soldati M, Gelso E. Alcoholic abstinence syndrome: short-term treatment with metadoxine. Int J Clin Pharmacol Res. 1991;11:35-40. [PubMed] |
| 50. | Manor I, Ben-Hayun R, Aharon-Peretz J, Salomy D, Weizman A, Daniely Y, Megiddo D, Newcorn JH, Biederman J, Adler LA. A randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy, safety, and tolerability of extended-release metadoxine in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2012;73:1517-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Manor I, Newcorn JH, Faraone SV, Adler LA. Efficacy of metadoxine extended release in patients with predominantly inattentive subtype attention-deficit/hyperactivity disorder. Postgrad Med. 2013;125:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Manor I, Rubin J, Daniely Y, Adler LA. Attention benefits after a single dose of metadoxine extended release in adults with predominantly inattentive ADHD. Postgrad Med. 2014;126:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, Augustin S, Mookerjee RP, Michelena J, Smyrk TC. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231-9.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Fernandez-Rodriguez CM, Hauser G, Park YM S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN