Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4536
Peer-review started: October 25, 2014
First decision: November 14, 2014
Revised: December 9, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 21, 2015
Processing time: 178 Days and 4.4 Hours
AIM: To investigate whether the Chinese medicine Tong Xie Yao Fang (TXYF) improves dysfunction in an irritable bowel syndrome (IBS) rat model.
METHODS: Thirty baby rats for IBS modeling were separated from mother rats (1 h per day) from days 8 to 21, and the rectum was expanded by angioplasty from days 8 to 12. Ten normal rats were used as normal controls. We examined the effects of TXYF on defection frequency, colonic transit function and smooth muscle contraction, and the expression of 5-hydroxytryptamine (5-HT) and substance P (SP) in colonic and hypothalamus tissues by Western blot and RT-PCT techniques in both normal rats and IBS model rats with characterized visceral hypersensitivity.
RESULTS: Defecation frequency was 1.8 ± 1.03 in normal rats and 4.5 ± 1.58 in IBS model rats (P < 0.001). However, the defecation frequency was significantly decreased (3.0 ± 1.25 vs 4.5 ± 1.58, P < 0.05), while the time (in seconds) of colon transit function was significantly increased (256.88 ± 20.32 vs 93.36 ± 17.28, P < 0.001) in IBS + TXYF group rats than in IBS group rats. Increased colonic smooth muscle tension and contract frequency in IBS model rats were significantly decreased by administration of TXYF. Exogenous agonist stimulants increased spontaneous activity and elicited contractions of colon smooth muscle in IBS model rats, and all of these actions were significantly reduced by TXYF involving 5-HT and SP down-regulation.
CONCLUSION: TXYF can modulate the activity of the enteric nervous system and alter 5-HT and SP activities, which may contribute to the symptoms of IBS.
Core tip: Baby rats separating from mother rats (1 h per day) and rectum expanded with angioplasty were carried out to establish a rat irritable bowel syndrome (IBS) model. We examined the ability of the traditional Chinese medicine Tong Xie Yao Fang (TXYF) to improve the dysfunction of gastrointestinal motility in IBS rats. The effects of TXYF on defecation frequency and colonic motility were analyzed by recording contractions of colon smooth muscle and measurement of the expression of 5-hydroxytryptamine and substance P. Exogenous stimulants increased spontaneous activity and elicited contractions of colon smooth muscle in IBS model rats, which were significantly reduced by TXYF.
-
Citation: Yin Y, Zhong L, Wang JW, Zhao XY, Zhao WJ, Kuang HX. Tong Xie Yao Fang relieves irritable bowel syndrome in rats
via mechanisms involving regulation of 5-hydroxytryptamine and substance P. World J Gastroenterol 2015; 21(15): 4536-4546 - URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4536
Irritable bowel syndrome (IBS) comprises a group of functional bowel disorders in which abdominal discomfort or pain is associated with features of disordered defecation and a change in bowel habit[1]. This has been a global problem because about 1 in 10 people suffers from IBS at some time in their lives.
The exact etiology of IBS is still not clear, but recent research suggests that paresthesia, alterations in intestinal motility, the broadly characterized participation of visceral hypersensitivity including gastrointestinal hormones that regulate gastrointestinal tract (GIT) motility, intestinal inflammation and immune responses are involved[2]. The GIT is innervated by primary sensory neurons, intermediate sensory neurons and motor neurons, which control many gastrointestinal functions from relaxation and contraction of smooth muscle to endocrine and paracrine secretions. This system is independent of the central nervous system and is termed the enteric nervous system (ENS) or “micro-brain”. The ENS innervates the mucosa and the entire intestinal serosa as well as the circular and longitudinal smooth muscle layers and various types of secretory cells. One function of these neurons, nerve fiber tracts and neurotransmitters is to integrate the “feeling” of intestinal movement and urgency with central nervous system (CNS) control centers. The nerves that connect the GIT and the CNS at different levels are collectively known as the brain-gut axis[3]. They coordinate CNS and ENS functions including nerve pathways, endocrine systems and the immune response, and form a two-way pathway that effectively regulates gastrointestinal motility, visceral sensitivity, ghrelin secretion, the body’s stress response and CNS cognitive functions. For example, negative emotions can affect gastrointestinal functions through the brain-gut axis, causing or aggravating IBS[4].
5-hydroxytryptamine (5-HT) and substance P (SP) are monoamine neurotransmitters present in the CNS and GIT. As a stimulant, 5-HT is directly involved in pain modulation and can elicit gastrointestinal smooth muscle contraction, increase GIT tension and accelerate bowel movements, all features associated with IBS[5]. Previous studies have reported the abnormalities of 5-HT metabolism in patients with IBS. Patients with diarrhea-predominant IBS (D-IBS) had significantly higher postprandial plasma 5-HT levels, indicating that the acute postprandial abdominal pain and gastrointestinal symptoms may be associated with excessive secretion of 5-HT[6].
In the GIT, SP is found mainly in neurons of the ENS and is a pro-inflammatory sensory neuropeptide, which transmits sensory information to the ENS and is involved in triggering visceral pain sensitivity and accelerating gastrointestinal motility[7]. However, reports on the actions of SP are not entirely consistent in IBS. For example, Gui et al[8] reported that in IBS patients, plasma levels of SP were significantly higher, indicating that SP acts as a circulating hormone to increase peristalsis and produce diarrhea. On the other hand, Zhang et al[9] pointed out that in patients with IBS, SP levels did not change significantly in either the plasma or the mucosa compared to controls.
Enterochromaffin (EC) cells activate the mucosal processes of intrinsic and extrinsic primary afferent (sensory) neurons and secrete 5-HT (serotonin) in response to mucosal stimuli. Submucosal intrinsic primary afferent neurons, which secrete acetylcholine and calcitonin gene-related peptide, initiate peristaltic and secretory reflexes and are activated by 5-HT receptors. Release of neurotransmitters is enhanced by presynaptic 5-HT4 receptor activation which strengthens neurotransmission in prokinetic pathways. 5-HT3 receptors mediate signaling to the CNS and triggers cancer chemotherapy-associated nausea and the visceral hypersensitivity of D-IBS; however, because 5-HT3 receptors also mediate fast neurotransmission and activate myenteric intrinsic primary afferent neurons, they may also cause constipation[3]. Recently it has been postulated that altered 5-HT signaling in the central nervous system and in the gut contributes to hypersensitivity in IBS[5].
The traditional Chinese medicine Tong Xie Yao Fang (TXYF) has been used to treat IBS[10] and has been shown to improve the symptoms, but its therapeutic efficacy is controversial[10] and its mechanism of action remains unclear.
In the present study, the actions of TXYF in improving dysfunction of gastrointestinal motility in IBS were investigated by examining its potential effects on the regulation of 5-HT and SP activity. The mechanism of TXYF action on rat colon motility and the regulation of the brain-gut axis were also analyzed.
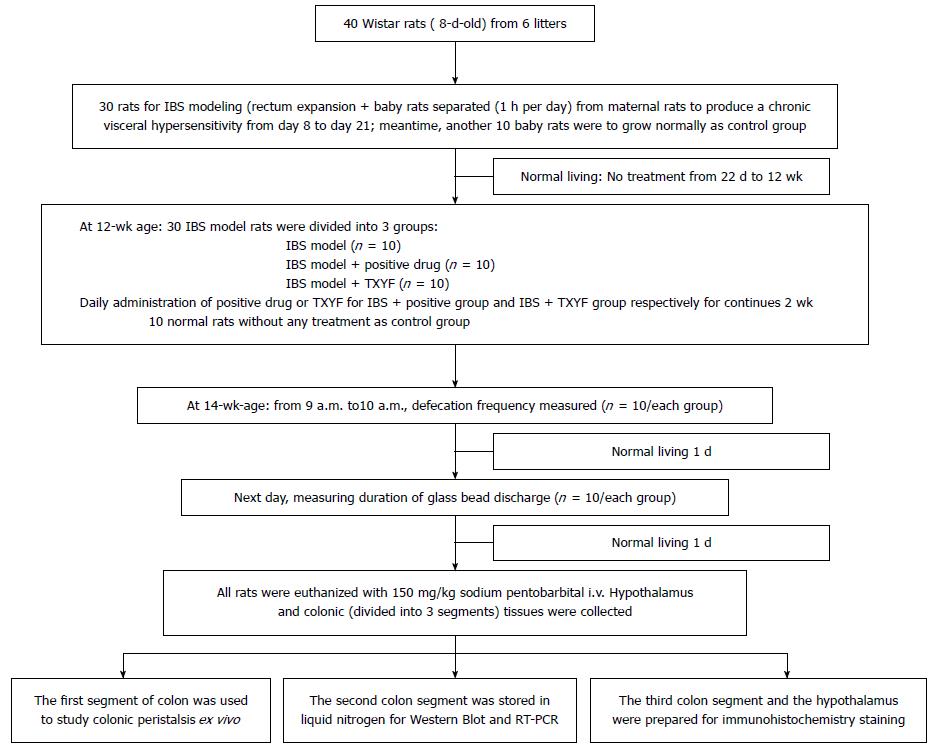
This study was approved by the Ethics Committee of the Heilongjiang University of Chinese Medicine. Eight-day-old Wistar rats (11 ± 2 g) were purchased from the Heilongjiang University of Chinese Medicine Experimental Animal Center. A distension stimulation method in neonatal rats was combined with short-term separation of neonatal and maternal animals to produce chronic IBS from a visceral hypersensitivity model[11,12] (Figure 1). Neonatal rats were reared together with their mothers in specially designed cages. On days 8 to 12, a balloon (sac length 15 mm, EV3 Inc., United States) was inserted into the rectum by angioplasty, with the end of the balloon being positioned 2 cm from the anus. Water was injected gradually into the balloon to expand the intestine twice a day, each extension lasting for 1 min, with 30 min intervals between trials. On days 13 to 17, a balloon (sac length 20 mm) was used to expand the rectum, while other conditions remained unchanged. On days 18 to 21, a children’s rectal dilatation catheter (E206Fr-3mL) (Shenyang Bosi Lin Medical Devices Co. Ltd., Shenyang, China) was used to inject 0.1 mL of water, while other conditions remained unaltered. Neonatal rats were separated from maternal rats for 1 h every day following the rectal distension, and then returned[13,14]. The experimental protocol was repeated for 14 d. Bowel sensitivity assessment was performed 14 and 28 d after the rectal distension was ceased, using the abdominal withdrawal reflex method (AWR)[11]. A score ≥ 2 points confirmed the triggering of visceral hypersensitivity. Rats with a score < 2 points were excluded from the study.
AWR operating procedure was as follows: Rats were fasted for 12 h and then, under general anesthesia, a catheter was inserted into the anus with its balloon end 6-8 cm from the anus. The catheter was taped to the base of the rat’s tail to fix the balloon in a stable position. Rats were then placed in clear plastic cages after regaining consciousness, which permitted them to move forwards and backwards, but they could not turn around. After 30 min of adaption, water was slowly injected into the bowel to expand bowel tract. Every expansion continued for 20 s and then the rat rectal distension response was observed and semi-quantitatively scored, based on their behavioral responses: 0 point, rat colon expanded with no behavioral response; 1 point, rat colon expanded when the body was stationary with reduced head movement, defined as the initial perception threshold; 2 points, rat colon expanded with abdominal muscle contraction but the abdomen was not lifted off the floor, defined as the discomfort threshold; 3 points, during the colon expansion the contraction of the abdominal muscles happened with abdominal lift off the floor, defined as the pain threshold; 4 points, during the colon expansion the rat pelvis was raised with an arched body, defined as the maximum tolerance threshold.
Thirty 12-wk-old rats were divided into three groups: an IBS model group, a positive control group (IBS model + positive drug) and a TXYF group (IBS model + TXYF). Another normal control group of 10 rats was set aside and not used for modeling. The normal control group and the IBS model group were treated with saline [10 mL/kg, intragastric (ig)]. The positive control group was treated with 13.5 × 10-3 g/kg ig pinaverium suspended liquid. The TXYF group was treated with 2.03 mg/kg of ig TXYF suspension. Each group was dosed for a total of 2 wk.
TXYF was prepared with largehead atractylodes rhizome (Rhizoma Atractylodis Macrocephalae), white peony root (Radix Paeoniae Alba), dried tangerine peel (Pericarpium Citri Reticulatae), and divaricate saposhnikovia root (Radix Saposhnikoviae), which were composed in 6:4:3:2 proportions. Raw components were soaked in a 10 times volume of distilled water for 0.5 h and boiled twice, first for 1.5 h and then for 1 h. Two of the boiled ingredients were filtered, mixed together and concentrated at a 1:1 ratio (100% concentration), and stored at 4 °C for later use. The TXYF was diluted in distilled water to a concentration of 0.203 g/mL (clinically equivalent) and stored at room temperature before use.
5-HT and SP immunohistochemistry kits were supplied by Beijing Biosynthesis Biotechnology Co., Ltd. DAB was supplied by Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., China. Atropine sulfate injection (Tianjin Jin-yao Amino Acid Co., Tianjin, China), acetylcholine (Beijing Chemical Reagent Company, Beijing, China), barium chloride (Tianjin Fucheng Chemical Reagent Factory, Tianjin, China), and histamine phosphate (Shanghai Lizhu Dongfeng Biotechnology Co., Ltd., Shanghai, China) were also used.
Thermo script TM reverse transcription-polymerase chain reaction (RT-PCR) kit, platinum tap DNA polymerase kit, and Trizol reagent were obtained from Invitrogen Co (New York, United States); 5-HT, SP, β-actin, the primer, oligo(T)18 and M-MuLV reverse transcriptase were obtained from Shanghai Biological Engineering Technology Services Co., Ltd., China; and DNA marker was purchased from Shanghai Shenergy Biocolor Bioscience and Technology Company (Shanghai, China).
Medlab-n/4cs biological signal acquisition and processing system (Nanjing Mei Yi Technology Co., Ltd., Nanjing, China), rotary tissue slicer 2145 (Leica, Wetzlar, Germany), OLYMPUS microscope-BX60 (Japan OLYMPUS Company, Tokyo, Japan), CMIAS series of digital medical image analysis system, Med 6.0 (Beijing Maikeaodi Image Technology Co., Ltd., Beijing, China), and Odyssey Infrared Fluorescence Scanning Imaging System (LI-COR Company, Lincoln, United States) were used.
IBS model rats treated with saline, positive drug or TXYF and normal rats without any treatment were reared for 2 wk, and reached the age of 14 wk.
Defecation frequency measurements in rats: All rats were denied food but not water for 12 h. Next day, the filter pad in the cage was cleaned and the degree of defecation noted between 9 and 10 am was recorded for statistical purposes.
Determination of the colonic transit function in rats: On the next day after stool frequency measurements, the colonic transit function in rats was measured using the glass bead discharging time method[15]. Rats were denied food but not water for 24 h and then anesthetized with ether. A 3 mm diameter glass bead was quickly but gently inserted into the rectum and positioned 3 cm from the anus. Each rat was then placed in a cage with a clean filter pad. After 2-5 min, the rats slowly regained consciousness. The glass bead discharge time (in second) was recorded from the time the rat regained consciousness.
After recording the glass bead discharge time, all rats were back to normal living for 1 d, followed by a fast for 18 h. Thereafter, all rats were euthanized with 150 mg/kg sodium pentobarbital intravenously. Colonic tissue (divided into 3 segments) and hypothalamus tissue were collected; two of the colonic segments and hypothalamus tissue were prepared for experiments of 5-HT and SP gene and protein expression. The rest of colonic segment was stored in ice-cold Krebs solution (NaCl 5.54 g, KCl 0.35 g, CaCl2 0.28 g, NaHCO3 2.1 g, KH2PO4 0.16 g, MgSO4.7H2O 0.29 g and glucose 2.1 g, dissolved in 1 L of distilled water). The Maxwell bath was filled with 25 mL of Krebs solution and maintained at a constant temperature (36.5 ± 0.5 °C), and bubbled with 95% O2/5% CO2 to pH 7.4. The bowel end was fixed on the bottom hook in a Maxwell bath and the other end was connected to an isometric tension transducer. The colonic segment was allowed to equilibrate for 15 min.
Normal control group: Baseline spontaneous colonic smooth muscle contraction was measured in the absence of drugs, then the contraction was recorded in the presence of TXYF. After the colon segment was washed with Krebs solution 3-4 times, the stimulants, 150 μL of 0.01% acetylcholine, 50 μL of 10% barium chloride and, and 100 μL of 1% histamine, were respectively added and the magnitudes of contraction were recorded. Then, TXYF (100 μL) was added again. The interval between drug additions was 3 min. Colonic smooth muscle contraction was recorded using a physiological recorder (Medlab-n/4cs) and contractions digitised at 50 Hz. The mean tension and frequency of colon muscle contractions were recorded for 3 min before and after drug additions.
IBS model groups: As same as above, baseline spontaneous colonic smooth muscle contraction was measured in the absence of drugs, then the contraction was recorded in the presence of TXYF. However, in the IBS model groups, TXYF was firstly added to observe the inhibition of colonic tissue hypersensitivity, then, stimulants was added, respectively.
Colonic and hypothalamus tissues were washed in normal saline, placed in 4% paraformaldehyde solution for fixation, then dehydrated in progressively stronger alcohol solutions and finally embedded in paraffin for sectioning. Part of the colon was placed in liquid nitrogen and then stored at -80 °C for subsequent RT-PCR and Western blot testing.
Paraffin sections were dewaxed, washed sequentially in tap water and rinsed once with double distilled water for 3 min before being washed twice for 3 min in PBS.
Sections were then placed in rapid antigen retrieval solution for frozen sections (the retrieval solution was boiled before use), microwaved for 20 min and removed after cooling. Each section was then rinsed in double-distilled water for 3 min followed by two rinses in PBS for 2 min each, and excess PBS was removed.
Sections were incubated in 3% H2O2 for 5-10 min to block endogenous peroxidase, and then rinsed twice in PBS for 2 min. Sections were exposed to goat blocking solution and incubated at 37 °C for 15 min to block nonspecific antigens. The goat blocking solution was removed, and then the 5-HT or SP primary antibody was added. Negative controls were treated with PBS instead of the primary antibody. Sections were incubated at 4 °C in a fridge overnight, and then placed in an incubator at 37 °C for 30 min and then rinsed 3 times in PBS for 2 min. Next, the HRP conjugated goat anti-rabbit IgG polyclonal antibody was added, and sections incubated at 37 °C for 30 min and then washed 3 times with PBS for 2 min.
DAB was used for visualization, with the development time being controlled by the degree of washing (which stops it). Slides were exposed to hematoxylin for 10 s for counterstaining and washed in PBS, and a conventional dehydration protocol was performed for transparency. Sections were mounted in resin on slides and the results analyzed using microscopy.
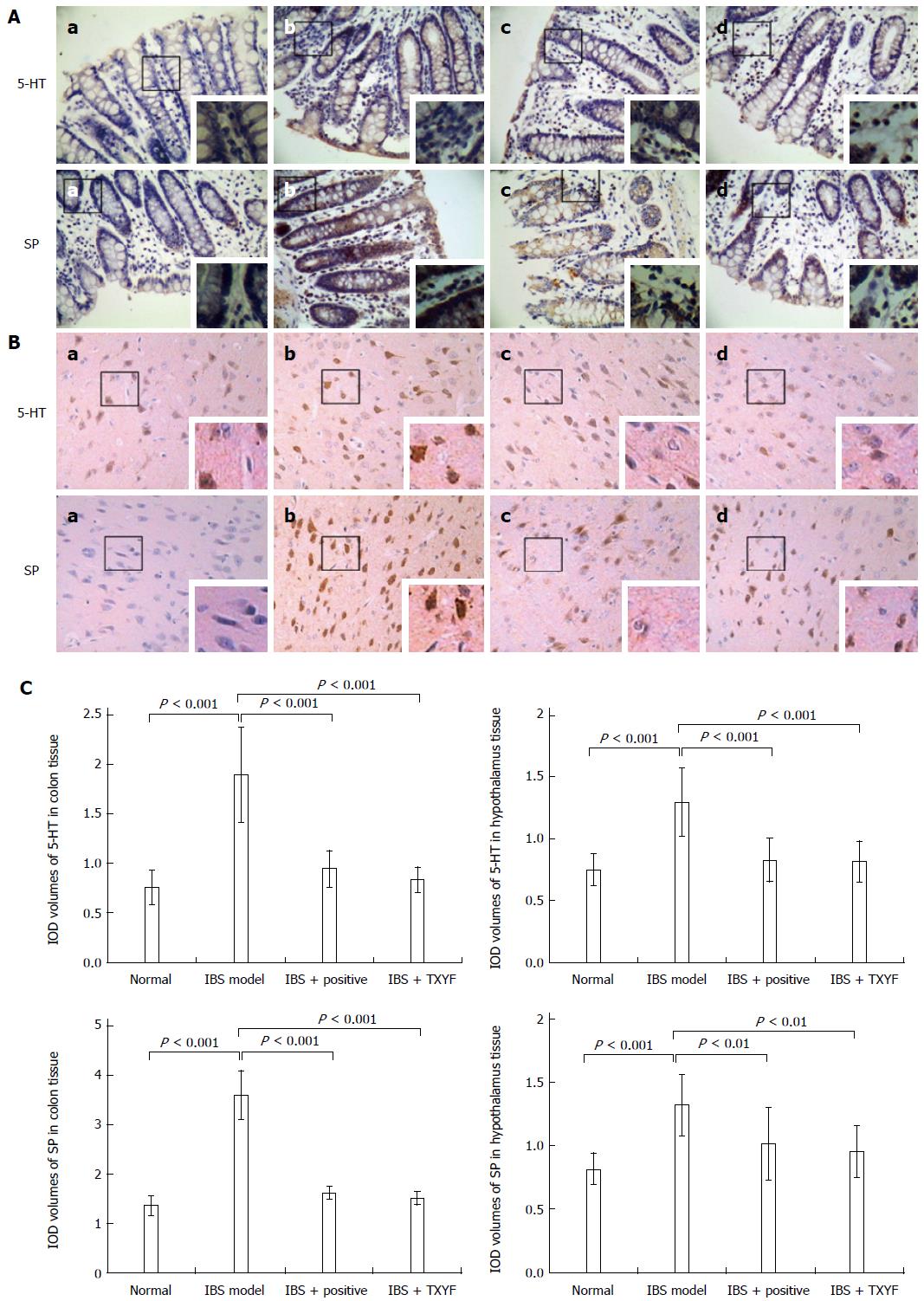
The positive expression of 5-HT and SP proteins was evaluated as follows: If brown particles were detected in the cytoplasm and nucleus of cells, the expression was deemed positive; the deeper the brown color, the stronger the expression. If the cell was not stained with brown particles, the expression was declared negative. The proportion of cells with stained brown nuclei (positive particles) was counted. Using an OLYMPUS BX60 microscope at 400 × magnification, 30 images were randomly taken. Image analysis was carried out on sections from each group of slides using a digital medical image analysis system. The integrated optical density (IOD) of positive targets was measured as the half quantitative indicator of the expression of 5-HT and SP.
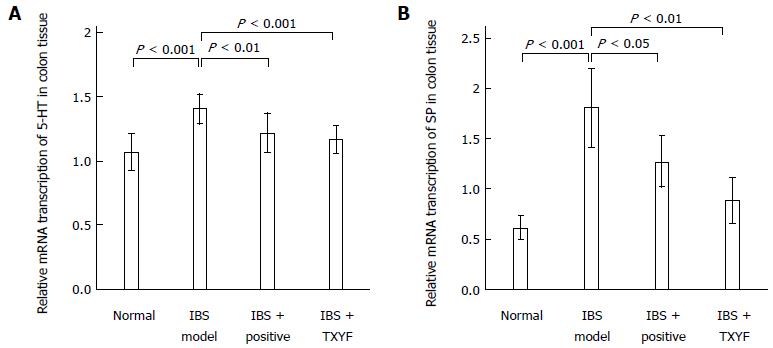
Colon tissue was stored in liquid nitrogen before being analyzed with a Trizol-step total RNA extraction kit. Following the detection of the purity of total RNA with a quantitative UV spectrophotometer, reverse transcription of total RNA was carried out to generate cDNA. Primers were designed using Premier 5.0 for rat 5-HT, SP and β-actin, based on mRNA sequences from the NCBI database (United States). Specific primers were synthesized by Shanghai Biological Engineering Technology Services Limited. Primer sequences were as follows: 5-HT forward primer, 5’-CGT CGA TGA CCA TGC TCG AT-3’, reverse primer, 5’-AGC ATG CGC TTG AAG TCA TG-3’ (amplified fragment length, 508 bp); SP forward primer 5’-CAT TTC CAG TGA ACG GTG GA-3’, reverse primer, 5’-ACG GGA CTG CGT CTG TGT TC-3’ (amplified fragment length, 599 bp); β-actin forward primer 5’-GCC ATT GGC CAG GAG GGC AG-3’, reverse primer, 5’-CGC CAC CAC GCT CTT CTG TC-3’ (amplified fragment length, 229 bp). PCR reactions, electrophoretic separation and color imaging were performed. Using a standard DNA marker, the sizes of the amplified product gene fragments were identified. A digital medical image analysis system was used for electrophoretic band analysis. The β-actin brightness value was used as the reference to determine the ratio of the relative expression of mRNA.
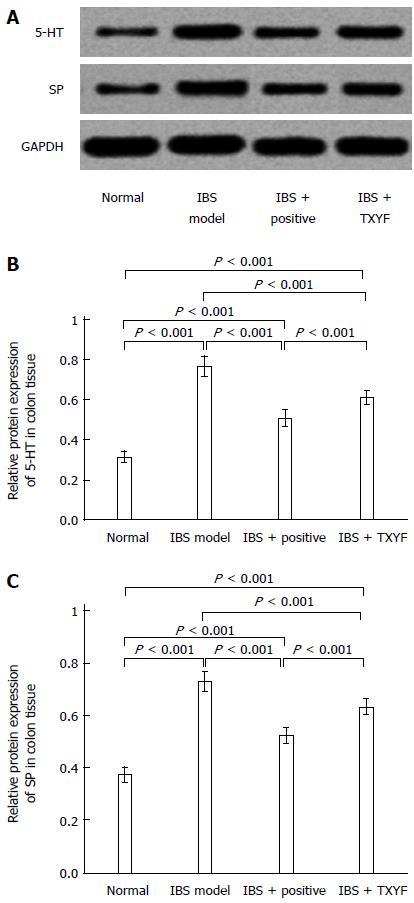
T-PER reagent (1 mL) was added to 100 mg of a colon sample for tissue homogenization. The supernatant was collected after the sample had been centrifuged for 5 min. The total protein concentration was determined using the BCA method. 0.1 mL of standard or sample was added to each tube together with 2 mL of WR, then mixed and incubated at 37 °C for 30 min. The absorbance of each tube was determined using a standard calibration curve. Protein samples were separated by SDS-PAGE electrophoresis, transferred to a membrane, blocked, and then incubated with diluted 5-HT and anti-SP (1:1000, 5% skim milk diluted) antibodies in a sealed hybridization bag at 4 °C, which was shaken overnight. After washing the membrane 3 times with Tris Buffered Saline containing Tween 20 (TBST), membranes were incubated with a diluted fluorescent secondary antibody (1:10000 dilution) in a sealed hybridization bag in a dark room and shaken for 1 h. The membrane was washed a further 3 times with TBST. An Odyssey Infrared Imaging System was used to detect fluorescence signals. The ratio to the reference protein band (ratio of the absorbance of GAPDH) was calculated as the relative amount of target protein in a semi-quantitative analysis.
First, we evaluated the distribution and the homogeneity of variance of all original data from each group and each treatment. All data used in the study were in accordance with a normal distribution and homogeneity of variance. Then, we used the one-way ANOVA with post hoc SNK (student Newman Keuls-q) test with Prism 6.0 software (GraphPad Software, San Diego, CA, United States), in which the SNK test is designed with a Bonferroni correction. P < 0.05 was considered to be statistically significant.
Defecation frequency in rats in the IBS model group was significantly increased (2.5 fold) compared to the normal group, indicating the successful establishment of the IBS model. After treatment with TXYF for 14 d, the defecation frequency in the IBS + TXYF group was found to be significantly lower, compared with the IBS model group (P < 0.05) (Table 1).
Compared with the normal group, rats in the IBS model group exhibited a significantly shorter colon glass bead discharge time (P < 0.001), indicating that IBS can affect the function of colonic transit in rats. Following the administration of TXYF for 14 d, the time of glass bead discharge from the rats in IBS + TXYF group was significantly increased compared with rats in the IBS model group (P < 0.001, Table 2), which indicates that TXYF can effectively improve the function of colonic transit in rats.
Drugs may affect motility, visceral sensation and other aspects of gut function such as secretion or absorption. Various 5-HT receptor subtypes are involved in gut motility, visceral sensation and other aspects of gut functions. SP is involved in motor adaptation and pain transmission associated with inflammation, which involve the control of acetylcholine release from neurons innervating the GIT[16]. Barium chloride (BaCl2) induces intestinal secretion by releasing Ca2+ from intracellular stores[16]. It has been reported that histamine induced neuronal hypertrophy and increased mast cell density in the GIT[17]. In the present study, we used these agonists to stimulate rat colonic smooth muscle and then tested the effectiveness of TXYF in preventing stimulation.
In the normal group, TXYF had only a minor effect on normal colonic smooth muscle tension and the frequency of spontaneous activity. However, the addition of stimulants significantly increased spontaneous activities and TXYF significantly reduced these increased activities (Table 3). In the IBS model group, smooth muscle tension and spontaneous activity were significantly higher (P < 0.05) than in normal rats. However, smooth muscle tension and spontaneous activity were significantly decreased after the addition of TXYF. In the IBS rat model groups, TXYF significantly decreased the tension and frequency of colonic tissue before histamine addition. However, these stimulatory actions were significantly regulated towards normal control levels after the application of TXYF (Table 3).
| Model | Treatment | Group | Dosage | Tension(g) | Frequency (n/min) |
| Normal | Basal | - | 3.59 ± 0.81 | 34.6 ± 6.3b | |
| TXYF | 2.03 mg/kg | 3.26 ± 0.68a | 31.8 ± 7.6 | ||
| Barium | Basal | - | 3.36 ± 0.89a,c | 32.7 ± 7.2d | |
| Barium | 10%, 50 μL | 4.45 ± 0.76c,f | 42.5 ± 6.4d,f | ||
| TXYF | 2.03 mg/kg | 3.44 ± 0.78f | 34.1 ± 6.7f | ||
| Acetylcholine | Basal | - | 2.51 ± 0.61b,c | 34.9 ± 6.7b | |
| Acetylcholine | 0.01%, 150 μL | 3.11 ± 0.42b,c,e | 36.9 ± 7.1 | ||
| TXYF | 2.03 mg/kg | 2.42 ± 0.48b,e | 35.9 ± 7.9 | ||
| Histamine | Basal | - | 3.76 ± 0.58d | 34.9 ± 6.2b | |
| Histamine | 1%, 100 μL | 4.76 ± 0.69d,f | 38.1 ± 6.9 | ||
| TXYF | 2.03 mg/kg | 3.89 ± 0.73f | 35.2 ± 6.2 | ||
| IBS model | Basal | - | 4.23 ± 0.62 | 43.2 ± 5.7b | |
| TXYF | 2.03 mg/kg | 3.99 ± 0.73a | 38.2 ± 6.2 | ||
| Barium | Basal | - | 4.23 ± 0.62a | 43.2 ± 5.7 | |
| TXYF | 2.03 mg/kg | 3.73 ± 0.63 | 37.2 ± 6.7 | ||
| Barium | 10%, 50 μL | 4.25 ± 0.73 | 43.5 ± 7.4 | ||
| Acetylcholine | Basal | - | 4.23 ± 0.62b | 43.2 ± 5.7b | |
| TXYF | 2.03 mg/kg | 3.81 ± 0.64b,e | 37.9 ± 7.1 | ||
| Acetylcholine | 0.01%, 150 μL | 4.69 ± 0.78b,e | 39.2 ± 6.3 | ||
| Histamine | Basal | - | 4.23 ± 0.62g | 43.2 ± 5.7b,g | |
| TXYF | 2.03 mg/kg | 3.41 ± 0.64f,g | 34.9 ± 7.1g | ||
| Histamine | 1%, 100 μL | 4.39 ± 0.73f | 38.2 ± 7.2 |
5-HT and SP expression in colon and hypothalamic tissues, as well as the semi-quantitative immunohistochemistry results, indicated that in the 4 groups, there was low positive expression of 5-HT and SP in colon and hypothalamic tissues in the normal control group. In the IBS group, colon and hypothalamus tissues showed strongly positive 5-HT and SP expression, which was approximately two times greater than that in the controls (P < 0.001, Figure 2). After treatment of the IBS model group with TXYF, 5-HT and SP, staining was decreased in rat colon and hypothalamus to a level not significantly different than that in the normal control. Compared with the IBS model group, after the administration of TXYF to the IBS model group, there was a significant decrease of 5-HT (P < 0.001) and SP expression in colon (P < 0.001) and hypothalamus tissues (P < 0.01, Figure 2).
Figures 3 and 4 show the different mRNA and protein levels of colon 5-HT and SP in each experimental group. The 5-HT and SP mRNA transcriptions in the IBS model group were highest compared with those in other groups (Figure 3). The increased transcriptions were reduced after treatment with TXYF. Similar patterns were reflected in the measurements of 5-HT and SP protein expression (Figure 4).
TXYF has been demonstrated to be effective in alleviating the symptoms of IBS[10]. However, its efficacy has been questioned, so it is necessary to investigate further the effectiveness of TXYF in IBS[10]. In the present study, using neonatal rat rectal stimulation combined with a 14-d maternal separation model, the number of bowel movements was increased 2.5-fold compared with the normal control group, indicating that the model was successfully established. This model could therefore be used to investigate IBS and the mechanisms of action of drugs used to alleviate its symptoms. We clearly demonstrated that TXYF has a significant effect on IBS and improves the symptoms. TXYF significantly reduced the number of bowel movements and improved the colon glass bead discharge time in the IBS rat model. The number of defecations was significantly increased in our IBS model compared to the normal control group. The increased number of defecations was decreased after the administration of TXYF, suggesting that it may be an effective treatment for IBS, a finding consistent with a previous study[10].
We further validated the effects of TXYF on colon smooth muscle tension and spontaneous activity. Surprisingly, TXYF significantly reduced both the increased tension and spontaneous activity elicited by stimulants in normal control group rats and increased tension and spontaneous activity in IBS model rats. Previously, similar research has reported that TXYF can reduce the enhancement of colon smooth muscle tension induced by pilocarpine[9].
IBS rats exhibited significantly increased expression of 5-HT and SP in both the colon and hypothalamus, which was reduced by TXYF. We analyzed the mechanism of high-sensitivity IBS in rat colon and detected a two-fold increase in the levels of 5-HT and a one-to-two-fold increase in the level of SP in the hypothalamus and colonic tissues. Preliminary reports suggested that elevated 5-HT levels resulted in D-IBS and also led to the formation of constipation-predominant IBS (C-IBS) (IBS is characterized by two completely different stool and bowel habits), the mechanism probably involving different tissue distributions and the proportion of 5-HT receptor subtypes expressed. SP, as a critical neurotransmitter, effectively links the gut nervous system to the immune system, stimulating a wide range of effectors in the digestive tract to facilitate proper gastrointestinal motility, sensibility, secretion and absorption. The mechanism by which SP mediates visceral hypersensitivity may involve the promotion of the mast cell degranulation response and the release of histamines, leukotrienes, prostaglandins and bradykinin, all of which can cause inflammatory reactions leading to neuropathic pain[18]. TXYF can significantly decrease 5-HT and SP levels in both the colon and hypothalamus in IBS model rats, suggesting that 5-HT and SP are involved in the functions of the ENS. Shang et al[18] recently reported that TXYF attenuated PI-IBS symptoms by attenuating behavioral hyperalgesia and diarrhea, the underlying mechanism being mediated by an inhibition of protease activated receptor-2 (PAR-2) receptor expression. This inhibition reduces the levels of SP and other cytokines in colonic mucosa and decreases fecal serine protease activity[18]. In the present study, TXYF not only inhibited the expression of 5-HT and SP but also affected brain-gut peptide levels in the hypothalamus, suggesting that TXYF may well regulate the rat brain-gut axis by modifying the activity of 5-HT and SP in the CNS.
Brain-gut peptides and other hormones affect colonic motility by action on two fundamentally different pathways: one is action on neurons in the myenteric plexus and the other direct effects on smooth muscle cells. Which of these two effects predominates depends on the density of receptors expressed for these peptides and hormones in the myenteric plexus. The subtypes of receptors expressed in these tissues will depend on many parameters including environmental factors[19]. However, SP is a gastrointestinal peptide hormone present in the CNS and GIT and acts as a signaling molecule that connects the nervous system to the immune system. Wang et al[7] reported that the expression of SP in both the ENS and the CNS of the C-IBS rat model is abnormal, indicating that an abnormal change in SP levels may be involved in the pathogenesis of IBS. It is likely that SP neural pathways may play an important role in the regulation of gastrointestinal functions.
Increased motility of colon smooth muscle in IBS model rats was significantly reduced by TXYF. These stimulatory actions were significantly regulated towards normal control levels after the application of TXYF. The results showed that TXYF can modulate the activity of the ENS by altering 5-HT and SP activity and that these hormones/neurotransmitters may contribute to the symptoms of IBS.
About 1 in 10 people suffers from irritable bowel syndrome (IBS) at some time in their lives. The paresthesia, alterations in intestinal motility, visceral hypersensitivity, intestinal inflammation and immune responses were reported to relate to the etiology of IBS. However, the exact pathogenesis and the mechanism of IBS are still not clear, which leads to the difficulty of therapeutic strategy. The traditional Chinese medicine Tong Xie Yao Fang (TXYF) showed the evidence to improve dysfunction of gastrointestinal motility in IBS by actions on 5-hydroxytryptamine (5-HT) and substance P (SP) activity.
Although many studies have investigated the mechanism of IBS and effects of medicine treatment on the IBS patients, the results have not been satisfied in terms of etiology and efficient treatment of IBS.
This study investigated the ability of the traditional Chinese medicine TXYF to improve dysfunction of gastrointestinal motility in IBS rats. The improvement on the IBS rat model was involving the actions on 5-HT and SP activity.
The traditional Chinese medicine TXYF may contain the potency of treatment on IBS.
IBS comprises a group of functional bowel disorders in which abdominal discomfort or pain is associated with defecation or a change in bowel habit, and with features of disordered defecation. This is an important problem because worldwide it has been estimated that about 1 in 10 people suffers from IBS at some time in their lives. TXYF was prepared with traditional Chinese herbs, including largeheadatractylodes rhizome (RhizomaAtractylodisMacrocephalae), white peony root (Radix Paeoniae Alba), dried tangerine peel (PericarpiumCitriReticulatae), and divaricate saposhnikovia root (Radix Saposhnikoviae), which were composed in 6:4:3:2 proportions.
This is an important issue. IBS is a common disease that is associated with negative events. The manuscript is very well written and the English seems correct.
| 1. | Hou WR, Hou YL, Wu GF, Song Y, Su XL, Sun B, Li J. cDNA, genomic sequence cloning and overexpression of ribosomal protein gene L9 (rpL9) of the giant panda (Ailuropoda melanoleuca). Genet Mol Res. 2011;10:1576-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Heitkemper M, Jarrett M, Jun SE. Update on irritable bowel syndrome program of research. J Korean Acad Nurs. 2013;43:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184-S193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Med Sci Monit. 2004;10:RA55-RA62. [PubMed] |
| 5. | Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol. 2014;18:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Whitehead WE, Palsson OS, Gangarosa L, Turner M, Tucker J. Lubiprostone does not influence visceral pain thresholds in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:944-e400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Wang WF, Yang YS, Peng LH, Sun G. Alternation of substance P-containing neural pathways in a rat model of irritable bowel syndrome with rectal distension. Chin J Dig Dis. 2006;7:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Gui XY, Pan GZ, Ke MY. Effect of gastrointestinal peptides in colonic motility disorders induced bystress. Zhongguo Xiaohua Zazhi. 1997;2:94-96. |
| 9. | Zhang Y, Bo P, Li X. Increased incidence of bowel and psychological symptoms in Chinese female D-IBS patients with premenstrual syndrome. Gastroenterol Nurs. 2014;37:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Bian Z, Wu T, Liu L, Miao J, Wong H, Song L, Sung JJ. Effectiveness of the Chinese herbal formula TongXieYaoFang for irritable bowel syndrome: a systematic review. J Altern Complement Med. 2006;12:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 565] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Vázquez DM, Eskandari R, Zimmer CA, Levine S, López JF. Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology. 2002;27:245-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Lambás-Señas L, Mnie-Filali O, Certin V, Faure C, Lemoine L, Zimmer L, Haddjeri N. Functional correlates for 5-HT(1A) receptors in maternally deprived rats displaying anxiety and depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Rentesi G, Antoniou K, Marselos M, Fotopoulos A, Alboycharali J, Konstandi M. Long-term consequences of early maternal deprivation in serotonergic activity and HPA function in adult rat. Neurosci Lett. 2010;480:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Sato Y, Yamada M, Yoshida S, Soneda T, Ishikawa M, Nizato T, Suzuki K, Konno F. Benzoxazole derivatives as novel 5-HT3 receptor partial agonists in the gut. J Med Chem. 1998;41:3015-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hardcastle J, Hardcastle PT, Noble JM. The effect of barium chloride on intestinal secretion in the rat. J Physiol. 1983;344:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Keles N, Yavuz Arican R, Coskun M, Elpek GO. Histamine induces the neuronal hypertrophy and increases the mast cell density in gastrointestinal tract. Exp Toxicol Pathol. 2012;64:713-716. |
| 18. | Shang JJ, Yuan JY, Xu H, Tang RZ, Dong YB, Xie JQ. Shugan-decoction relieves visceral hyperalgesia and reduces TRPV1 and SP colon expression. World J Gastroenterol. 2013;19:8071-8077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 Suppl 7:33-46. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen JX, Chiba T, Kohen R, Poli-Neto OB S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM