Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4358
Peer-review started: July 16, 2014
First decision: August 15, 2014
Revised: September 12, 2014
Accepted: October 21, 2014
Article in press: October 21, 2014
Published online: April 14, 2015
Processing time: 273 Days and 11.4 Hours
AIM: To rationally evaluate the effect of S-1 vs capecitabine for the treatment of gastric cancer.
METHODS: MEDLINE, EMBASE, Cochrane Controlled Trials Register, Google Scholar, and China Journal Full Text Database were accessed to collect clinical randomized controlled trials regarding the effect of S-1 vs capecitabine for the treatment of gastric cancer patients. Statistical analysis was performed by meta-analysis. Four randomized controlled trials met the inclusion criteria.
RESULTS: Compared with capecitabine regimens, the 1-year survival rate in gastric cancer patients was 0.80 (95%CI: 0.52-1.21, P = 0.29). The overall response rate of S-1 vs capecitabine was 0.94 (95%CI: 0.59-1.51, P = 0.93). Compared with capecitabine regimens, the most frequent hematologic toxicities were neutropenia (OR = 0.99, 95%CI: 0.65-1.49, P = 0.94) and thrombocytopenia (OR = 0.72, 95%CI: 0.31-1.67, P = 0.44). The most frequent non-hematologic toxicities included nausea (OR = 0.85, 95%CI: 0.56-1.28, P = 0.43) and hand-foot syndrome (OR = 0.16, 95%CI: 0.10-0.27, P < 0.00001).
CONCLUSION: The existing studies suggest that S-1 is not more effective than capecitabine in the treatment of gastric cancer patients, but does exhibit less toxicity with regard to hand-foot syndrome.
Core tip: Systemic chemotherapy has proven to be an important treatment for advanced gastric cancer patients. A combination regimen containing 5-fluorouracil is most commonly used worldwide. S-1 and capecitabine are both oral fluoropyrimidine carbamates, and have proven to be effective for the treatment of gastric cancer patients. This is the first meta-analysis to systematically compare the effects between S-1 and capecitabine against gastric cancer in order to better understand the efficacy, safety, and feasibility of these anticancer drugs. The results may contribute to better treatment and quality of life for patients with advanced gastric cancer.
-
Citation: He AB, Peng XL, Song J, Zhang JX, Dong WG, Luo RF, Tang Y. Efficacy of S-1
vs capecitabine for the treatment of gastric cancer: A meta-analysis. World J Gastroenterol 2015; 21(14): 4358-4364 - URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4358
Gastric cancer is the second-leading cause of cancer-related deaths worldwide[1]. Although mortality rates of gastric cancer have declined over the past few decades, the disease still has a poor prognosis and remains a major health problem[2]. Surgical resection has been accepted as the gold standard and only possible curative treatment for patients with early stage gastric cancer[3,4]. However, most symptoms of gastric cancer are nonspecific, and screening strategies are unavailable in many areas; thus most patients with gastric cancer are diagnosed in an incurable stage.
Over the past two decades, multiple therapies, including systemic chemotherapy, have demonstrated efficacy in decreasing the risk of relapse and improving survival and quality of life for patients with advanced gastric cancer[5,6]. Although there is no single agent or globally-accepted standard chemotherapy treatment strategy for gastric cancer, combination regimens containing 5-fluorouracil (5-FU) are commonly used worldwide[7-11]. The efficacy of oral capecitabine in gastrointestinal cancers has been investigated in a series of studies[12-17], while adjuvant chemotherapy with S-1 is recommended in Japan[18,19].
In this study, we performed a meta-analysis to systematically compare the effects of S-1 and capecitabine in the treatment of gastric cancer in order to better understand the efficacy, safety, and feasibility of these anticancer drugs.
Randomized trials comparing S-1 with capecitabine regimen (single agent, doublet, or triplet) for the treatment of gastric cancer were searched MEDLINE, EMBASE, Cochrane Controlled Trials Register, and China Journal Full Text Database up to 1 October 2013. The language was limited to English and Chinese. The following keywords were used: gastric cancer, capecitabine, and S-1. We also searched the reference lists of pertinent manuscripts in order to identify other potentially relevant articles.
The inclusion criteria for selected articles were as follows: (1) all were random control tests; (2) adult studies were selected; (3) the experiments compared S-1 with capecitabine for the treatment of gastric cancer; and (4) full texts were selected. Preclinical studies, reviews, and case reports not covering the disease being studied were excluded.
Two reviewers selected papers, evaluated their quality, and then extracted the data independently. A third individual was consulted if there were any disagreements. Data on details pertaining to the patients, number of patients at the start of the study, and completed subjects, treatment type, outcomes, and adverse effects were extracted.
Statistical analyses were conducted via the Cochrane Collaboration’s Review Manager version 5.1. Relative risks (RR) and 95%CI were calculated as summary statistics. The estimate of RR from individual studies was calculated. Statistical heterogeneity was assessed by using the I2 test to quantify heterogeneity across studies. If the results of heterogeneity were significant, the random effects model was used to perform analysis, otherwise, the fix effects model was employed. Statistical significance was indicated by a P value less than 0.05.
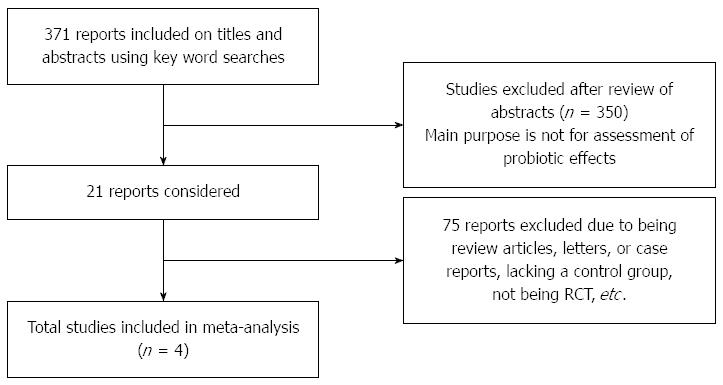
A total of 371 papers were initially identified using the search strategy described above. After a thorough screening of the papers, 4 studies were ultimately selected based on the inclusion/exclusion criteria (Figure 1). All four papers assessed the one year survival rate, while three papers evaluated the overall response rate. The study duration ranged from 0.5 to 39.2 mo. The number of patients in each of the included studies ranged from 81 to 129. Two papers were published in English and two in Chinese. The characteristics of the selected studies are presented in Tables 1 and 2[20-23]. Two studies used SOX compared with XEOLX in gastric cancer[21,23]. One study compared S-1 alone with capecitabine[22]. One study compared TS with TC in patients with gastric cancer[20].
| Study | Patients (S-1/capecitabine) | S-1 regimen | Capecitabine regimen |
| Kim et al[23] | 65/64 | S-1 80 mg/m2 per day d1-14 + oxaliplatin 130 mg/m2 d1 21-d cycle | Capecitabine 2000 mg/m2 per day d1-14 + oxaliplatin 130 mg/m2 d1 21-d cycle |
| Lee et al[22] | 42/44 | S-1: BSA < 1.25 m2, 80 mg/d; | Capecitabine |
| BSA: 1.25-1.5 m2, 100 mg/d; | 2500 mg/m2 per day | ||
| BSA > 1.5 m2, 120 mg/d; d1-28 42-d cycle | d1-14 21-d cycle | ||
| Zhang et al[21] | 41/40 | S-1 80 mg/m2 per day d1-14 + oxaliplatin 130 mg/m2 d1 21-d cycle | Capecitabine 2000 mg/m2 per day d1-14 + oxaliplatin 130 mg/m2 d1 21-d cycle |
| Xiong et al[20] | 42/44 | S-1 80 mg/m2 per day d1-14 + docetaxel 25 mg/m2 d1, 8, 15 28-d cycle | Capecitabine 1250 mg/m2 per day d1-14 + docetaxel 25 mg/m2 d1, 8, 15 28-d cycle |
| Study | Disease stage | Follow-up months | Trial randomization | Lost to follow-up | Survival analysis |
| Kim et al[23] | Advanced gastric cancer, chemotherapy-naive | 0.5-39.2 | Yes | Recorded | ITT |
| Lee et al[22] | Elderly patients (aged ≥ 65 yr) Advanced gastric cancer | Capecitabine: 21.9; S-1: 21.7 | Yes | Recorded | ITT |
| Zhang et al[21] | Gastric cancer after surgery | 24 | Yes | Recorded | Evaluable |
| Xiong et al[20] | Advanced gastric cancer, chemotherapy-naive | 2-28 | Yes | Recorded | ITT |
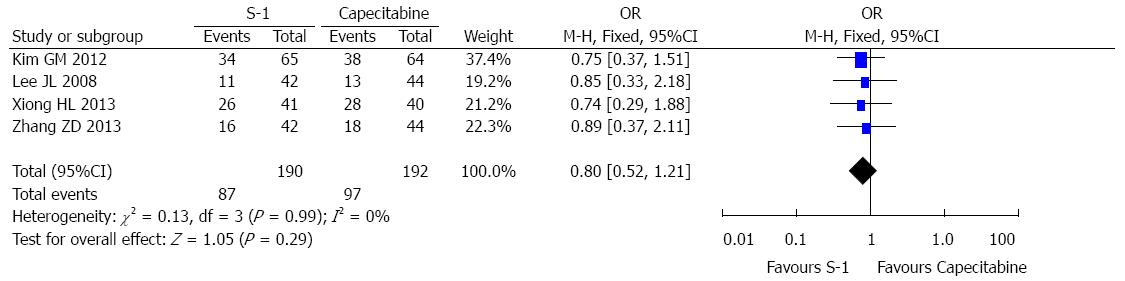
A total of 382 patients from 4 RCTs were included in the 1-year survival analysis; 190 patients were in the S-1 group and 192 were in the capecitabine group. The total recurrence rate of gastric cancer was 45.8% in the S-1 group and 50.5% in the capecitabine group. The pooled OR for the four studies was 0.80 (OR = 0.80, 95%CI: 0.52-1.21, P = 0.29) (Figure 2), suggesting no statistically significant difference between the S-1 and capecitabine groups. No heterogeneity was observed between the selected studies for the treatment analysis (I2 = 0%).
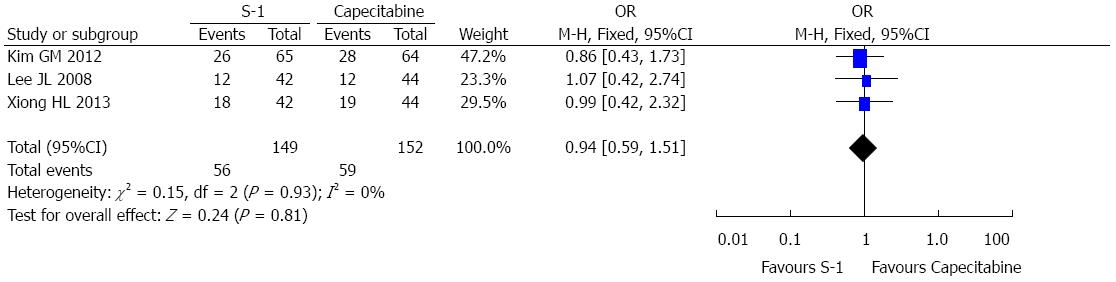
A total of 301 randomized patients from 3 RCTs were included in the overall response rate analysis; 149 patients were in the S-1 group and 152 were in the capecitabine group. A summary of the individual studies and pooled results from the primary analysis of overall response rate are presented in Figure 3. The total overall response rate was 37.6% in the S-1 group and 38.8% in the capecitabine group. The pooled OR for the three studies was 0.94 (OR = 0.94, 95%CI: 0.59-1.51, P = 0.93), suggesting no statistically significant difference between the S-1 and capecitabine groups. No heterogeneity was observed between the selected studies with regard to the treatment analysis (I2 = 0%).
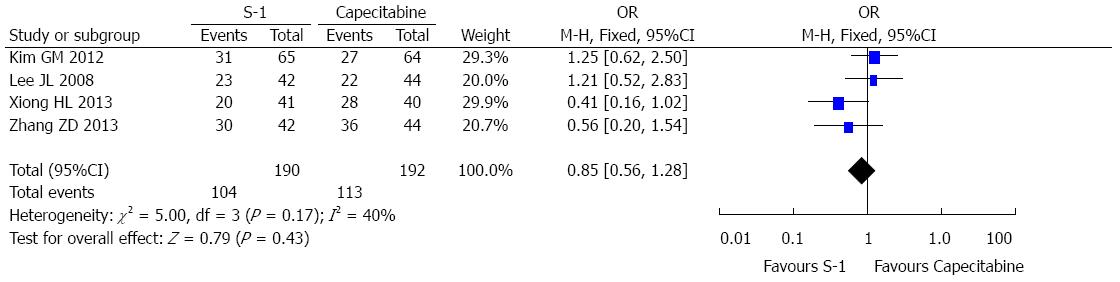
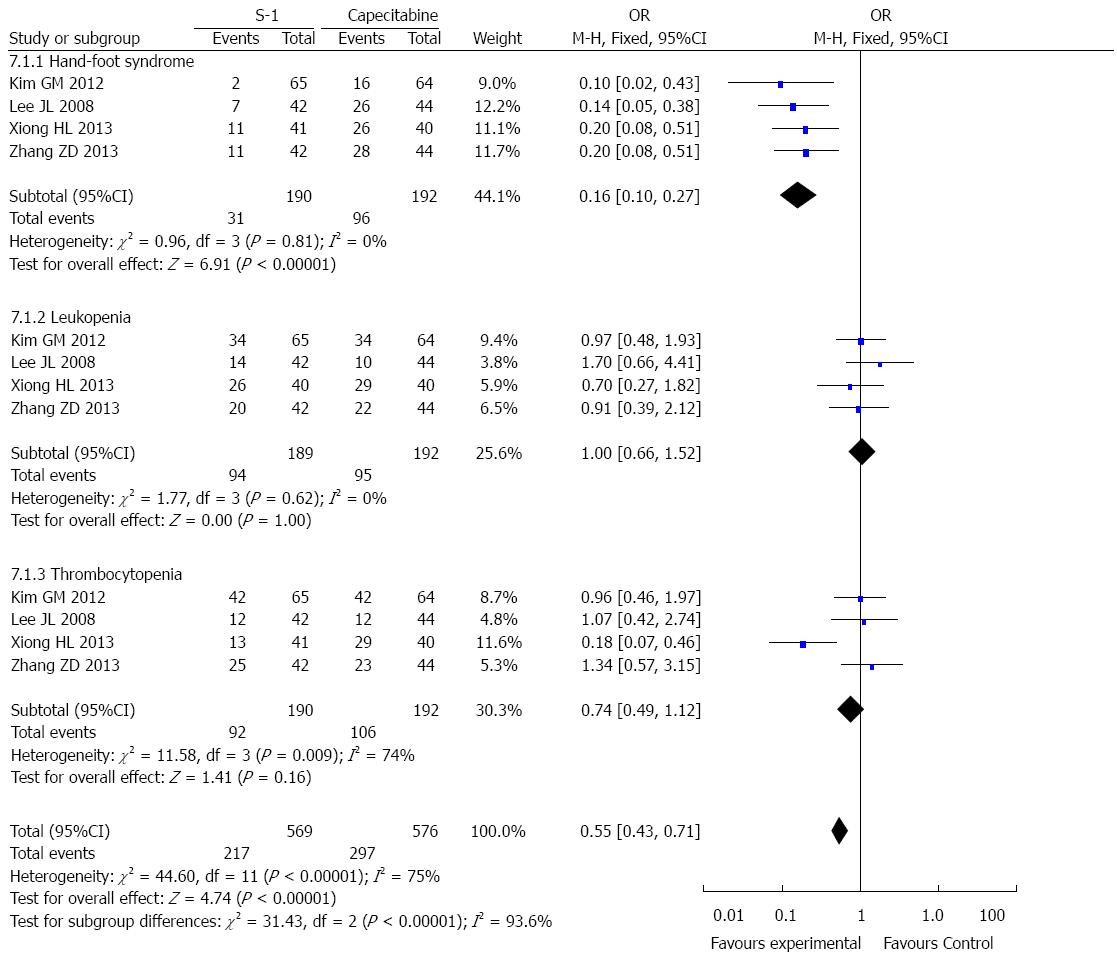
Overall, the toxicities observed in the 4 selected RCTs were tolerable. The most common grade 3-4 hematologic toxicities were neutropenia and thrombocytopenia. The most common grade 3-4 non-hematologic toxicities included nausea and vomiting. The pooled results suggested no significant difference between two treatment groups (Figures 4 and 5). In addition, hand-foot syndrome at any grade was more frequently noted in the capecitabine group than in the S-1 group (Figure 5).
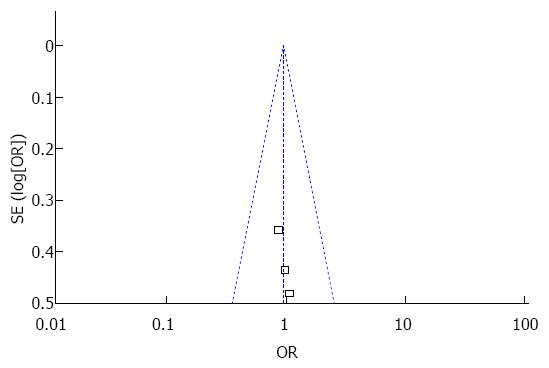
Publication bias was assessed by funnel plot. The funnel plots exhibited symmetry (Figure 6), thereby suggesting no publication bias among the selected studies.
Treatment of gastric cancer has been a major challenge in the past decades given its high incidence. A large number of patients are diagnosed with advanced or metastatic disease. A wide range of studies determined that combination chemotherapy containing fluoropyrimidine prolongs survival in patients with advanced gastric cancer[24-28]. Combination regimes containing fluoropyrimidine have therefore made undeniable gains in improving survival rates for patients with advanced gastric cancer. Given the relatively short overall survival of advanced gastric cancer patients and the palliative nature of systemic chemotherapy, chemotherapeutic agents should be selected based on efficacy, low toxicity, and convenient administration.
S-1 is an oral combination anticancer drug consisting of: 5-fluorouracil prodrug tegafur; 5-chloro-2, 4-dihydroxypyridine; and potassium oxonate. In this combination 5-chloro-2, 4-dihydroxypyridine acts as a dihydropyrimidine dehydrogenase inhibitor, whereas potassium oxonate suppresses the gastrointestinal toxicity of tegafur[29]. In several studies of gastric cancer patients, S-1 has exhibited a similar efficacy and reduced toxicity compared with infusional 5-FU[9,30,31]. Capecitabine is also an oral fluoropyrimidine carbamate that is metabolized primarily in the liver and enzymatically converted to 5-fluorouracil by thymidine phosphorylase in tumor tissues. The levels of the enzyme thymidine phosphorylase are considerably higher in gastric cancers compared with normal tissue, which allows 5-fluorouracil to be concentrated in tumor tissues[32]. The efficacy and safety of capecitabine for advanced gastric cancer has been demonstrated[33], and a randomized phase III trial indicated that capecitabine can replace 5-FU for the treatment of advanced esophagogastric cancer[34].
This meta-analysis focused on the comparison of survival outcomes and toxicity between S-1-based regimens and capecitabine for the treatment of patients with gastric cancer. Only four randomized trials with 382 patients met our eligible criteria. Among the four studies, 1-year survival and overall response rate were selected as the primary study endpoints. Although overall survival is considered to be the most clinically meaningful measure of treatment effect in cancer patients, only two of the studies analyzed overall survival; these results did not exhibit sufficient robustness for the meta-analysis. The results indicate that treatment with regimens containing capecitabine were equally as effective as S-1-containing chemotherapies in patients with gastric cancer with regard to 1-year survival and overall response rate. This result was consistent with the results of the four included studies[20-23].
The most suitable treatment regimen for an individual patient is not only dependent on treatment efficacy, but also involves other factors, such as toxicity[35]. S-1 and capecitabine were both well-tolerated, and no treatment-related deaths were reported in the selected studies. The most frequent hematological toxicities were neutropenia and thrombocytopenia, with no meaningful differences in hematologic toxicities being noted between the two treatment agents. The most frequently observed grade 3 or 4 non-hematologic toxicities included nausea and vomiting, and no meaningful differences were noted between the two treatment agents. The only notable non-hematologic difference in adverse events was the increased incidence of hand-foot syndrome in the capecitabine group compared with the S-1 group. Hand-foot syndrome is a characteristic non-hematologic toxicity of capecitabine that leads to treatment delays or dose reductions in many patients. Given these findings, we suggest that S-1 is superior to capecitabine for the treatment of gastric cancer.
Although this meta-analysis was based on RCTs and properly conducted, there were still some limitations to our study. One major limitation was that the number of selected studies was quite small, with this small sample size therefore likely not reflecting the actual situation. In addition, only two studies analyzed overall survival, with these results failing to exhibit sufficient robustness for the meta-analysis. However, 1-year survival and overall response rate, which are the most meaningful clinical measures of treatment efficacy in cancer patients, were analyzed.
In summary, although S-1 demonstrated no survival advantage over capecitabine, it resulted in a considerably lower incidence of hand-foot syndrome than capecitabine, thereby suggesting that S-1 is superior to capecitabine for the treatment of gastric cancer.
Gastric cancer is the second leading cause of cancer-related deaths globally, with most gastric cancer patients being diagnosed in an incurable stage. Systemic chemotherapy have been proved to decrease relapse risk and improve survival and quality of life for advanced gastric cancer patients, with a combination regimen containing 5-fluorouracil being most commonly used worldwide. S-1 and capecitabine are both oral fluoropyrimidine carbamates. The efficacy of oral capecitabine in gastrointestinal cancers has been investigated in a series of studies. Adjuvant chemotherapy with S-1 has been recommended in Japan. We aim to systematically compare the effects of S-1 and capecitabine in the treatment of gastric cancer for a better understanding of the efficacy, safety, and feasibility of these anticancer drugs.
Systemic chemotherapy is an important treatment for advanced gastric cancer patients; S-1 and capecitabine were both proved to be effective in the treatment of gastric cancer, but the efficacy, safety, and feasibility of S-1 vs capecitabine remains unknown.
The authors performed a meta-analysis to systematically compare the effects of S-1 and capecitabine in the treatment of gastric cancer in order to better understanding the efficacy, safety, and feasibility of these anticancer drugs. This may contribute to better treatment and quality of life for gastric cancer patients.
The results showed that S-1 is not more effective than capecitabine in the treatment of gastric cancer patients, but does result in fewer instances of hand-foot syndrome.
In this manuscript, Peng et al determined that S-1 was not more effective than capecitabine in the treatment of gastric cancer patients, but does show fewer instances of hand-foot syndrome. The efficacy of oral capecitabine in gastrointestinal cancers has been investigated in a series of studies, with adjuvant chemotherapy with S-1 being recommended in Japan. The authors compared the effects of S-1 and capecitabine in the treatment of gastric cancer, resulting in a meaningful conclusion.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8987] [Article Influence: 641.9] [Reference Citation Analysis (3)] |
| 3. | Wu HL, Tian Q, Peng CW, Liu SP, Li Y. Multivariate survival and outcome analysis of 154 patients with gastric cancer at a single Chinese institution. Asian Pac J Cancer Prev. 2011;12:3341-3345. [PubMed] |
| 4. | O’Connor KG. Gastric cancer. Semin Oncol Nurs. 1999;15:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kim YH. Chemotherapy for advanced gastric cancer: slow but further progress. Cancer Res Treat. 2005;37:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 901] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 7. | Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, Zhang B, Chen JP. The effectiveness of intravenous 5-fluorouracil-containing chemotherapy after curative resection for gastric carcinoma: A systematic review of published randomized controlled trials. J Chemother. 2007;19:359-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1471] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 9. | Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 320] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Bouché O, Ychou M, Burtin P, Bedenne L, Ducreux M, Lebreton G, Baulieux J, Nordlinger B, Martin C, Seitz JF. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol. 2005;16:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 11. | Shitara K, Sawaki A, Matsuo K, Kondo C, Takahari D, Ura T, Tajika M, Niwa Y, Muro K. A retrospective comparison of S-1 plus cisplatin and capecitabine plus cisplatin for patients with advanced or recurrent gastric cancer. Int J Clin Oncol. 2013;18:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 12. | Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097-4106. [PubMed] |
| 13. | Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282-2292. [PubMed] |
| 14. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 868] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 15. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 16. | Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. 2008;19:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2297] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 18. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1979] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 20. | Xiong HL, Liu XQ, Sun AH, He Y, Li J, Yuan X. Clinical comparison of Docetaxel combined with S-1 or Capecitabine in treating advanced gastric carcinoma. Xiandai Zhongliu Yixue. 2013;21:581-584. |
| 21. | Zhang ZD, Kong Y, MA F, Liu HX, Zhang B, Huang JX, Ma EM, Hua YW. Adjuvant chemotherapy with Oxaliplatin plus S-1 versus XELOX regimen for postoperative gastric cancer. Zhongguo Putong Waike Zazhi. 2013;22:747-751. |
| 22. | Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY, Kim JG, Park SR, Kang WK, Shin DB. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Janunger KG, Hafström L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Oba K, Morita S, Tsuburaya A, Kodera Y, Kobayashi M, Sakamoto J. Efficacy of adjuvant chemotherapy using oral fluorinated pyrimidines for curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials in Japan. J Chemother. 2006;18:311-317. [PubMed] |
| 26. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1435] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 27. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 28. | Jeung HC, Rha SY, Kim HK, Lim HY, Kim S, Kim SY, Gong SJ, Park CH, Ahn JB, Noh SH. Multi-institutional phase II study of S-1 monotherapy in advanced gastric cancer with pharmacokinetic and pharmacogenomic evaluations. Oncologist. 2007;12:543-554. [PubMed] |
| 29. | Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003;6 Suppl 1:2-8. [PubMed] |
| 30. | Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715-1720. [PubMed] |
| 31. | Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 32. | Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344-1347. [PubMed] |
| 33. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1713] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 34. | Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274-1281. [PubMed] |
| 35. | Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-98. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen L S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Liu XM