Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4216
Peer-review started: October 27, 2014
First decision: November 14, 2014
Revised: November 27, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 14, 2015
Processing time: 170 Days and 22 Hours
AIM: To investigate the expression of Th22 cells and related cytokines in colorectal cancer (CRC) tissues, and the probably mechanism.
METHODS: CRC tumor and paratumor tissues were collected to detect the expression levels of Th22 cells and of related cytokines by immunohistochemistry, flow cytometry and real-time quantitative polymerase chain reaction (RT-qPCR). Interleukin (IL)-22 alone or with a STAT3 inhibitor was co-cultured with RKO cells in vitro to study the effects of IL-22 on colon cancer cells. IL-22 alone or with a STAT3 inhibitor was injected into a BALB/c nude mouse model with subcutaneously transplanted RKO cells to study the effects of IL-22 on colon cancer growth.
RESULTS: The percentage of Th22 cells in the CD4+ T subset was significantly higher in tumor tissues compared with that in paratumor tissues (1.47% ± 0.083% vs 1.23% ± 0.077%, P < 0.05) as determined by flow cytometry. RT-qPCR analysis revealed that the mRNA expression levels of IL-22, aryl hydrocarbon receptor, CCL20 and CCL22 were significantly higher in tumor tissues compared with those in paratumor tissues. CCL27 mRNA also displayed a higher expression level in tumor tissues compared with that in paratumor tissues; however, these levels were not significantly different (2.58 ± 0.93 vs 2.30 ± 0.78, P > 0.05). IL-22 enhanced colon cancer cell proliferation in vitro and displayed anti-apoptotic effects; these effects were blocked by adding a STAT3 inhibitor. IL-22 promoted tumor growth in BALB/c nude mice; however, this effect was reversed by adding a STAT3 inhibitor.
CONCLUSION: Th22 cells that accumulate in CRC may be associated with the chemotactic effect of the tumor microenvironment. IL-22 is associated with CRC development, most likely via STAT3 activation.
Core tip: Although the functional characteristics of Th22 cells in inflammatory and autoimmune diseases have been extensively studied, their role in colorectal cancer (CRC) remains unclear. This study demonstrated the differences in the expression of Th22 cells and their related cytokines between colorectal tumor and paratumor tissues and the accumulation of Th22 cells in CRC may be associated with the functions of chemotactic factors that are secreted by the tumor microenvironment. Interleukin-22 was found to be the functional factor of Th22 cells that is associated with CRC development in both in vitro and in vivo experiments, most likely via STAT3 pathway activation.
- Citation: Huang YH, Cao YF, Jiang ZY, Zhang S, Gao F. Th22 cell accumulation is associated with colorectal cancer development. World J Gastroenterol 2015; 21(14): 4216-4224
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4216
Colorectal cancer (CRC) is the third most commonly occurring cancer in males and the second most commonly occurring cancer in females[1]. An increased overall survival rate has been observed in patients with CRC due to the detection of early stage CRC and to the improvement of therapeutic strategies[2]. However, over 1 million people develop CRC every year worldwide, and more than 500000 patients die, particularly those patients with advanced cancer[1,3]. Currently, the incidence rates of CRC are increasing in developing countries, including China[4].
Understanding the molecular pathways involved in CRC will help to improve cancer prevention and treatment[5]. Increasing evidence has shown that the dysregulation of different CD4+ T lymphocyte subpopulations and cytokine networks is involved in the pathogenesis and progression of CRC[6-8]. In situ analysis of tumor-infiltrating immune cells may be a valuable prognostic tool in the treatment of CRC and possibly of other malignant tumors[9,10].
Traditionally, CD4+ T helper cells (Th cells) include Th1, Th2, Th7, and regulatory T cells according to their cytokine milieu. Interleukin (IL)-22, which is a member of the IL-10 cytokine family, is regarded as a cytokine produced by Th1 cells and Th17 cells. Recently, two studies have shed new light on the unique features of this cytokine. IL-22-producing CD4+ T cells (Th22 cells), which are a new T helper cell subset, differ from Th1, Th2, or Th17 cells because this population only produces IL-22 and has low or undetectable expression of the Th17 and Th1 transcription factors ROR-γ and T-bet. Th22 cells express the chemokine receptors CCR4, CCR6 and CCR10 in human skin, and the transcription factor aryl hydrocarbon receptor (AHR) is required for IL-22 production[11,12]. The functional characteristics of Th22 cells in inflammatory and autoimmune diseases have been extensively studied[13]. Nevertheless, knowledge regarding the role of Th22 cells in malignant tumor immunity is limited; further research elucidating the pathogenesis of and therapy for carcinoma will be of interest. In the current study, we investigated the expression of Th22 cells and their related cytokines in colorectal tumor and paratumor tissues and determined their effects on colorectal cancer using in vivo and in vitro experiments.
All patients enrolled in this study provided written informed consent. This study protocol conformed to the ethical guidelines of the Declaration of Helsinki (Fortaleza, Brazil, October 2013) and was approved by the ethical committees and institutional Review Board of the First Affiliated Hospital of Guangxi Medical University, PRC.
Fifty patients diagnosed with CRC who received surgical resection at The First Affiliated Hospital of Guangxi Medical University from April 2013 to March 2014 were enrolled in this study. None of the patients had received radiotherapy or chemotherapy before sampling. Individuals with an autoimmune disease, infectious disease, or multiple primary cancers were excluded. The basic data regarding the study population are shown in Table 1. The tumor and paratumor tissues (at least 5 cm away from the tumor site) were collected immediately after surgical resection and stored in liquid nitrogen for polymerase chain reaction (PCR), fixed with 4% paraformaldehyde for immunohistochemistry (IHC) or immediately isolated for flow cytometry.
| Characteristics | Value |
| Sex | |
| Male | 33 |
| Female | 17 |
| Age (yr), mean (range) | 60 (38-81) |
| Colon | 22 |
| Rectum | 28 |
| TNM stage | |
| Stage I-II | 23 |
| Stage III-IV | 27 |
Fresh tumor and paratumor tissues were fixed in 4% paraformaldehyde, embedded with paraffin and sectioned at 4-μm thickness. IHC was performed as previously described[14]; the sectioned slides were stained using IL-22 antibody, which was purchased from Bioss Company (Beijing, China).
Fresh tumor and paratumor tissue samples for determining cytokine expression were stored at -80 °C until analysis. Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The cDNA was immediately reverse transcribed from the extracted total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen). Real-time quantitative PCR (RT-qPCR) was performed using a SYBR Green PCR kit (Roche). Amplification was performed using standard conditions and was normalized to transcripts of the housekeeping gene β-actin. The primer sequences for PCR are shown in Table 2. Relative expression levels of mRNA were calculated using the 2-ΔΔCt method as described by Livak et al[15] and adjusted by the level of β-actin mRNA for each sample.
| Gene | Sequence (5’ to 3’) | Product (bp) | Tm (°C) |
| IL-22 | F: GTTCTCCTTCCCCAGTCACCA | 145 | 60 |
| R: AGCTGCTCCTCCCTGTACCAA | |||
| AHR | F: ACATCACCTACGCCAGTCG | 94 | 60 |
| R: CGCTTGGAAGGATTTGACTTGA | |||
| CCL20 | F: ATCCAAAACAGACTTGGGTGAA | 89 | 60 |
| R: TCCATTCCAGAAAAGCCACA | |||
| CCL22 | F: ATTACGTCCGTTACCGTCTGC | 100 | 60 |
| R: TCCCTGAAGGTTAGCAACACC | |||
| CCL27 | F: TCCTGAGCCCAGACCCTACA | 175 | 60 |
| R: CGTTGAGCCAGGTGAAGCA | |||
| β-actin | F: TGACGTGGACATCCGCAAAG | 205 | 60 |
| R: CTGGAAGGTGGACAGCGAGG |
Tumor and paratumor tissues were washed three times in RPMI 1640 before being cut into small pieces (1 mm tissue samples). Then, the specimens were collected in RPMI 1640 containing 1 mg/mL collagenase IV, 30 μg/mL DNase I and 0.1 mg/mL hyaluronidase, and then a magnetic stirrer was used for stirring the digestion mixture for 3 h. Next, the dissociated cell suspensions were filtered through 150-μm and 70-μm cell strainers to obtain cell suspensions, which were centrifuged in a discontinuous Percoll gradient (75% and 40%). The cells at the interface were harvested and resuspended at 1 × 106 cells/mL in RPMI 1640 containing 10% fetal calf serum. Cell viability was determined by trypan blue exclusion.
The cell suspensions were stimulated in culture for 4 h with 50 ng/mL PMA, 1 μg/mL ionomycin and 0.7 μL/mL GolgiStop reagent at 37 °C in a CO2 incubator (5% CO2 in humidified air). The cultured cell suspensions were stained with surface and intracellular anti-human-specific antibodies, which were conjugated with PE, PE-Cy5 or APC. These human antibodies included anti-CD4, IL-22 and IL-17, which were purchased from BD Biosciences (Franklin Lakes, NJ, United States) or eBioscience (San Diego, CA, United States). Then, the cells were resuspended and analyzed using a FACSCaliburflow cytometer (BD Bioscience). The data were analyzed using FlowJo software (TreeStar, Ashland, OR, United States). Cellular debris was eliminated from the analysis using a gate set at forward and side scatter.
The human colon cancer cell line RKO was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Recombinant human IL-22 was purchased from PeproTech Company, United States. STAT3 inhibitor (S3I-201) was purchased from Selleck Chemicals, United States. RKO cells were cultured in complete DMEM medium supplemented with 10% FBS and 1% antibiotic/antimycotic in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% CO2 for 24 h. Then, IL-22 (50 ng/mL) or S3I-201 (50 μmol/L) was added to the experimental medium for co-culture. After 24 h, the RKO cells were trypsinized and then stained with intracellular Ki-67 (BD Bioscience) to detect cell proliferation or stained with Annexin V and 7-amino-actinomycin (7-AAD) (BD Bioscience) to detect apoptosis.
BALB/c nude mice (6-8 wk of age) were obtained from Guangxi Medical University Animal Experiment Center, and all animal experiments conformed to the National Guidelines of the Animal Care Committee. Twenty-one BALB/c nude mice were injected subcutaneously with RKO cells; each mouse was injected with 5 × 106 cells in 300 μL of saline solution. Tumor growth was monitored every two days. Tumor volume was calculated by the following formula: (major circumference×minor circumference2)/2. After the tumor volumes reached 60 mm3, the 21 mice were divided into 3 groups. The IL-22 group was injected intraperitoneally with IL-22 (1 μg/100 μL) and DMSO (100 μL) every other day, the IL-22 + S3I-201 group was injected with IL-22 (1 μg/100 μL) and S3I-201 (100 μg/100 μL), and the control group was injected with saline solution (100 μL) and DMSO (100 μL) simultaneously, each group for a total of 5 times. The mice were sacrificed at 48 h after the last intervention.
The data are expressed as the mean ± SE. Data comparisons between the different groups were performed using Student’s t-test, a paired t-test or one-way ANOVA. Analysis was completed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, United States), and P values that were less than 0.05 were considered statistically significant.
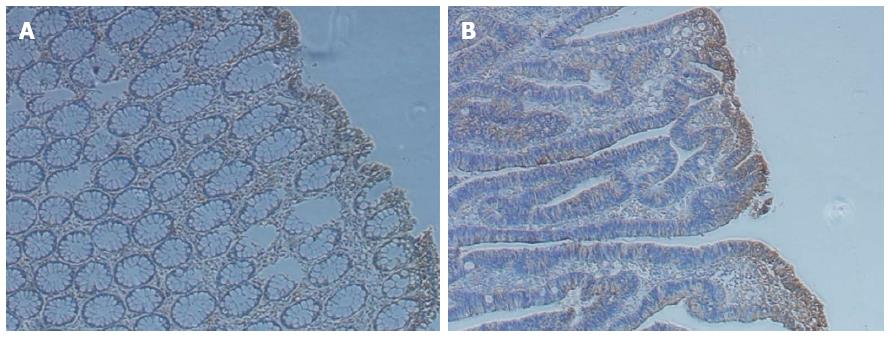
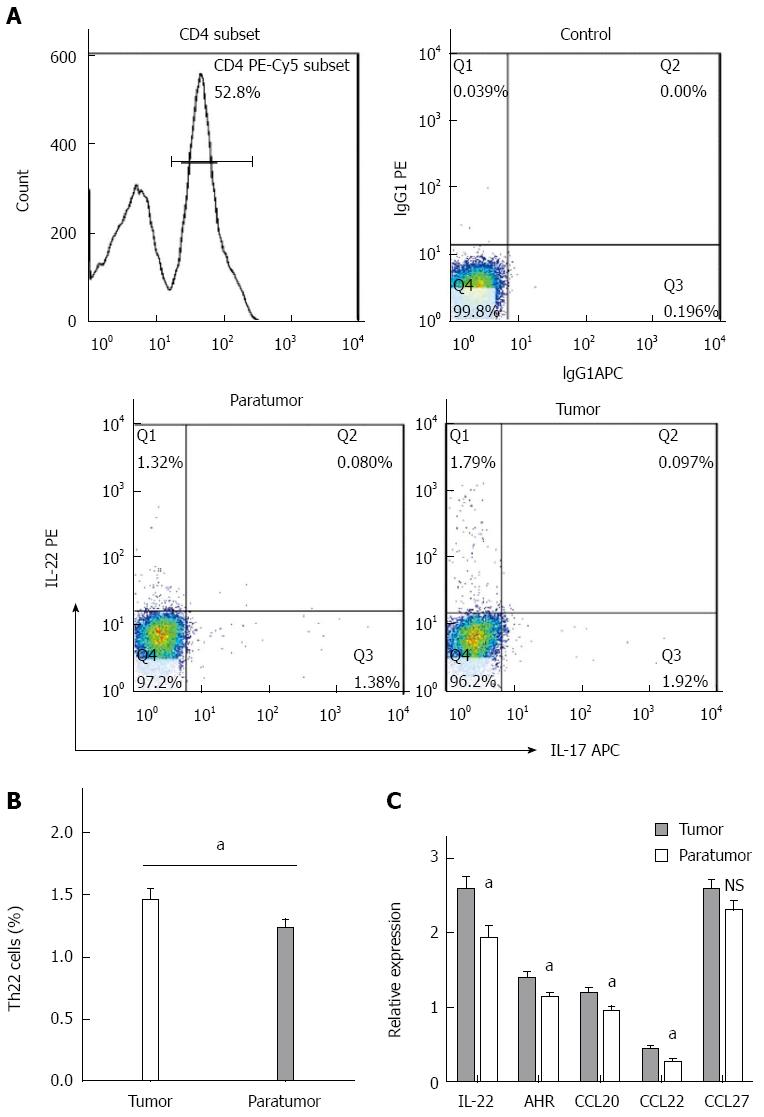
IL-22 is a functional cytokine that is primarily produced by Th22 cells. By IHC, we observed that IL-22 was present in both tumor and paratumor tissues and was particularly enriched in tumor tissues (Figure 1). To further understand the roles of Th22 cells in the tumor microenvironment in patients with CRC, the proportion of Th22 cells in tumor and paratumor tissue was detected by flow cytometry (Figure 2A). As shown in Figure 2B, the prevalence of Th22 cells in the CD4+ T subset was higher in tumor tissues compared with that in paratumor tissues (P < 0.05).
The relative expression levels of IL-22, AHR, CCL20, CCL22 and CCL27 in colorectal tumor and paratumor tissues were measured by RT-qPCR. CCL20, CCL22 and CCL27 are common chemokines that have been identified as attractants of different types of leukocytes to sites of tumors and of inflammation. As shown in Figure 2C, the mRNA expression levels of IL-22, AHR, CCL20 and CCL22 were significantly higher in tumor tissues compared with those in paratumor tissues (P < 0.05). CCL27 mRNA also displayed a higher expression level in tumor tissues compared with that in paratumor tissues; however, these levels were not significantly different (P > 0.05).
The effects of IL-22 on colon cancer cells were assessed by co-culturing with RKO cells in vitro. As shown in Figure 3A and C, compared with control medium, the proliferation of RKO cells was significantly promoted by IL-22 treatment (P < 0.05). This enhanced proliferation was blocked when S3I-201 was added to the RKO cell culture in the presence of IL-22. In contrast, the apoptosis of RKO cells was significantly inhibited by IL-22 treatment (P < 0.05) compared with the control medium. The inhibition of STAT3 signaling by S3I-201 completely abrogated this suppression of apoptosis (Figure 3B and 3D).
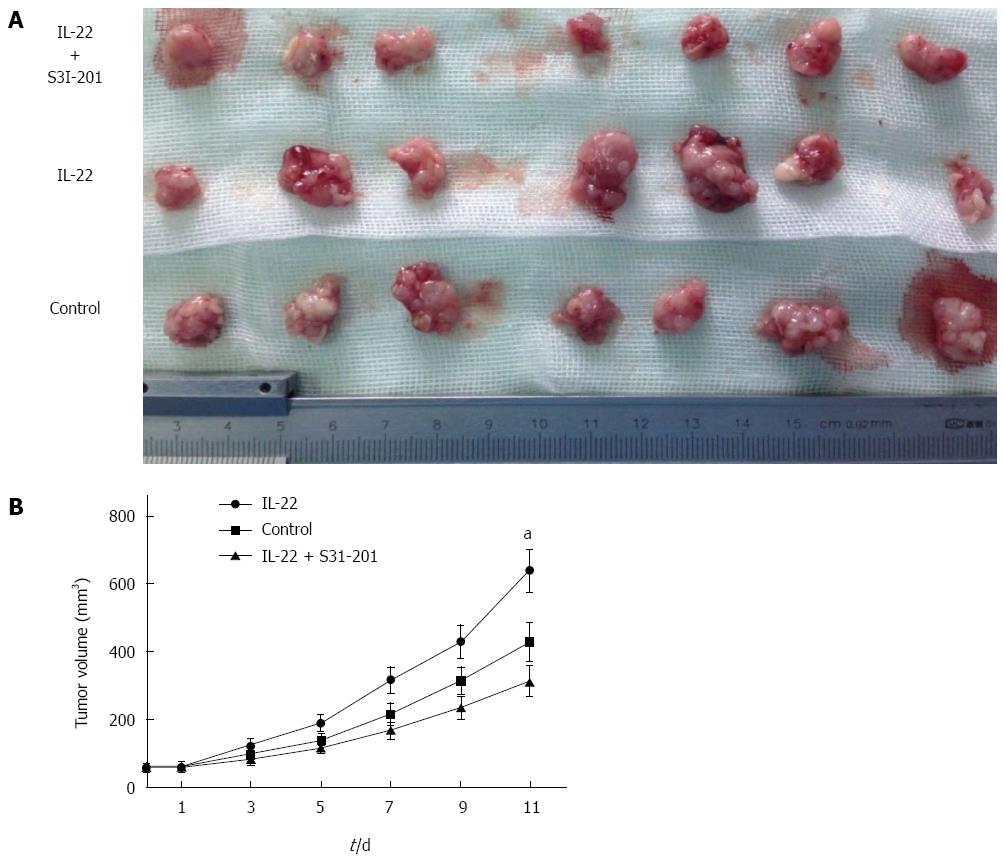
BALB/c nude mice transplanted subcutaneously with RKO cells were used to investigate the effects of IL-22 on colon cancer growth in vivo. As shown in Figure 4, the tumor growth of nude mice was significantly promoted (P < 0.05) after intraperitoneal injection with IL-22 every other day compared with that of the control mice. However, this promoting effect induced by IL-22 treatment could be completely reversed by S3I-201 treatment.
The roles of tumor antigen-specific CD4+ T cells in cancer immunity have been extensively studied in recent years[16,17]. Th22 cells, which are a newly described subset of CD4+ T cells, play important roles in a variety of carcinomas. The percentage of Th22 cells is significantly increased in both the peripheral blood and tumor tissues in patients with gastric cancer; this percentage correlates with gastric cancer progression and can predict poor patient survival[18,19]. The over-expression of Th22 cells is also present in hepatocellular carcinoma[20], pancreatic cancer[21] and malignant pleural effusion[22]. In the current study, we demonstrated that the proportion of Th22 cells was enriched in tumor tissues relative to paratumor tissues in patients with CRC. By IHC and RT-qPCR, we observed that the expression level of IL-22 was significantly higher in tumor tissues than in paratumor tissues. AHR is known as the key transcription factor of Th22 cells[11,12]; in the present study, AHR displayed a higher level of expression in tumor tissues compared with that in paratumor tissues. These results are similar to those of aforementioned reports that indicated that the accumulation and differentiation of Th22 cells are induced by the tumor microenvironment.
The phenotypic characteristics of Th22 cells have been described as CCR4+CCR6+CCR10+, and the chemotactic factors CCL22, CCL20 and CCL27 are their corresponding ligands[12]. In this study, we observed that the colorectal tumor microenvironment expressed higher levels of CCL22, CCL20 and CCL27 compared with those of the paratumor tissues, suggesting that the accumulation of Th22 cells in tumor tissues may be mediated by the chemotactic cytokines that are secreted by the tumor microenvironment. This result is similar to that of a study of malignant pleural effusion[22].
Many studies have demonstrated the constitutive activation of STAT3 in a wide variety of human carcinomas, including hematological malignancies and diverse solid tumors[23]. Abundant evidence has suggested that the dysregulation of IL-22 is associated with aberrant STAT3 signaling in liver injury[24], ulcerative colitis[25], oral squamous cell carcinoma[26], and gastric cancer[27]. STAT3 activation in CRC correlates with adverse clinical results[28]. In this study, we co-cultured RKO cells with IL-22 in vitro to investigate the effects of IL-22 on colon cancer cells. We observed that IL-22 enhanced RKO cell proliferation and had anti-apoptotic effects; these effects were blocked by adding S3I-201, suggesting that IL-22 exerts its functions in CRC via STAT3 signaling. These results are similar to those found in studies of lung cancer cells[22] and of Hct-116 colon cancer cells[29]. Moreover, by activating the STAT3 pathway, IL-22 may act as a novel chemoresistance cytokine that prevents CRC patients from benefiting from FOLFOX chemotherapy[30], and promote CRC invasiveness and stemness[31]. Finally, we verified this effect in a subcutaneous tumor model. We observed that tumor growth in nude mice could be significantly promoted by IL-22 but completely reversed by adding a STAT3 inhibitor.
In conclusion, we measured the proportion of Th22 cells in the colorectal tumor microenvironment and found that the accumulation of Th22 cells in tumor sites may be related to the functions of chemotactic factors that are secreted by the tumor microenvironment. In addition, IL-22 was associated with CRC development in both in vitro and in vivo experiments, most likely by activating the STAT3 signaling pathway. The correlation between immunology and malignant tumors has become an important research area[32,33]. Further understanding the regulation and mechanism of Th22 cells in tumor microenvironments may provide new insights into immune therapeutic strategies for patients with CRC.
Colorectal cancer (CRC) is one of the most commonly occurring cancers worldwide. In recent years, tumor immunology has become a research hotspot, and understanding the molecular pathway involved in CRC will help to improve cancer prevention and treatment. Th22 cells were first introduced in 2009, and the functional characteristics of these cells in inflammatory and autoimmune diseases have been extensively studied. However, knowledge regarding their role in tumor immunity is relatively limited, particularly in CRC.
Studies have shown that Th22 cells are involved in the progression of many digestive malignant tumors. However, the specific participation mechanism of these cells remains unclear.
The authors analyzed the relation between Th22 cells and the colorectal tumor microenvironment from a new perspective, verified their effects on CRC via in vivo and in vitro experiments, and attempted to demonstrate the specific signaling pathway by which Th22 cells participate in carcinogenesis.
The results of this study indicated that Th22 cells might be a prognostic factor and a potential therapeutic target for patients with CRC.
Flow cytometry, which is a biophysical technology employed in cell counting, cell sorting and biomarker detection, widely used in basic research, clinical trials and blood cancer diagnosis.
This is a well conducted study on very timely topics. The authors can improve this paper with more thorough literature review in the context of tumor changes and immunity.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 921] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 3. | Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 956] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 4. | Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055-6072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365-16385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huang C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol. 2012;18:971-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol. 2006;12:7233-7238. [PubMed] |
| 9. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 5009] [Article Influence: 250.5] [Reference Citation Analysis (19)] |
| 10. | Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20:3738-3750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 832] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 12. | Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 794] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 13. | Tian T, Yu S, Ma D. Th22 and related cytokines in inflammatory and autoimmune diseases. Expert Opin Ther Targets. 2013;17:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Li L, Huang YH, Li Y, Wang FQ, Shang BY, Zhen YS. Antitumor activity of anti-type IV collagenase monoclonal antibody and its lidamycin conjugate against colon carcinoma. World J Gastroenterol. 2005;11:4478-4483. [PubMed] |
| 15. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 138902] [Article Influence: 5556.1] [Reference Citation Analysis (2)] |
| 16. | Zhang S, Li W, Xia Z, Mao Y. CD4 T cell dependent tumor immunity stimulated by dendritic cell based vaccine. Biochem Biophys Res Commun. 2011;413:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20:4586-4596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Guo G, Chen W, Liu XF, Zhang JY, Liu T. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother. 2012;61:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Qin S, Ma S, Huang X, Lu D, Zhou Y, Jiang H. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res. 2014;26:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 21. | Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B, Wang H. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing CD4+ T cells correlate with pancreatic cancer progression. Pancreas. 2014;43:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ye ZJ, Zhou Q, Yin W, Yuan ML, Yang WB, Xiang F, Zhang JC, Xin JB, Xiong XZ, Shi HZ. Interleukin 22-producing CD4+ T cells in malignant pleural effusion. Cancer Lett. 2012;326:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 24. | Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, Li Y, Park O, Dooley S, Ju C. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 2014;193:2512-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY, Sun C, Shi RH. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol. 2013;19:2638-2649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Naher L, Kiyoshima T, Kobayashi I, Wada H, Nagata K, Fujiwara H, Ookuma YF, Ozeki S, Nakamura S, Sakai H. STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma. Int J Oncol. 2012;41:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Fukui H, Zhang X, Sun C, Hara K, Kikuchi S, Yamasaki T, Kondo T, Tomita T, Oshima T, Watari J. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br J Cancer. 2014;111:763-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 30. | Wu T, Wang Z, Liu Y, Mei Z, Wang G, Liang Z, Cui A, Hu X, Cui L, Yang Y. Interleukin 22 protects colorectal cancer cells from chemotherapy by activating the STAT3 pathway and inducing autocrine expression of interleukin 8. Clin Immunol. 2014;154:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 31. | Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40:772-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 325] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 32. | Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 33. | Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1121] [Cited by in RCA: 1104] [Article Influence: 92.0] [Reference Citation Analysis (9)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ogino S S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Liu XM