Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.4030
Peer-review started: September 4, 2014
First decision: October 29, 2014
Revised: November 26, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 7, 2015
Processing time: 215 Days and 19 Hours
AIM: To determine the value of computed tomographic angiography (CTA) for diagnosis and therapeutic planning in lower gastrointestinal (GI) bleeding.
METHODS: Sixty-three consecutive patients with acute lower GI bleeding underwent CTA before endovascular or surgical treatment. CTA was used to determine whether the lower GI bleeding was suitable for endovascular treatment, surgical resection, or conservative treatment in each patient. Treatment planning with CTA was compared with actual treatment decisions or endovascular or surgical treatment that had been carried out in each patient based on CTA findings.
RESULTS: 64-row CTA detected active extravasation of contrast material in 57 patients and six patients had no demonstrable active bleeding, resulting in an accuracy of 90.5% in the detection of acute GI bleeding (57 of 63). In three of the six patients with no demonstrable active bleeding, active lower GI bleeding recurred within one week after CTA, and angiography revealed acute bleeding. The overall location-based accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the detection of GI bleeding by 64-row CTA were 98.8% (249 of 252), 95.0% (57 of 60), 100% (192 of 192), 100% (57 of 57), and 98.5% (192 of 195), respectively. Treatment planning was correctly established on the basis of 64-row CTA with an accuracy, sensitivity, specificity, PPV and NPV of 98.4% (248 of 252), 93.3% (56 of 60), 100% (192 of 192), 100% (56 of 56), and 97.5% (192 of 196), respectively, in a location-based evaluation.
CONCLUSION: 64-row CTA is safe and effective in making decisions regarding treatment, without performing digital subtraction angiography or surgery, in the majority of patients with lower GI bleeding.
Core tip: The best modality for the initial diagnosis of acute lower gastrointestinal bleeding (GI) bleeding is controversial. We determined the clinical value of computed tomography angiography (CTA) for diagnosis and therapeutic planning in patients with lower GI bleeding. Sixty-three consecutive patients with acute lower GI bleeding underwent CTA before endovascular or surgical treatment. We found a high overall location-based accuracy, sensitivity, and specificity for the diagnosis and therapeutic planning of acute GI bleeding. We suggest that 64-row CTA is safe and effective in diagnosis and therapeutic planning, without performing digital subtraction angiography or surgery, in patients with lower GI bleeding.
- Citation: Ren JZ, Zhang MF, Rong AM, Fang XJ, Zhang K, Huang GH, Chen PF, Wang ZY, Duan XH, Han XW, Liu YJ. Lower gastrointestinal bleeding: Role of 64-row computed tomographic angiography in diagnosis and therapeutic planning. World J Gastroenterol 2015; 21(13): 4030-4037
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/4030.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.4030
Lower gastrointestinal (GI) bleeding, defined as a bleeding source below the ligament of Treitz in the duodenum, is responsible for approximately 20% of all GI bleeding[1]. Although up to 90% of acute lower GI hemorrhage stops spontaneously without any intervention, a small proportion of these patients will experience exsanguinating hemorrhage and will require invasive procedures to control the bleeding[2,3]. Lower GI bleeding is a common surgical or interventional emergency requiring immediate diagnosis to determine the site and cause of bleeding in order to provide optimal treatment. However, there is considerable controversy regarding the best modality for the initial diagnosis of acute lower GI bleeding.
The current literature suggests that colonoscopy is usually preferred, but the procedure in most cases is technically difficult due to fresh blood or feces; moreover, it is more often a diagnostic rather than a therapeutic modality[4,5]. Over the past few decades, mesenteric angiography and embolization of the bleeding vessels have slowly been accepted as an integral part in the management of patients with acute lower GI hemorrhage[6-10]. However, these procedures are more invasive and require a longer time to perform compared with investigations such as computed tomography (CT). Moreover, colonoscopy and angiography can be relatively inaccessible outside of normal working hours, particularly in some countries or regions due to lack of trained personnel or infrastructure.
Recently, helical computerized tomography has increasingly been used to compare the sensitivity and specificity of computed tomography angiography (CTA) to digital subtraction angiography (DSA) as screening tools for the detection of acute GI bleeding[11-15]. However, the use of CTA in the diagnosis and preoperative planning of acute GI bleeding has received little attention. Therefore, the purpose of the present study was to determine the clinical value of 64-row CTA in the diagnosis and therapeutic planning of patients with acute lower GI bleeding compared with conventional angiography.
The institutional review board approved the study protocol, and patients or qualifying family members provided informed consent before participation. From June 2010 to June 2014, 63 consecutive patients with suspected acute lower GI bleeding detected by CTA underwent interventional embolization, surgical resection or conservative treatment. DSA or surgery, regarded as the gold standard, was performed after CTA to confirm the diagnosis of lower GI bleeding.
The patients included 37 men and 26 women aged 18-89 years (median age, 66 years). Acute GI bleeding was defined as hematemesis, melena, or hematochezia that occurred 24 h prior to CTA. Massive bleeding was considered to have occurred if either of the following two criteria was met: the patients required transfusion of at least 4 units of blood during a 24-h period in the hospital, or they were hemodynamically unstable (hypotension with systolic blood pressure < 90 mmHg). The causes of lower GI tract bleeding (n = 63) were stress ulceration (n = 7), diverticulum (n = 18), trauma (n = 26), arteriovenous malformation (AVM, n = 4), angiodysplasia (n = 1), stromal tumor of the GI tract (n = 1), Crohn’s disease (n = 3), colon cancer (n = 1), nonspecific colitis (n = 1), and unknown (n = 1).
All CTA examinations were performed using a 64-row CT scanner (LightSpeed VCT or Discovery CT750 HD, GE Healthcare, United States). The smart preset scan technique was used for enhancement CTA with the following parameters: 0.625 mm × 64 slice, 120 kVp, 100-600 mA, 1.375:1 helical pitch, and an acquisition time of 30.6 s. The standard dosage for enhancement CTA was 0.7 mL/kg body weight in addition to 40 mL normal sodium, and the contrast material was administered using a power injector at a rate of 4 mL/s through a 22-gauge needle into the antecubital vein. When the concentration of contrast medium in the ascending aorta was 100 HU, the CT scanner automatically scanned from the diaphragmatic dome to the pubic symphysis and collected images. The acquired image data sets were then transferred to a workstation (GE AW4.3 or GE AW4.3, GE Medical, United States), where 3D image reconstruction, including oblique, coronal and sagittal maximum-intensity projection (MIP), multiplanar reconstruction (MPR), and three-dimensional volume-rendered (VR) images of the GI tract and abdominal vascular structures, were performed with a 732 × 732 matrix.
With arterial phase 64-row CT, the following two features were considered diagnostic of lower GI bleeding: (1) the presence of contrast material extravasation in the bowel lumen; and (2) extravasated contrast material with an attenuation level greater than 90 HU.
Three observers were blinded to all clinical, DSA and surgical data. They independently analyzed all CTA datasets on an offline workstation from multiple on-screen viewing angles. The source images, MIPs, MPR, and VR were presented on-screen, thus allowing adjustment of the appropriate threshold of the window width and level. In the presence of interobserver discrepancies in the detection of lower GI bleeding, a consensus or a majority decision was obtained. Two radiologists (K.Z. and G.H.H.) with 7 and 2 years of experience of abdominal CT, respectively, analyzed the CT images. Final decisions regarding the CT findings were made by consensus. In 56 patients, both observers independently reached the same interpretation. In the remaining seven patients, a decision was reached by consensus.
For this assessment, the location of lower GI bleeding was recorded in the following anatomic locations: jejunum, ileum, ascending colon, transverse colon, descending colon, and rectum. The locations of active bleeding were individually recorded by two authors.
After diagnostic CTA, treatment decisions regarding interventional embolization with coil and/or gelatin sponge or glue, surgical resection or conservative treatment were made by consensus by the attending surgeon and interventional radiologists. There were four treatment options: (1) embolization with coils or glue; (2) surgery; (3) unsuccessful attempt to place coils or surgical resection, no further therapy; and (4) conservative treatment. Treatment methods and treatment criteria based on CTA are shown in Table 1.
| Location | Acute lower GI bleeding present1 (n = 60) | Acute lower GI bleedingabsent (n = 192) | ||
| True-positive findings | False-negative findings | True-negative findings | False-positive findings | |
| Jejunum | 15 | 1 | 47 | 0 |
| Ileum | 29 | 2 | 32 | 0 |
| Colon | 12 | 0 | 51 | 0 |
| Rectum | 1 | 0 | 62 | 0 |
| Total (n = 252) | 57 | 3 | 192 | 0 |
In our hospital, surgical resection is considered first-line treatment for lower GI bleeding. Surgical resection was considered when lower GI bleeding was confirmed by CTA and associated with diverticular disease, angiodysplasia or AVM, malignancy or bowel ischemia. In patients with massive bleeding, endovascular embolization was often needed to stop bleeding with subsequent radical surgical resection of the lesions.
Patients were considered for endovascular embolization when lower GI bleeding was confirmed by CTA and associated with stress ulceration or trauma.
Patients were considered suitable for conservative treatment when they had no lower GI bleeding on CTA, had lower GI bleeding that was too difficult for endovascular treatment or surgical resection, or when the treatment had been unsuccessful.
The categorical demographic and basic characteristic variables, expressed as numbers and percentages, were compared using the χ2 test. Continuous variables were expressed as mean ± SD and compared using an unpaired t test, if normally distributed. The calculation of sensitivity, specificity, accuracy, PPV and NPV for the detection of acute lower GI bleeding with 64-row CTA was performed on the basis of a per location analysis in relation to results at angiography or surgery. For the purposes of statistical analysis, a true-positive finding was defined as depiction on 64-row CTA of the presence of contrast material extravasation when the results of angiography were positive for active bleeding. A false-positive finding was defined as depiction on 64-row CTA of the presence of active bleeding that was not detected at angiography. A true-negative finding was defined as the lack of identification of a bleeding focus on 64-row CTA images when the results of angiography were negative for active bleeding. A false-negative finding was defined as depiction on 64-row CTA of the absence of active bleeding despite detection of active bleeding at angiography. The diagnostic performance parameters of CTA for the diagnosis of lower GI bleeding compared with those of DSA or surgery (that is, accuracy, sensitivity, specificity, PPV and NPV) were expressed as percentages.
A good correlation from the prospective 64-row CTA protocol was defined as treatment planning by radiologists or surgeons based on CTA that correlated with the actual treatment decision or treatment performed by the interventional radiologists or surgeon based on CTA. A deviation from the protocol was defined as treatment planning by the radiologists or surgeon based on CTA that was changed or differed from the actual treatment decision or procedures performed by the interventional radiologists or surgeon based on DSA. On the basis of this dichotomization, accuracy, sensitivity, specificity, PPV and NPV were also calculated. Statistical analyses were performed using SPSS (version 13.0, SPSS Inc., Chicago, IL, United States).
64-row CTA detected active extravasation of contrast material in 57 patients and six patients had no demonstrable active extravasation of contrast material. During 64-row CTA, contrast material extravasation was identified in the jejunum in 17 patients, in the ileum in 33 patients, in the colon in 12 patients, and in the rectum in one patient. Of these 57 patients with contrast material extravasation depicted on 64-row CTA, findings at angiography or surgery confirmed acute GI bleeding in all 57 patients. In three patients, angiography revealed acute duodenal bleeding that was not detected on 64-row CTA (false-negative 64-row CTA findings). Thus, the overall patient-based accuracy of 64-row CTA in the detection of acute GI bleeding was 90.5% (57 of 63). In 57 patients in whom 64-row CTA depicted extravasation of contrast material, the mean attenuation level was 276 HU (attenuation range, 115-378 HU).
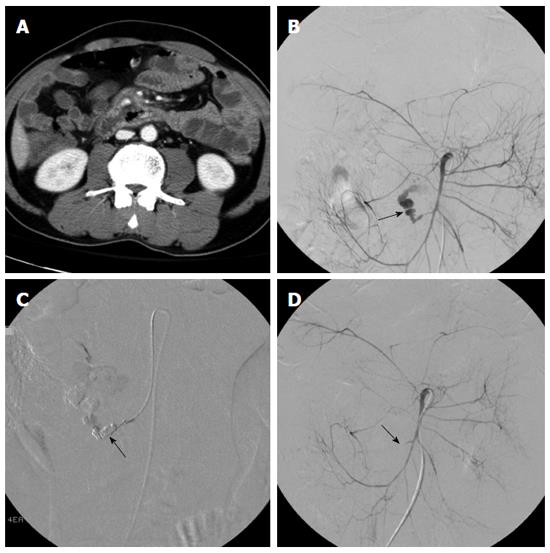
We evaluated 252 anatomic locations in 63 patients for the presence or absence of acute GI bleeding (Table 1). The overall location-based accuracy, sensitivity, specificity, PPV and NPV for the detection of GI bleeding by 64-row CTA were 98.8% (249 of 252), 95.0% (57 of 60), 100% (192 of 192), 100% (57 of 57), and 98.5% (192 of 195), respectively. Of the 252 locations evaluated, 57 had evidence of acute GI bleeding on both 64-row CTA and angiography or surgery (true-positive CTA findings) (Figure 1). In three cases without evidence of acute GI bleeding on 64-row CTA, findings were positive at angiography (false-negative findings).
64-row CTA had an accuracy of 100% for localization of acute GI bleeding. The site of contrast material extravasation on 64-row CTA images corresponded exactly with the angiographically or surgically depicted site of bleeding in all patients in whom a focus of bleeding was detected on 64-row CTA.
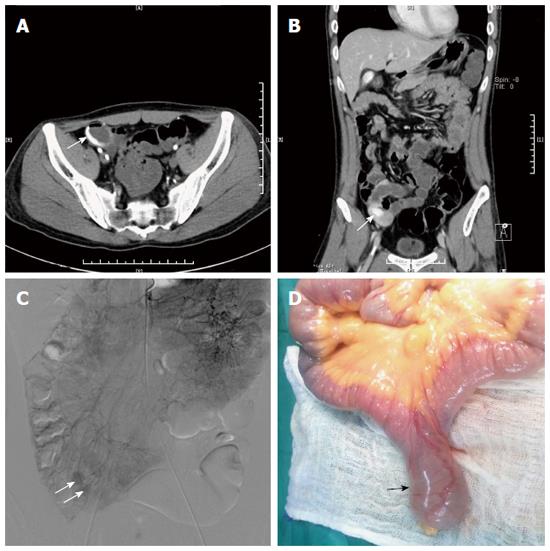
When actual treatment decisions were compared with treatment performed based on DSA or surgery, a good correlation with the prospective CTA protocol was obtained in all patients (Table 2, Figure 2). Of the 63 patients with acute lower GI bleeding on 64-row CTA, treatment planning in 32 patients consisted of coils and/or gelatin sponge or glue, 25 patients were managed with surgical resection, and the remaining 6 patients with no acute lower GI bleeding received conservative treatment. In three of 6 patients who received conservative treatment, active lower GI bleeding recurred within one week after CTA examination, and angiography revealed acute lower GI bleeding that was not detected on 64-row CTA (false-negative findings). Embolization was performed in three patients. In one patient with colon cancer, surgical resection could not be performed due to massive bleeding and endovascular embolization was carried out (false-negative finding). Treatment planning was correctly established on the basis of 64-row CTA with an accuracy, sensitivity, specificity, PPV and NPV of 98.4% (248 of 252), 93.3% (56 of 60), 100% (192 of 192), 100% (56 of 56), and 97.5% (192 of 196), respectively, in a location-based evaluation (Table 3).
| Treatment method | Treatment planning by CTA | Actual treatment performed |
| Endovascular treatment | 32 | 36 |
| Surgical resection | 25 | 24 |
| Conservative treatment | 6 | 3 |
| Location | Endovascular or surgical treatment (n = 60) | Conservative treatment(n = 192) | ||
| True-positive findings | False-negative findings | True-negative findings | False-positivefindings | |
| Jejunum | 15 | 1 | 47 | 0 |
| Ileum | 29 | 2 | 32 | 0 |
| Colon | 11 | 1 | 51 | 0 |
| Rectum | 1 | 0 | 62 | 0 |
| Total (n = 252) | 56 | 4 | 192 | 0 |
Lower GI bleeding may involve the small bowel, colon, and rectum, and carries a mortality rate of 3.6%[16]. Lower GI bleeding is less common than upper GI bleeding and accounts for approximately 30% of all GI bleeding[17,18]. Lower GI bleeding tends to affect more elderly patients than young patients[19]. Common causes of lower GI bleeding are diverticular disease, angiodysplasia, neoplasms, colitis, and benign anorectal lesions[16,17,20].
Many diagnostic approaches are available to detect and locate the source of lower GI bleeding, each with its own advantages and weaknesses[17]. Although endoscopy is considered to be the first-line diagnostic modality for lower GI bleeding, endoscopy often fails to depict the exact focus of bleeding when excessive blood or clots impair visualization[21]. Capsular endoscopy is a relatively new method for establishing the cause of small bowel bleeding, but is not useful in an urgent situation[22]. Although colonoscopy is frequently used in lower GI bleeding, its value in the diagnosis of massive bleeding remains controversial, and appears to be most efficacious only when massive bleeding has stopped, allowing time for a bowel preparation[23]. Radionuclide imaging is noninvasive, simple to perform, sensitive, and has the ability to carry out delayed scans up to 24 h after radioisotope injection to detect re-bleeding, but it is a time-consuming method with a high false localization rate of up to 22%[24]. Catheter-directed angiography is considered accurate in the diagnosis of acute GI bleeding, and may be employed advantageously for immediate therapeutic transcatheter embolization[25]. However, it is an invasive procedure, and negative results are common in patients with a stable hemodynamic status, slower GI bleeding or no active bleeding present at the time of contrast material administration[26]. Therefore, fast and accurate detection, and localization of the bleeding source are crucial for effective hemostatic treatment in patients with rapid acute massive lower GI bleeding.
Recent technical advances in CT have led to the increased use of CTA as a first-line modality in the detection and localization of GI bleeding, particularly within the lower GI tract[27-30]. 64-row CTA allows thinner collimation, faster scanning times, greater anatomic coverage and better multi-planar reformatted (MPR) images, which have greatly expanded the diagnostic role of CTA for various pathologic processes. CTA provides several distinct advantages over nuclear scintigraphy and catheter angiography: CTA is readily available at all times and has been shown to detect rates of bleeding greater than the threshold of first-order selective mesenteric angiography[11-15]. When positive, 64-row CTA provides precise anatomic localization of the bleeding site as well as an exquisite map of the mesenteric vasculature. Preoperative knowledge of the bleeding site and the patient’s mesenteric vascular anatomy facilitates rapid catheterization of the source mesenteric trunk, directs subselective mesenteric catheterization, and obviates the need for aortography. Evaluation with CTA may also demonstrate the etiology of the bleed or additional unsuspected pathology that may require deviation from customary management. Conversely, when active hemorrhage is excluded by CTA, temporarily deferring angiography and continuing supportive care appears safe with no adverse events directly attributable to angiographic deferment in our series. This may prevent unnecessary invasive tests or mobilization of the surgical or interventional radiology team. Therefore, we propose that CTA should be adopted in the clinic as the first-line examination for the evaluation of acute lower GI bleeding.
This prospective study was based on our hypothesis that CTA could replace DSA or surgery as a reliable diagnostic and pretreatment planning tool for patients with lower GI bleeding. The results of our study indicated that arterial phase 64-row CTA is highly accurate for both detection and localization of acute massive lower GI bleeding. For the detection of acute GI bleeding, 64-row CTA had a sensitivity of 95.0% and a specificity of 100%, and for treatment planning, 64-row CTA had a sensitivity of 93.3% and a specificity of 100%. Although there were three false-negative diagnoses, we found that 64-row CTA not only accurately identified the presence of lower GI bleeding, but also demonstrated a good correlation with DSA or surgery in treatment planning for lower GI bleeding. It appears that treatment planning on the basis of CTA, before DSA or surgery, is a feasible and effective option for patients with lower GI bleeding.
Although there have been many reports in the literature assessing the validity of CTA as a diagnostic technique for the detection of lower GI bleeding[11-15], few have evaluated the clinical implications of a protocol that uses CTA instead of DSA or surgery as a diagnostic and pretreatment planning tool. If CTA is to serve as a non-invasive replacement for DSA or surgery in pretreatment planning, it must provide precise visualization of the location of the lower GI bleeding and its surrounding structures. In addition, it is essential to identify the cause of bleeding and the bleeding artery before performing endovascular treatment or surgical resection. A combination of the patient’s history and CTA results showed that the cause of bleeding in most patients was clearly known prior to surgical or interventional management. For instance, four patients with AVM, one with angiodysplasia, one with stromal tumor of the GI tract, two with Crohn’s disease, one with colon cancer, and 16 with diverticular bleed were treated surgically without angiographic or endoscopic intervention.
The overall sensitivity and specificity of CTA in the detection of GI hemorrhage in our series was 95% and 100%, respectively. These values are similar to the recently published data by Yoon et al[12] (sensitivity, 90.9%; specificity, 99%). Yoon et al[12] demonstrated that arterial phase CTA performed well in patients with massive GI hemorrhage in their prospective series of 26 patients. These authors found that the results of their study indicated that arterial-phase 64-row CTA is highly accurate for both the detection (sensitivity of 90.9% and specificity of 99%) and localization (accuracy of 100%) of acute massive GI bleeding as compared with angiograms performed in all patients with GI bleeding. In addition, 64-row CTA had an accuracy of 100% for localization of acute GI bleeding. The site of contrast material extravasation on 64-row CTA scans corresponded to the site of bleeding identified on angiograms or surgical resection in patients with acute GI bleeding. This agreement is of particular importance in the performance of angiography and subsequent embolization procedures in critically ill patients with acute massive lower GI bleeding[12].
The present study had some limitations. Firstly, this was a single-center study, and the patient population was relatively small. The small sample prevents us from generalizing the results. Secondly, the risks of CTA scanning are minor and arise from exposure of the patient to ionizing radiation and the administration of intravenous contrast material. Thirdly, CT artifacts can obscure contrast material extravasation in the bowel lumen which may be misdiagnosed as acute GI bleeding. Lastly, intermittent GI hemorrhage, even in cases of massive bleeding, is not uncommon, and CTA may fail to depict any abnormalities during periods of quiescence[12]. This phenomenon of intermittent hemorrhage may account for the false-negative CT angiograms reported in this study. Therefore, repeated CTA or catheter angiography is often necessary before a bleeding source is definitively localized. In addition, there was a lack of a true gold standard for comparison in three negative CTA cases, as most were successfully observed without additional intervention and the exact site of hemorrhage was never determined.
In conclusion, our findings suggest that 64-row CTA could act as an accurate first-line screening method for the detection and localization of acute lower GI bleeding sites. The procedure is safe and effective for making decisions regarding treatment, without performing DSA or surgery, in the majority of patients with lower GI bleeding.
Lower gastrointestinal (GI) bleeding is a common surgical or interventional emergency requiring immediate diagnosis to determine the site and cause of bleeding in order to provide optimal treatment. However, there is controversy regarding the best modality for the initial diagnosis of acute lower GI bleeding.
Computerized tomography has increasingly been used to compare the sensitivity and specificity of computed tomographic angiography (CTA) with digital subtraction angiography (DSA) as screening tools for depicting acute GI bleeding. However, the use of CTA in the diagnosis and preoperative planning of acute GI bleeding has received little attention. The authors determined the clinical value of CTA for diagnosis and therapeutic planning in patients with lower GI bleeding.
CTA was used as a diagnostic and preoperative planning tool in patients with lower GI bleeding. The results of 64-row CTA were compared with those of DSA and surgical resection. This is the first study to determine the value of CTA for diagnosis and therapeutic planning in lower GI bleeding.
64-row CTA could act as an accurate first-line screening method for the detection and localization of acute lower GI bleeding sites. The procedure was safe and effective for making decisions regarding treatment, without performing DSA or surgery, in the majority of patients with lower GI bleeding.
GI bleeding, defined as a bleeding source below the ligament of Treitz in the duodenum, is responsible for approximately 20% of all GI bleeding.
The authors present a nonrandomized prospective trial evaluating the value of CTA for diagnosis and therapeutic planning in lower GI bleeding. The results reveal a high overall location-based accuracy, sensitivity, specificity, and positive and negative predictive values for the diagnosis and therapeutic planning in patients with acute GI bleeding by 64-row CTA. These results suggest 64-row CTA is safe and effective in making decisions regarding treatment on the basis of CTA, without performing DSA or surgery, in the majority of patients with lower GI bleeding and could act as an accurate first-line screening method for detection and localization of acute lower GI bleeding sites.
| 1. | Friedman LS, Martin P. The problem of gastrointestinal bleeding. Gastroenterol Clin North Am. 1993;22:717-721. [PubMed] |
| 2. | Peter DJ, Dougherty JM. Evaluation of the patient with gastrointestinal bleeding: an evidence based approach. Emerg Med Clin North Am. 1999;17:239-261, x. [PubMed] |
| 3. | Schuetz A, Jauch KW. Lower gastrointestinal bleeding: therapeutic strategies, surgical techniques and results. Langenbecks Arch Surg. 2001;386:17-25. [PubMed] |
| 4. | Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82. [PubMed] |
| 5. | Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402. [PubMed] |
| 6. | DeBarros J, Rosas L, Cohen J, Vignati P, Sardella W, Hallisey M. The changing paradigm for the treatment of colonic hemorrhage: superselective angiographic embolization. Dis Colon Rectum. 2002;45:802-808. [PubMed] |
| 7. | Tan KK, Wong D, Sim R. Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg. 2008;32:2707-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Gillespie CJ, Sutherland AD, Mossop PJ, Woods RJ, Keck JO, Heriot AG. Mesenteric embolization for lower gastrointestinal bleeding. Dis Colon Rectum. 2010;53:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Tan KK, Nallathamby V, Wong D, Sim R. Can superselective embolization be definitive for colonic diverticular hemorrhage? An institution’s experience over 9 years. J Gastrointest Surg. 2010;14:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Koh DC, Luchtefeld MA, Kim DG, Knox MF, Fedeson BC, Vanerp JS, Mustert BR. Efficacy of transarterial embolization as definitive treatment in lower gastrointestinal bleeding. Colorectal Dis. 2009;11:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16:3957-3963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160-167. [PubMed] |
| 13. | Sun H, Jin Z, Li X, Qian J, Yu J, Zhu F, Zhu H. Detection and localization of active gastrointestinal bleeding with multidetector row computed tomography angiography: a 5-year prospective study in one medical center. J Clin Gastroenterol. 2012;46:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol. 2013;23:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Chang WC, Tsai SH, Chang WK, Liu CH, Tung HJ, Hsieh CB, Huang GS, Hsu HH, Yu CY. The value of multidetector-row computed tomography for localization of obscure acute gastrointestinal bleeding. Eur J Radiol. 2011;80:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Lim JK, Ahmed A. Endoscopic approach to the treatment of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:123-129. [PubMed] |
| 17. | Laing CJ, Tobias T, Rosenblum DI, Banker WL, Tseng L, Tamarkin SW. Acute gastrointestinal bleeding: emerging role of multidetector CT angiography and review of current imaging techniques. Radiographics. 2007;27:1055-1070. [PubMed] |
| 18. | Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92:924-928. [PubMed] |
| 19. | Lee EW, Laberge JM. Differential diagnosis of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:112-122. [PubMed] |
| 20. | Lieberman D. Gastrointestinal bleeding: initial management. Gastroenterol Clin North Am. 1993;22:723-736. [PubMed] |
| 21. | Vreeburg EM, Snel P, de Bruijne JW, Bartelsman JF, Rauws EA, Tytgat GN. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236-243. [PubMed] |
| 22. | Davis BR, Harris H, Vitale GC. The evolution of endoscopy: wireless capsule cameras for the diagnosis of occult gastrointestinal bleeding and inflammatory bowel disease. Surg Innov. 2005;12:129-133. [PubMed] |
| 23. | Al Qahtani AR, Satin R, Stern J, Gordon PH. Investigative modalities for massive lower gastrointestinal bleeding. World J Surg. 2002;26:620-625. [PubMed] |
| 24. | Fallah MA, Prakash C, Edmundowicz S. Acute gastrointestinal bleeding. Med Clin North Am. 2000;84:1183-1208. [PubMed] |
| 25. | Lipof T, Sardella WV, Bartus CM, Johnson KH, Vignati PV, Cohen JL. The efficacy and durability of super-selective embolization in the treatment of lower gastrointestinal bleeding. Dis Colon Rectum. 2008;51:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Shin JH, Yoon HK, Chae EY, Myung SJ, Ko GY, Gwon DI, Sung KB. Angiographically negative acute arterial upper and lower gastrointestinal bleeding: incidence, predictive factors, and clinical outcomes. Korean J Radiol. 2009;10:384-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Artigas JM, Martí M, Soto JA, Esteban H, Pinilla I, Guillén E. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. Radiographics. 2012;33:1453-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Martí M, Artigas JM, Garzón G, Alvarez-Sala R, Soto JA. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology. 2012;262:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Lee SS, Oh TS, Kim HJ, Chung JW, Park SH, Kim AY, Ha HK. Obscure gastrointestinal bleeding: diagnostic performance of multidetector CT enterography. Radiology. 2011;259:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Huprich JE, Fletcher JG, Alexander JA, Fidler JL, Burton SS, McCullough CH. Obscure gastrointestinal bleeding: evaluation with 64-section multiphase CT enterography--initial experience. Radiology. 2008;246:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (4)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gong JS, Peparini N, Pescatori M S- Editor: Ma YJ L- Editor: Logan S E- Editor: Wang CH