Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3936
Peer-review started: November 4, 2014
First decision: November 26, 2014
Revised: December 7, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 7, 2015
Processing time: 154 Days and 22.5 Hours
AIM: To investigate the eradication rate and histological changes after Helicobacter pylori (H. pylori) eradication treatment following subtotal gastrectomy for gastric cancer.
METHODS: A total of 610 patients with H. pylori infection who had undergone surgery for either early or advanced gastric adenocarcinoma between May 2004 and December 2010 were retrospectively studied. A total of 584 patients with proven H. pylori infection after surgery for gastric cancer were enrolled in this study. Patients received a seven day standard triple regimen as first-line therapy and a 10 d bismuth-containing quadruple regimen as second-line therapy in cases of eradication failure. The patients underwent an esophagogastroduodenoscopy (EGD) between six and 12 mo after surgery, followed by annual EGDs. A further EGD was conducted 12 mo after confirming the result of the eradication and the histological changes. A gastric biopsy specimen for histological examination and Campylobacter-like organism testing was obtained from the lesser and greater curvature of the corpus of the remnant stomach. Histological changes in the gastric mucosa were assessed using the updated Sydney system before eradication therapy and at follow-up after 12 mo.
RESULTS: Eradication rates with the first-line and second-line therapies were 78.4% (458/584) and 90% (36/40), respectively, by intention-to-treat analysis and 85.3% (458/530) and 92.3% (36/39), respectively, by per-protocol analysis. The univariate and multivariate analyses revealed that Billroth II surgery was an independent factor predictive of eradication success in the eradication success group (OR = 1.53, 95%CI: 1.41-1.65, P = 0.021). The atrophy and intestinal metaplasia (IM) scores 12 mo after eradication were significantly lower in the eradication success group than in the eradication failure group (0.25 ± 0.04 vs 0.47 ± 0.12, P = 0.023; 0.27 ± 0.04 vs 0.51 ± 0.12, P = 0.015, respectively). The atrophy and IM scores 12 mo after successful eradication were significantly lower in the Billroth II group than in the Billroth I group (0.13 ± 0.09 vs 0.31 ± 0.12, P = 0.029; 0.32 ± 0.24 vs 0.37 ± 0.13, P = 0.034, respectively).
CONCLUSION: Patients with H. pylori following subtotal gastrectomy had a similar eradication rate to patients with an intact stomach. H. pylori eradication is recommended after subtotal gastrectomy.
Core tip: This is the first study to investigate the eradication rate and histological changes after Helicobacter pylori (H. pylori) eradication treatment in patients following subtotal gastrectomy for gastric cancer. The patients with H. pylori infection who had undergone a subtotal gastrectomy for gastric cancer had a similar eradication rate to patients with an intact stomach. H. pylori eradication in gastric cancer patients following subtotal gastrectomy resulted in histological improvement, especially in the Billroth II group.
-
Citation: Hwang JJ, Lee DH, Kang KK, Lee AR, Yoon H, Shin CM, Park YS, Kim N. Eradication rate and histological changes after
Helicobacter pylori eradication treatment in gastric cancer patients following subtotal gastrectomy. World J Gastroenterol 2015; 21(13): 3936-3943 - URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3936
Helicobacter pylori (H. pylori) infection is a leading cause of gastric cancer[1,2] and H. pylori eradication therapy is thought to convey beneficial effects in preventing metachronous cancer after endoscopic resection of early gastric cancer[3]. As H. pylori infection remains a risk factor for malignancy after subtotal gastrectomy, several guidelines recommend H. pylori eradication therapy in patients who have undergone surgery for gastric cancer[4,5].
Several guidelines recommend H. pylori eradication in patients following surgery for gastric cancer[4,5] but its beneficial effects have not been established. In general, glandular atrophy from H. pylori infection is reversible after eradication but intestinal metaplasia (IM) cannot be improved in patients who have not undergone surgery[6,7]. Onoda et al[8] reported significant changes in glandular atrophy after eradication in the remnant stomach. However, Matsukura et al[9] determined no significant improvements in glandular atrophy or IM. Cho et al[10] reported that H. pylori eradication following subtotal gastrectomy significantly reduced both glandular atrophy and IM scores, 36 mo after eradication.
In this retrospective study, we investigated whether H. pylori eradication resulted in histological improvement in patients who underwent a subtotal gastrectomy for gastric cancer. We also compared the H. pylori eradication rates in patients who underwent subtotal gastrectomy with the recently reported eradication rates in a group that did not undergo surgery.
A total of 610 patients with H. pylori infection who had undergone surgery for either early or advanced gastric adenocarcinoma at Seoul National University Bundang Hospital between May 2004 and December 2010 were retrospectively studied. The patients underwent either open or laparoscopically-assisted distal gastrectomy and either Billroth I (gastroduodenostomy) or II (gastrojejunostomy) surgery was used for reconstruction. The exclusion criteria were as follows: (1) age below 18 years; (2) previous H. pylori eradication before diagnosis of malignancy; (3) previous gastric surgery or endoscopic resection for gastric cancer; (4) severe concurrent disease (hepatic, renal, respiratory or cardiovascular); (5) pregnancy; (6) palliative therapy; and (7) any condition probably associated with poor compliance (e.g., alcoholism or drug addiction).
All patients gave written informed consent and the study was performed according to the directions of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee at Seoul National University Bundang Hospital (Institutional Review Board number: B-1306/206-109).
The patients underwent an esophagogastroduodenoscopy (EGD) between six and 12 mo after surgery, followed by annual EGDs. Of the 610 patients who were offered eradication therapy, all consented to the treatment. A further EGD was conducted 12 mo after confirming the result of the eradication and the histological changes. H. pylori infection and eradication failure were defined on the basis of at least one of the following three tests: first, a positive 13C-urea breath test (13C-UBT); second, histological evidence of H. pylori by modified Giemsa staining in the lesser and greater curvature of the corpus of the remnant stomach; and third, a positive rapid urease test (CLOtest; Delta West, Bentley, Australia) in gastric mucosa biopsy samples from the lesser and greater curvatures of the corpus of the remnant stomach. An endoscopic specialist performed the biopsies and described the endoscopic findings (Dong Ho, Lee). A gastric biopsy specimen for histological examination and Campylobacter-like organism testing was obtained from the lesser and greater curvature of the corpus of the remnant stomach and immediately fixed in formalin. The tumor location was determined with reference to the Japanese Classification of Gastric Cancer[11]. The degree of atrophy and IM, polymorphonuclear neutrophil activity and mononuclear cell count were graded according to the updated Sydney system (0 = none, 1 = mild, 2 = moderate and 3 = marked)[12].
All the patients received seven day standard triple therapy [amoxicillin 1000 mg twice a day (b.i.d.), clarithromycin 500 mg b.i.d. and esomeprazole 20 mg b.i.d.] as first-line therapy. Patients who failed to respond to first-line therapy received a 10 d bismuth-containing quadruple regimen [tripotassium dicitratobismuthate 300 mg four times a day (q.i.d.), tetracycline 500 mg q.i.d., metronidazole 500 mg three times a day and esomeprazole 20 mg b.i.d.] as second-line therapy, with a subsequent 13C-UBT for evaluation of eradication. Patients who took < 85% of the prescribed medication were considered to have low compliance.
H. pylori eradication rates were determined using intention-to-treat (ITT) and per-protocol (PP) analyses. ITT analysis compared treatment groups, including all the patients as originally allocated. PP analysis compared treatment groups, including only those patients who completed the treatment as originally allocated. The mean ± SD was calculated for quantitative variables. The Student’s t test was used to evaluate continuous variables and the χ2 test and Fisher’s exact test were used to assess non-continuous variables. Additionally, univariate and multivariate analyses were conducted to assess the effects of factors on the eradication rate. Updated Sydney system scores were compared using the Wilcoxon signed-rank test and Mann-Whitney U test for unpaired data. All of the statistical analyses were performed using Predictive Analytics Software (PASW) version 20.0 for Windows (SPSS Inc., IBM, Chicago, IL, United States). A P value of less than 0.05 was defined as carrying statistical significance. The statistical methods of this study were reviewed by Medical Research Collaborating Center at Seoul National University Bundang Hospital.
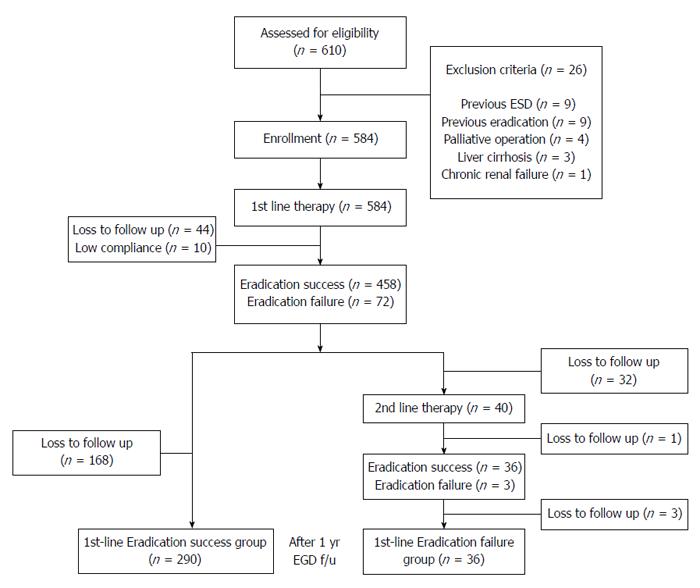
A schematic diagram of the study is provided in Figure 1. Of the 610 patients, 26 were excluded from the study because of previous H. pylori eradication before diagnosis of malignancy (nine patients), endoscopic submucosal dissection before surgery (nine patients), palliative surgery (four patients), liver cirrhosis (three patients) and chronic renal failure (one patient). A total of 584 patients underwent first-line eradication treatment. Forty-four were lost to follow-up and 10 had low treatment compliance. Eradication was surveyed using a 13C-UBT in all the patients after treatment but histological changes were surveyed in only 326 patients after 12 mo. The remaining 204 patients were not examined for histological changes after eradication because they were lost to follow-up. Finally, 326 patients had histological changes of their gastric mucosa analyzed after eradication of H. pylori. Of these, 290 patients in whom H. pylori was successfully eradicated with first-line therapy were assigned to the eradication success group and 36 patients in whom first-line therapy failed were assigned to the eradication failure group. In patients in the eradication failure group, second-line therapy was used to eradicate H. pylori infection. However, this was not successful in most patients because of either poor compliance or adverse events. The mean ages of the eradication success and failure groups were 56.7 ± 10.4 and 56.9 ± 10.7 years (P = 0.124), respectively. The enrolled patients’ baseline demographic and clinical characteristics are provided in Table 1. The eradication rate of patients who underwent Billroth II surgery (96.7%, 89/92) was significantly higher than that of patients who underwent Billroth I surgery in the eradication success group (85.8%, 201/234, P = 0.012). There were no statistically significant differences in gender distribution, smoking status, alcohol use, underlying disease, early gastric cancer/advanced gastric cancer or tumor location between the two groups (P > 0.05).
| Eradication success(n = 290) | Eradication failure(n = 36) | P value | Univariate analysis | |
| Age | 56.7 ± 10.4 | 56.9 ± 10.7 | 0.124 | 0.127 |
| Gender | 0.229 | 0.233 | ||
| Male | 183 (63.1) | 19 (52.7) | ||
| Female | 107 (36.9) | 17 (47.3) | ||
| Smoking | 0.789 | 0.792 | ||
| Non-smoker | 228 (78.6) | 29 (80.5) | ||
| Smoker | 62 (21.4) | 7 (19.5) | ||
| Alcohol | 0.213 | 0.219 | ||
| Non-drinker | 224 (77.2) | 23 (63.8) | ||
| Drinker | 66 (22.8) | 13 (36.2) | ||
| Underlying disease | ||||
| Hypertension | 65 (22.4) | 6 (16.6) | 0.431 | 0.437 |
| Diabetes mellitus | 24 (8.2) | 4 (11.1) | 0.531 | 0.539 |
| EGC/AGC | 0.451 | 0.463 | ||
| EGC | 217 (74.8) | 29 (80.5) | ||
| AGC | 73 (25.2) | 7 (19.5) | ||
| Tumor location | 0.695 | 0.707 | ||
| Lower (Antrum) | 147 (50.6) | 17 (47.2) | ||
| Middle (Body) | 143 (49.3) | 19 (52.7) | ||
| Surgery | 0.012 | 0.016 | ||
| Billroth-I | 201 (69.4) | 33 (91.7) | ||
| Billroth-II | 89 (30.6) | 3 (8.3) |
Table 2 shows the rates of eradication of H. pylori infection according to the ITT and PP analyses. The eradication rates of first-line therapy by ITT and PP analyses were 78.4% (95%CI: 74.9-81.6%) and 85.3% (95%CI: 82.1-88.1%), respectively. Forty of the 72 patients who failed first-line therapy underwent second-line treatment and their eradication rates by ITT and PP analyses were 90% (95%CI: 77.0-96.0%) and 92.3% (95%CI: 79.7-97.4%), respectively.
| First-line therapy | Second-line therapy | P value | |
| ITT analysis | |||
| %, Eradication(ratio) | 78.4 (458/584) | 90 (36/40) | 0.027 |
| 95%CI | 74.9-81.6 | 77.0-96.0 | |
| PP analysis | |||
| %, Eradication(ratio) | 85.3 (458/530) | 92.3 (36/39) | 0.034 |
| 95%CI | 82.1-88.1 | 79.7-97.4 |
To evaluate the clinical factors influencing the efficacy of H. pylori eradication, univariate analyses were performed, which are listed in Table 1. The eradication rate of patients who underwent Billroth II surgery was significantly higher than that of patients who underwent Billroth I surgery (P = 0.012). The other factors did not affect the eradication response. The multivariate analysis revealed that Billroth II surgery (OR = 1.53, 95%CI: 1.41-1.65, P = 0.021) was an independent factor predictive of eradication success in the eradication success group (Table 3).
| Odds ratio | 95%CI | P value | |
| Billroth-II vs Billroth-I | 1.53 | 1.41-1.65 | 0.021 |
| Male vs female | 1.01 | 0.87-1.15 | 0.125 |
| Smoking (+) vs (-) | 1.07 | 0.89-1.25 | 0.163 |
| Alcohol (+) vs (-) | 0.98 | 0.94-1.08 | 0.187 |
Table 4 shows the histological changes of the gastric mucosa according to eradication. Histological changes were compared before and after eradication and scored according to the updated Sydney system. The atrophy scores were significantly lower than the baseline after eradication in the eradication success group (0.25 ± 0.04 vs 0.46 ± 0.04, P < 0.001). The atrophy scores were not significantly different compared to the baseline after eradication in the eradication failure group (0.47 ± 0.12 vs 0.50 ± 0.11, P = 0.698). The atrophy scores after eradication were significantly lower in the eradication success group than in the eradication failure group (0.25 ± 0.04 vs 0.47 ± 0.12, P = 0.023). The IM scores were significantly lower than the baseline after eradication in the eradication success group (0.27 ± 0.04 vs 0.41 ± 0.05, P < 0.001). The IM scores were not significantly different compared to the baseline after eradication in the eradication failure group (0.51 ± 0.12 vs 0.56 ± 0.14, P = 0.226). The IM scores after eradication were significantly lower in the eradication success group than in the eradication failure group (0.27 ± 0.04 vs 0.51 ± 0.12, P = 0.015). Activity and chronic inflammation scores decreased in all the groups. In metachronous cancer patients (n = 7), the atrophy and IM scores were lower than the baseline after eradication but the differences were not statistically significant (P > 0.05; Table 5).
| Eradication success (n = 290) | Eradication failure (n = 36) | P value | |||||
| Baseline | 12 mo | P value | Baseline | 12 mo | P value | ||
| Atrophy | 0.46 ± 0.04 | 0.25 ± 0.04 | < 0.001 | 0.50 ± 0.11 | 0.47 ± 0.12 | 0.698 | 0.023 |
| IM | 0.41 ± 0.05 | 0.27 ± 0.04 | < 0.001 | 0.56 ± 0.14 | 0.51 ± 0.12 | 0.226 | 0.015 |
| Neutrophil count | 2.12 ± 0.04 | 0.40 ± 0.04 | < 0.001 | 2.08 ± 0.12 | 1.11 ± 0.18 | < 0.001 | < 0.001 |
| Mononuclear cells | 2.10 ± 0.03 | 1.45 ± 0.03 | < 0.001 | 2.08 ± 0.08 | 1.64 ± 0.11 | 0.004 | 0.085 |
| Baseline | 12 mo | P value | |
| Atrophy | 1.29 ± 0.42 | 0.71 ± 0.36 | 0.436 |
| Intestinal metaplasia | 1.34 ± 0.41 | 1.00 ± 0.38 | 0.457 |
| Neutrophil count | 2.00 ± 0.22 | 0.86 ± 0.34 | 0.071 |
| Mononuclear cell | 2.00 ± 0.31 | 1.85 ± 0.20 | 0.289 |
Table 6 shows the histological changes of the gastric mucosa according to reconstructive surgery method after successful eradication. Thirty-six eradication failure patients were excluded to analyze the histological changes of the gastric mucosa after successful eradication between the Billroth I and Billroth II groups. The atrophy scores were significantly lower than the baseline after successful eradication in the Billroth II group (0.13 ± 0.09 vs 0.53 ± 0.19, P < 0.001). The atrophy scores were not significantly different compared to the baseline after successful eradication in the Billroth I group (0.31 ± 0.12 vs 0.33 ± 0.10, P = 0.831). The atrophy scores after successful eradication were significantly lower in the Billroth II group than in the Billroth I group (0.13 ± 0.09 vs 0.31 ± 0.12, P = 0.029). The IM scores were significantly lower than the baseline after successful eradication in the Billroth II group (0.32 ± 0.24 vs 0.67 ± 0.21, P < 0.001). The IM scores were not significantly different compared to the baseline after successful eradication in the Billroth I group (0.37 ± 0.13 vs 0.39 ± 0.13, P = 0.572). The IM scores after successful eradication were significantly lower in the Billroth II group than in the Billroth I group (0.32 ± 0.24 vs 0.37 ± 0.13, P = 0.034). Activity and chronic inflammation scores were decreased in all the groups.
| Billroth-I (n = 201) | Billroth-II (n = 89) | P value | |||||
| Baseline | 12 mo | P value | Baseline | 12 mo | P value | ||
| Atrophy | 0.33 ± 0.10 | 0.31 ± 0.12 | 0.831 | 0.53 ± 0.19 | 0.13 ± 0.09 | < 0.001 | 0.029 |
| IM | 0.39 ± 0.13 | 0.37 ± 0.13 | 0.572 | 0.67 ± 0.21 | 0.32 ± 0.24 | < 0.001 | 0.034 |
| Neutrophil count | 2.24 ± 0.10 | 0.21 ± 0.08 | < 0.001 | 2.33 ± 0.16 | 0.33 ± 0.16 | < 0.001 | < 0.001 |
| Mononuclear cells | 2.21 ± 0.10 | 1.33 ± 0.12 | 0.831 | 1.93 ± 0.19 | 1.47 ± 0.09 | 0.540 | 0.085 |
Anatomical and physiological changes after surgery are inevitable and may affect the H. pylori eradication rate and histological findings. The effects on H. pylori eradication of bacterial overgrowth due to low acidity, impairment of absorption as a result of intestinal hurry, changes in blood flow and bile reflux caused by interruption of the pyloric sphincter are well-known[13-19]. However, some questions remain about the effects of H. pylori eradication therapy after gastric surgery.
First, it is uncertain whether differences in histological improvements between patients undergoing and patients not undergoing gastric surgery are associated with H. pylori eradication therapy. One study reported no significant changes in either glandular atrophy or IM scores after eradication in the remnant stomach[9], whereas another study showed significant improvements in glandular atrophy compared with normal mucosa[8]. Second, it is unknown whether the eradication rate differs between patients who have undergone and those who have not undergone gastric surgery and whether the eradication rate in patients after surgery decreases, as in the current study[20], because histological improvement was significantly higher in the eradicated group[8,9].
In this study, we evaluated histological changes, particularly glandular atrophy and IM, and the eradication rate of H. pylori infection in patients who underwent a subtotal gastrectomy for gastric cancer. Our data indicated that only the eradication success group had significantly improved glandular atrophy and IM scores 12 mo after treatment, suggesting the importance of H. pylori eradication rate. The eradication rate in patients who underwent Billroth II surgery was significantly higher than that of patients who underwent Billroth I surgery. The multivariate analysis revealed that Billroth II surgery was an independent factor predictive of eradication success in the eradication success group. The atrophy and IM scores were significantly lower than the baseline after eradication in the eradication success group. The atrophy and IM scores after eradication were significantly lower in the eradication success group than in the eradication failure group. Moreover, the atrophy and IM scores were significantly lower than the baseline after successful eradication in the Billroth II group. These results suggest that H. pylori eradication would result in histological improvement in patients who underwent surgery for gastric cancer, especially Billroth II surgery.
At least 50% of the Korean population has a H. pylori infection and its eradication rate with standard triple therapy is reported to be between 72.5% and 83.7%[21]. A 14 d bismuth-containing quadruple therapy as the second-line treatment resulted in an eradication rate of 82.6%-93.6% in Korea[22]. After surgery, the efficacy of eradication therapy varies from 70% to 90%[9,23]. In our results, the eradication rates of the seven day standard triple therapy as first-line therapy and the 10 d bismuth-containing quadruple therapy as second-line therapy were 78.4% and 90%, respectively, using ITT analysis and 85.3% and 92.3%, respectively, using PP analysis. Our data suggest a slightly higher result than previous studies. As histological improvement was seen only in the H. pylori negative group, the eradication rate is important[8,10]. In our results, there were also significant histological improvements in the eradication success group. Therefore, H. pylori eradication following subtotal gastrectomy might lead to histological improvement.
Billroth II surgery has been reported to result in a higher reflux rate than Billroth I surgery[24] and bile reflux plays a role in the eradication of H. pylori after subtotal gastrectomy. Rino et al[25] reported that the overall rate of H. pylori infection was 37.1%: 39.6% in Billroth I reconstruction, 0% in Billroth II reconstruction and 55.6% in pylorus-preserving gastrectomy. We hypothesized that the reconstructive surgery method would influence eradication and histological changes. In our study, atrophy and IM scores were significantly lower than the baseline after successful eradication in the Billroth II group. The atrophy and IM scores after successful eradication were also significantly lower in the Billroth II group than in the Billroth I group, suggesting that the reconstructive surgery method influences eradication and histological changes. Previous studies have shown that the H. pylori infection rate was significantly lower in Billroth II patients than Billroth I due to the role of bile reflux which interferes with H. pylori colonization. If H. pylori is still left after Billroth II surgery, the gastric carcinogenesis process is promoted because of the synergistic effect of bile reflux and H. pylori infection[24-26]. Therefore, H. pylori eradication should be strongly recommended following subtotal gastrectomy, especially in the Billroth II group.
Metachronous cancer developed in seven patients in this study and all of these were patients in which H. pylori eradication had been successful. The atrophy and IM scores were lower than the baseline after eradication but the differences were not statistically significant. However, the atrophy and IM scores in this group were higher than the mean for all the patients and increased after eradication. These results indirectly indicate that H. pylori eradication alone does not ensure prevention of metachronous cancer after surgery.
This study had several limitations. First, due to its retrospective nature at a single center, only two thirds of the patient population underwent histological examination after eradication and only 40 of the 72 patients who failed first-line treatment went on to second-line eradication. Second, the follow-up time to evaluate the changes in atrophy and IM was relatively short.
Although our study was limited by its retrospective nature, we enrolled a large number of patients and evaluated the eradication rate of second-line treatment and histological changes according to eradication and reconstructive surgery method. The patients with H. pylori infection who underwent subtotal gastrectomy for gastric cancer had a similar eradication rate when compared with the patients with an intact stomach. The eradication success group showed histological improvement in glandular atrophy and IM. After successful eradication, the Billroth II group showed a significant decrease in atrophy and IM scores over the Billroth I group. Therefore, H. pylori eradication is needed for these patients and more active treatment is required in the Billroth II group. Our study may support the recommendation that H. pylori should be treated even after gastrectomy for gastric cancer, especially after Billroth II reconstruction.
It is well established that Helicobacter pylori (H. pylori) infection is a strong risk factor for gastric cancer. Several guidelines recommend H. pylori eradication in patients after surgery for gastric cancer but its beneficial effects have not been established.
There is controversy as to whether H. pylori eradication results in histological improvement in patients following subtotal gastrectomy for gastric cancer.
This is the first study to investigate the eradication rate and histological changes after H. pylori eradication treatment in patients following subtotal gastrectomy for gastric cancer. The patients with H. pylori infection who had undergone a subtotal gastrectomy for gastric cancer had a similar eradication rate when compared with the patients with an intact stomach. H. pylori eradication in gastric cancer patients following a subtotal gastrectomy resulted in histological improvement, especially in the Billroth II group.
This study urges clinicians to confirm H. pylori infection and to start eradication therapy to prevent gastric cancer recurrence or metachronous cancer in patients following subtotal gastrectomy.
H. pylori is a bacterium found in the stomach. It is linked to the development of gastritis, peptic ulcers and stomach cancer. To prevent recurrence in patients following subtotal gastrectomy, it is necessary to eradicate H. pylori infections.
H. pylori eradication alone does not ensure prevention of metachronous cancer after surgery but after successful eradication, the Billroth II group showed a significant decrease in atrophy and IM scores compared with the Billroth I group. So, they should make H. pylori eradication an important factor in the treatment of patients following Billroth II reconstruction.
| 1. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2761] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1934] [Article Influence: 80.6] [Reference Citation Analysis (3)] |
| 3. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 952] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (7)] |
| 5. | Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Ohkusa T, Fujiki K, Takashimizu I, Kumagai J, Tanizawa T, Eishi Y, Yokoyama T, Watanabe M. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann Intern Med. 2001;134:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Onoda N, Katsuragi K, Sawada T, Maeda K, Mino A, Ohira M, Ishikawa T, Wakasa K, Hirakawa K. Efficacy of Helicobacter pylori eradication on the chronic mucosal inflammation of the remnant stomach after distal gastrectomy for early gastric cancer. J Exp Clin Cancer Res. 2005;24:515-521. [PubMed] |
| 9. | Matsukura N, Tajiri T, Kato S, Togashi A, Masuda G, Fujita I, Tokunaga A, Yamada N. Helicobacter pylori eradication therapy for the remnant stomach after gastrectomy. Gastric Cancer. 2003;6:100-107. [PubMed] |
| 10. | Cho SJ, Choi IJ, Kook MC, Yoon H, Park S, Kim CG, Lee JY, Lee JH, Ryu KW, Kim YW. Randomised clinical trial: the effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther. 2013;38:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1947] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 12. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3622] [Article Influence: 120.7] [Reference Citation Analysis (6)] |
| 13. | Greenlee HB, Vivit R, Paez J, Dietz A. Bacterial flora of the jejunum following peptic ulcer surgery. Arch Surg. 1971;102:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | ILLINGWORTH CF. Post-gastrectomy syndromes: a review. Gut. 1960;1:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992;72:445-465. [PubMed] |
| 17. | O’Connor HJ, Newbold KM, Alexander-Williams J, Thompson H, Drumm J, Donovan IA. Effect of Roux-en-Y biliary diversion on Campylobacter pylori. Gastroenterology. 1989;97:958-964. [PubMed] |
| 18. | Bechi P, Amorosi A, Mazzanti R, Romagnoli P, Tonelli L. Gastric histology and fasting bile reflux after partial gastrectomy. Gastroenterology. 1987;93:335-343. [PubMed] |
| 19. | O’Connor HJ, Dixon MF, Wyatt JI, Axon AT, Ward DC, Dewar EP, Johnston D. Effect of duodenal ulcer surgery and enterogastric reflux on Campylobacter pyloridis. Lancet. 1986;2:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 21. | Kim SY, Jung SW. [Helicobacter pylori eradication therapy in Korea]. Korean J Gastroenterol. 2011;58:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010;15:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Bertoli Neto JL, Lourenço LG, Bertoli CF, Ulbrich FS, Sabbi AR, Bueno AG. Evaluation of the efficacy of triple therapy regimen for Helicobacter pylori eradication in gastrectomized patients with gastric adenocarcinoma. Gastric Cancer. 2006;9:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Bair MJ, Wu MS, Chang WH, Shih SC, Wang TE, Chen CJ, Lin CC, Liu CY, Chen MJ. Spontaneous clearance of Helicobacter pylori colonization in patients with partial gastrectomy: correlates with operative procedures and duration after operation. J Formos Med Assoc. 2009;108:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Rino Y, Imada T, Shiozawa M, Takahashi M, Fukuzawa K, Hasuo K, Nagano A, Tanaka J, Hatori S, Amano T. Helicobacter pylori of the remnant stomach and its eradication. Hepatogastroenterology. 1999;46:2069-2073. [PubMed] |
| 26. | Abe H, Murakami K, Satoh S, Sato R, Kodama M, Arita T, Fujioka T. Influence of bile reflux and Helicobacter pylori infection on gastritis in the remnant gastric mucosa after distal gastrectomy. J Gastroenterol. 2005;40:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Da MX, Memon MA S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wang CH