Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3893
Peer-review started: August 24, 2014
First decision: September 27, 2014
Revised: October 25, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: April 7, 2015
Processing time: 226 Days and 6.9 Hours
AIM: To investigate the hepatoprotective effects and antioxidant activity of caffeic acid phenethyl ester (CAPE) in rats with liver fibrosis.
METHODS: A total of 75 male Sprague-Dawley rats were randomly assigned to seven experimental groups: a normal group (n = 10), a vehicle group (n = 10), a model group (n = 15), a vitamin E group (n = 10), and three CAPE groups (CAPE 3, 6 and 12 mg/kg, n = 10, respectively). Liver fibrosis was induced in rats by injecting CCl4 subcutaneously, feeding with high fat forage, and administering 30% alcohol orally for 10 wk. Concurrently, CAPE (3, 6 and 12 mg/kg) was intraperitoneally administered daily for 10 wk. After that, serum total bilirubin (TBil), aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured to assess hepatotoxicity. To investigate antioxidant activity of CAPE, malondialdehyde (MDA), glutathione (GSH) levels, catalase (CAT) and superoxide dismutase (SOD) activities in liver tissue were determined. Moreover, the effect of CAPE on α-smooth muscle actin (α-SMA), a characteristic hallmark of activated hepatic stellate cells (HSCs), and NF-E2-related factor 2 (Nrf2), a key transcription factor for antioxidant systems, was investigated by immunohistochemistry.
RESULTS: Compared to the model group, intraperitoneal administration of CAPE decreased TBil, ALT, and AST levels in liver fibrosis rats (P < 0.05), while serum TBil was decreased by CAPE in a dose-dependent manner. In addition, the liver hydroxyproline contents in both the 6 and 12 mg/kg CAPE groups were markedly lower than that in the model group (P < 0.05 and P < 0.001, respectively). CAPE markedly decreased MDA levels and, in turn, increased GSH levels, as well as CAT and SOD activities in liver fibrosis rats compared to the model group (P < 0.05). Moreover, CAPE effectively inhibited α-SMA expression while increasing Nrf2 expression compared to the model group (P < 0.01).
CONCLUSION: The protective effects of CAPE against liver fibrosis may be due to its ability to suppress the activation of HSCs by inhibiting oxidative stress.
Core tip: It has been demonstrated that caffeic acid phenethyl ester (CAPE) has several biological and pharmacological properties. The aim of this study was to evaluate the hepatoprotective effects and antioxidant activity of CAPE in rats with liver fibrosis, as well as the underlying mechanism.
- Citation: Li M, Wang XF, Shi JJ, Li YP, Yang N, Zhai S, Dang SS. Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World J Gastroenterol 2015; 21(13): 3893-3903
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3893.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3893
Caffeic acid phenethyl ester (CAPE), a flavonoid-like compound, is one of the major components of honeybee propolis. Honeybee propolis possesses a highly complex and variable chemical composition, and approximately 300 components have been identified[1,2]. CAPE has been widely accepted as a hepatoprotective folk medicine for many years. Its protective effects are attributed to its cytoprotective, anti-oxidant, anti-proliferative, and anti-inflammatory effects, and it has been shown to inhibit both lipoxygenase activity and lipid peroxidation[3-9]. CAPE can also inhibit phorbol ester-induced H2O2 production and tumor promotion[10,11], and it was reported that many CAPE activities were related to transcription factor inhibition of nuclear factor-kappa B (NF-κB)[10,12-14].
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix proteins, which is a characteristic of many chronic liver diseases[15]. Oxidative stress can cause excessive damage to hepatocytes through lipid peroxidation and protein alkylation. In addition, oxidative stress can liberate mediators and assist in the activation of hepatic stellate cells (HSCs), which are the predominant fibrogenic cell type producing collagen type I in the liver[16,17]. Therefore, oxidative stress has been recognized as a fundamental factor in the pathological process of liver fibrosis. NF-E2-related factor 2 (Nrf2) is a key transcription factor that regulates the induction of genes encoding antioxidant proteins and phase 2 detoxifying enzymes, which is involved in drug metabolism, detoxification and antioxidant defenses[18,19]. In nuclear protein extracts from normal and activated HSCs, Nrf1 and Nrf2 proteins were identified as part of the NAD(P)H quinine oxidoreductase 1 antioxidant response element (ARE) DNA/protein complex. Concomitant activation of the ARE defense and enhanced cell proliferation may confer protection against electrophile-mediated HSC toxicity during liver fibrosis[20]. All of these factors have led us to propose the following hypothesis: CAPE is more effective in inhibiting liver fibrosis and oxidative stress by activating Nrf2 expression compared to the effects of vitamin E (vit E), which is one of the well-known antioxidant agents.
CAPE was obtained from Yuancheng Technology Co. (Wuhan, China; purity 98%). Vit E was obtained from Sigma Chemical Co. (St. Louis, MO). Serum total bilirubin (TBil), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured with an auto chemistry analyzer (Hitachi Co., Japan). Antibodies against α-smooth muscle actin (α-SMA) and Nrf2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and hydroxyproline detection kits, CCl4, olive oil, and alcohol were also obtained from commercial sources.
Seventy-five male Sprague-Dawley rats (254.50 ± 16.22 g) were obtained from Xi’an Jiaotong University Medical College. The rats were reared in a controlled environment at 21 ± 2 °C and 50% ± 5% relatively humidity under a 12-h dark/light cycle and were acclimatized for at least 1 wk prior to use. The experimental procedures were performed according to the animal care guidelines of the National Institutes of Health and performed under the Xi’an Jiaotong University Guidelines for Animal Experimentation.
The rats were randomly assigned to seven experimental groups as follows: a normal group that was given water and a standard chow diet ad libitum (n = 10); a vehicle group that was treated by intraperitoneal injection of 10% alcohol (vehicle for CAPE) and subcutaneous injection of olive oil (vehicle for CCl4; n = 10); a model group (10% alcohol via the intraperitoneal route, once a day; n = 15); a vit E group (vit E dissolved in olive oil was administered intraperitoneally at 10 mg/kg, once daily; n = 10); and three CAPE groups (CAPE dissolved in 10% alcohol was administered intraperitoneally at 3, 6 or 12 mg/kg, once daily, respectively; n = 10). Except for the normal and vehicle groups, the other groups were injected with CCl4 dissolved in 40% v/v olive oil at 1 mg/kg body weight (2 mg/kg for the first time) twice a week via the subcutaneous route, fed rich-fat forage, and given alcohol (30%) orally for 10 wk.
At the conclusion of the experiment, all rats were fasted for 12 h before sacrifice. Liver, kidneys and spleen were removed and weighed, and blood was collected for serum isolation. Liver tissue samples (0.5 g) were homogenized (1:10, w/v) in ice-cold normal saline (NS). Homogenates were centrifuged at 5000 rpm for 10 min to remove debris. The supernatants, including the cytosolic fraction, were obtained and used for the analyses of select parameters. Protein concentrations of supernatants were measured by the Coomassie brilliant blue method[21].
Serum TBil, ALT and AST levels were measured to assess hepatotoxicity. Briefly, blood samples were kept overnight in a 4 °C refrigerator before centrifugation at 3000 g for 10 min. Afterwards, serum samples were stored in a -80 °C freezer before analysis. TBil, ALT and AST in serum were evaluated by an autoanalyzer (Hitachi-7170, Hitachi, Japan).
Liver samples were frozen at -20 °C before analysis. Hepatic collagen content was evaluated by hydroxyproline (Hyp) quantification according to the method described and validated by Edwards and O’Brien[22] with some modifications. Briefly, liver tissues (75 to 100 mg) were homogenized in 1 mL of phosphate-buffered saline and hydrolyzed in 6 mol/L HCl overnight at 120 °C. Next, 5 μL of hydrolysates was mixed with 5 μL of citrate acetate buffer, and then 100 μL of chloramine T solution was added. After 20 min of incubation at room temperature, 100 mL of Ehrlich’s solution was added. The mixture was incubated for 15 min at 65 °C, and absorbance was read at 550 nm. Recovery of known standard amounts was performed using similar liver samples to provide quantification. The hepatic hydroxyproline content is expressed as g/g wet liver weight.
Hepatic lipid peroxidation levels were determined by measuring MDA (a thiobarbituric acid reactive substance) levels[23]. Briefly, 10% hepatic homogenate samples were mixed with a thiobarbituric acid (TBA) reagent consisting of 0.375% TBA and 15% trichloroacetic acid in 0.25 mol/L hydrochloric acid. The reaction mixtures were placed in a boiling water bath for 40 min and then centrifuged at 4000 g for 5 min. Supernatant absorbance was measured at 535 nm. Results are expressed as nmol/mg protein. Hepatic GSH level was estimated by a colorimetric method using 5,5’-dithio bis-(2-nitrobenzoic acid) (DTNB)[24]. Samples were mixed with sodium phosphate buffer and DTNB. The mixture was incubated for 5 min at room temperature. Absorbance was measured at 405 nm. GSH content was determined from a standard curve generated with known concentrations of GSH. Results are expressed as μmol/mg protein.
CAT and SOD assays were performed according to the manufacturer’s instructions as previously described[25]. Briefly, CAT activity was defined as the enzyme concentration required to decompose 1 μmol H2O2 within 1 min. This reaction was initiated by addition of 1.0 mL of freshly prepared 20 mmol H2O2. The rate of H2O2 decomposition was measured spectrophotometrically at 240 nm for 1 min. To measure SOD activity, xanthine and xanthine oxidase were used to generate superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium chloride to form a red formazan dye. Absorbance was then measured at 550 nm. CAT and SOD activities are expressed as UI/mg protein.
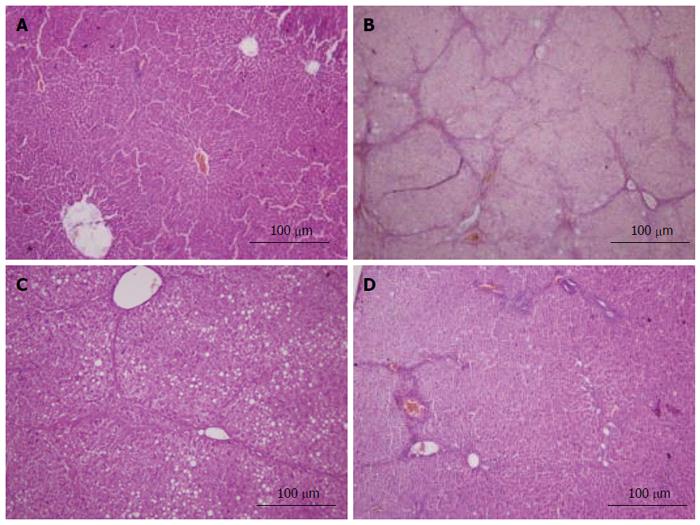
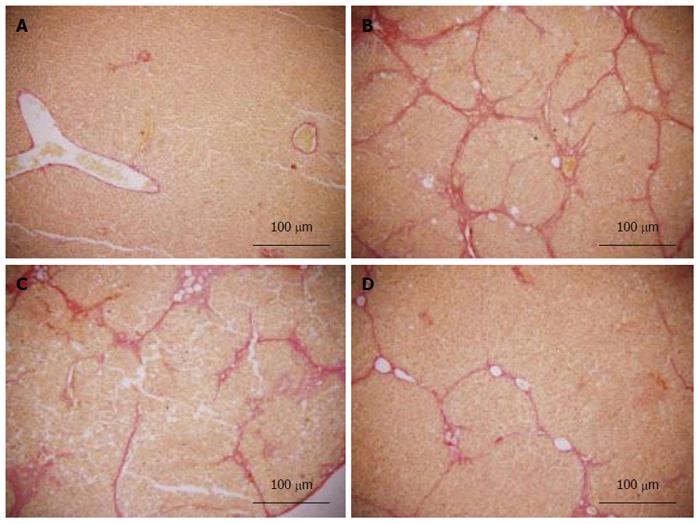
Fresh liver tissues that had been trimmed to a thickness of 3 mm were placed in plastic cassettes and immersed in neutral buffered formalin for 24 h. Fixed tissues were processed routinely, embedded in paraffin, sectioned, deparaffinized, and rehydrated using standard techniques. The extent of CCl4-induced necrosis was evaluated by assessing the morphological changes in liver sections stained with hematoxylin-eosin (HE) and van Gieson (VG). Fibrosis was scored semi-quantitatively as described by Zhang et al[26]. Accordingly, 0 was accepted as normal liver; 1, as thickened perivenular collagen and thin collagen septa; 2, as thin septa with incomplete bridging between portal regions; 3, as thin septa and extensive bridging; and 4, as thickened septa with complete bridging of portal regions and nodular appearance.
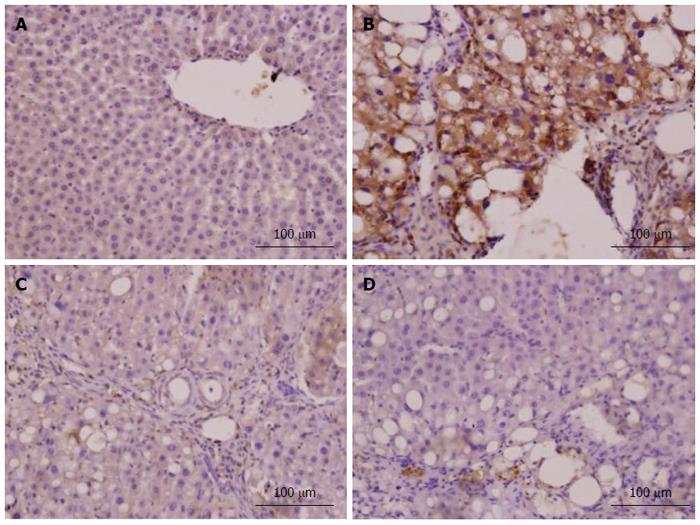
Immunohistochemistry for α-SMA was performed to identify HSC activation. Nrf2 expression in liver tissue was confirmed by immunohistochemistry, and the density of positive cells was measured with Image-Pro plus 6.0 software. Integrated optical density of positive staining of α-SMA and Nrf2 in each photograph was measured, and the average of all photographs in each group was calculated[27].
Immunohistochemical staining was performed on formalin-fixed paraffin-embedded liver sections (thickness, 5 mm). Following dehydration through xylene and a graded series of alcohol, the sections were washed with phosphate buffered saline (PBS; pH 7.4) and treated with 0.3% H2O2 for 20 min to block endogenous peroxidase activity. Tissue antigen retrieval was then accomplished by microwave heating for 2 min. After three washes with PBS, 10% goat non-immune serum was applied to liver sections for 30 min to prevent non-specific antibody binding. The sections were incubated with a primary mouse antibody (α-SMA or Nrf2, 1:100) in a humidified chamber at 37 °C for 3 h. A biotinylated secondary antibody and streptavidin peroxidase complex (ZSGB-BIO, Histostain-Plus Kit, Cat No: 85-9043) were consecutively applied at 37 °C for 15 min each, and three PBS washes were performed between applications. Antibody binding was visualized by color development using 3, 3 diaminobenzidine/H2O2. Finally, the sections were counter-stained with Mayer’s hematoxylin and rinsed with tap water, and the slides were mounted and observed under a light microscope.
The GraphPad.prism version 5.0 software package was used for statistical analysis. Results of measurement data are expressed as mean ± SD and tested by analysis of variance followed by the Tukey-Kramer test. Results of ranked data were statistically analyzed by the nonparametric Kruskal-Wallis H test followed by the Wilcoxon test for paired comparisons. Differences were considered significant if the P-value was less than 0.05.
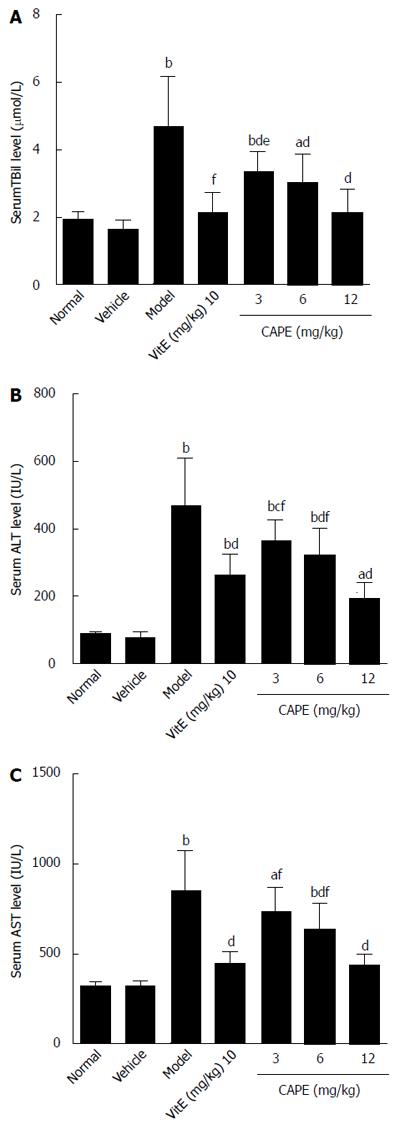
The biochemical results of analyses are presented in Figure 1. Serum TBil, ALT, and AST levels were significantly higher in the model group compared to the normal group (P < 0.01). Intraperitoneal administration of CAPE significantly reduced (P < 0.05) TBil, ALT, and AST levels in liver fibrosis rats when compared to the model group, and serum TBil was decreased by CAPE in a dose-dependent manner.
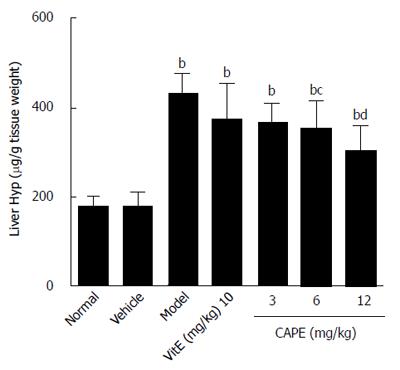
As demonstrated in Figure 2, liver hydroxyproline content in the model group was 1.5 times higher than that in the normal group, and the liver hydroxyproline contents in both the 6 and 12 mg/kg CAPE groups were significantly lower than that in the model group (P < 0.05 and P < 0.001, respectively).
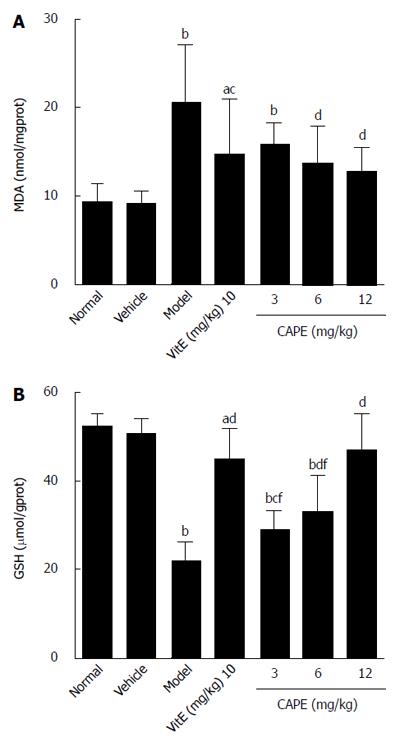
To evaluate the effect of CAPE on liver lipid peroxidation, MDA levels (an indicator of oxidative damage and one of the principal products of lipid peroxidation) were monitored. GSH is an important part of the non-enzymatic antioxidant system and is known to play an important role in liver injury; therefore, GSH levels were also determined in this study. The results of MDA and GSH are shown in Figure 3. In the CAPE (6 and 12 mg/kg) treated rats, the MDA levels were significantly lower, and the levels of GSH were significantly higher than those in the model group. Moreover, MDA and GSH concentrations in groups treated with 12 mg/kg CAPE were not significantly different from those in the normal group.
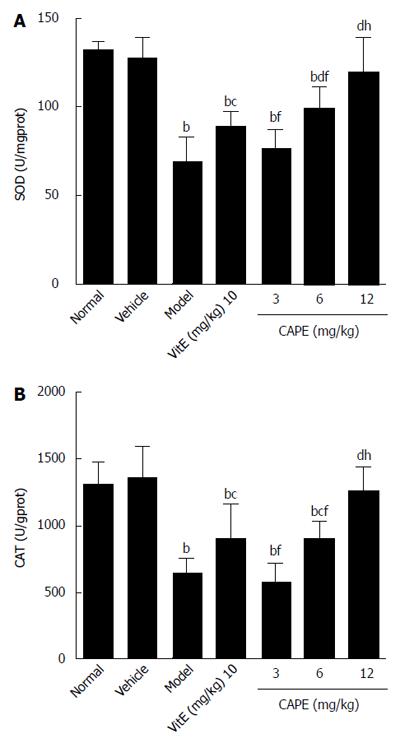
As shown in Figure 4, in a similar manner to GSH, CAT and SOD activities were significantly decreased in liver fibrosis rats induced with CCl4, alcohol and rich-fat forage. CCl4, alcohol and rich-fat forage administration significantly depleted antioxidant enzyme (CAT and SOD) activities, whereas CAPE (6 and 12 mg/kg) treatment significantly protected against the depletion of CAT and SOD, and the effects of treatment with 12 mg/kg CAPE were more obvious compared to 10 mg/kg vit E.
Histopathological changes in all of the groups are shown in Figures 5 and 6 (HE and VG staining). In microscopic examinations of the samples, liver sections in the normal and vehicle groups showed normal parenchymal cells, portal system, and blood sinusoids (no histopathological changes). Administration of CCl4, alcohol and rich-fat forage induced severe alterations in liver histopathology. The model group liver sections showed peri-portal apoptosis, lymphocytic infiltration, dilated central veins, thick bundles of proliferous collagenous fibers stained with eosin and typical pseudo-lobule. The liver did not show significant improvement in either the 3 or 6 mg/kg CAPE group, whereas the 12 mg/kg group had similar recovery as in the vit E positive control group. However, steatosis, edema and swelling of hepatocytes (cobble stone appearance), spotty necrosis and a small amount of collagenous fibers still could be observed in the CAPE (12 mg/kg) and Vit E groups. The degree of liver fibrosis in the groups is shown in Table 1.
| Group | 0 | 1 | 2 | 3 | 4 |
| Normal | 6 | 0 | 0 | 0 | 0 |
| Vehicle | 6 | 0 | 0 | 0 | 0 |
| Model | 0 | 0 | 0 | 3 | 3 |
| Vit E | 0 | 4 | 2 | 0 | 0 |
| CAPE (mg/kg) | |||||
| 3 | 0 | 0 | 2 | 3 | 1 |
| 6 | 0 | 1 | 3 | 2 | 0 |
| 12 | 0 | 5 | 1 | 0 | 0 |
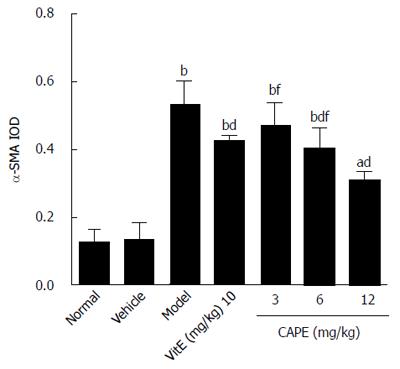
In the model group, immunohistochemistry localized the α-SMA protein predominantly to the fibrous septa, inflamed area and portal area, and we observed a significantly higher (P < 0.001) expression level than in the normal group (Figure 7). CAPE treatment could down-regulate the α-SMA expression in liver tissue. The expression level of α-SMA in the 12 mg/kg CAPE group was significantly lower than those in the model group and the vit E group (Figure 8).
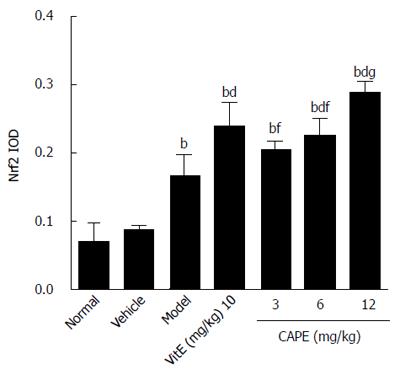
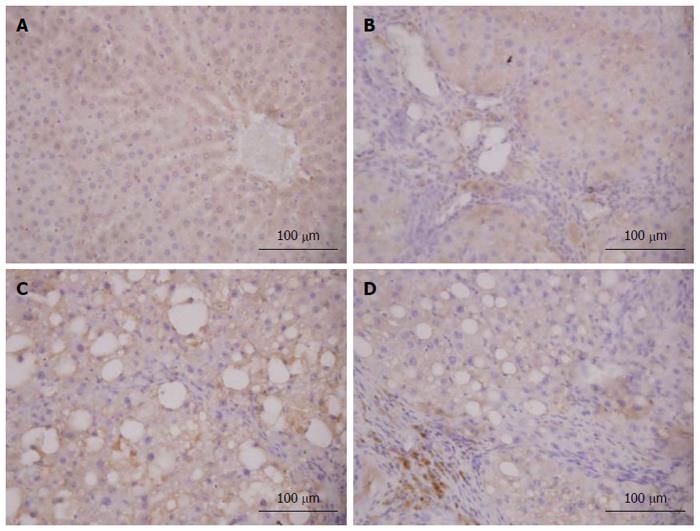
As shown in Figures 9 and 10, it was observed that Nrf2-positive cells detected by immunohistochemistry in liver tissue were increased in the model group compared to the normal group, and the expression levels of Nrf2 were increased in the 6 and 12 mg/kg CAPE groups compared with the model group. In addition, expression level of Nrf2 in the 12 mg/kg dose group was highest (P < 0.05) in all the groups.
CCl4 induced hepatotoxicity derives from the reductive dehalogenation of CCl4 that is catalyzed by CYP450 in the endoplasmic reticulum of liver cells to generate unstable trichloromethyl (CCl3+) and trichloromethyl peroxyl (CCl3O2-) radicals. Similarly, it was reported that alcohol-induced liver injury was associated with formation of the l-hydroxyl ethyl radical and lipid radicals[28]. Oxidative stress also plays an important role in high-fat forage induced liver injury. Free radicals attack and covalently bind to microsomal lipids and proteins, leading to lipid peroxidation and initiating secondary biochemical processes, which are the ultimate cause for unfolding the pathological consequences of CCl4.
In the current study, CCl4, alcohol and high-fat forage resulted in prominent hepatotoxicity as evidenced by the increases in serum TBil, ALT and AST levels and histopathology (HE and VG staining), and CAPE at all the three doses reduced TBil, ALT and AST levels in blood in a dose-dependent manner, indicating reduced hepatocellular damage. Histopathological observations also substantiated biochemical data of the study. CAPE treatment at 6 and 12 mg/kg resulted in the improvement in liver histoarchitecture and better recovery than that in the Vit E treated positive group.
Exogenous and endogenous protective agents with antioxidant properties demonstrated some protective effects against hepatotoxicity induced by various etiological factors. CAPE exhibits a number of beneficial effects against various types of degenerative diseases in humans, largely because the major components of CAPE have potent antioxidant activities[29,30]. GSH is a low molecular weight thiol antioxidant and a co-substrate for a variety of antioxidants and anti-xenobiotic enzymes of phase II pathway of the CYP450 cycle. It has been reported that the covalent binding of most toxicants to hepatic protein occurs only after depletion of GSH, and the severity of hepatic necrosis is related to the degree of covalent binding[31]. In addition, antioxidant enzymes such as CAT and SOD are easily inactivated by lipid peroxides or reactive oxygen species, which results in decreased activities of these enzymes in CCl4 toxicity. Thus, antioxidant enzymes also play an important role in the detoxification of xenobiotics, catalyzing their conjugation with reduced GSH[32]. Finally, it has been reported that antioxidants and flavonoids can act as inhibitors of LPO by scavenging peroxy radicals derived from polyunsaturated fatty acids and interrupt the chain reactions[33]. Therefore, LPO was monitored by measuring MDA - an end product of LPO in membrane components of cells[34].
In the present study, the model group showed significantly decreased GSH levels, CAT and SOD activities, and increased MDA levels compared with the normal group, indicating increased oxidative stress due to free-radical induced liver damage. CAPE treatment significantly reduced the elevated MDA levels and also increased tissue GSH levels, CAT and SOD activities. These results suggest that suppression of oxidative stress by CAPE in rats is an important aspect of the hepatoprotective effect of CAPE against hepatocytoxicity induced with CCl4, alcohol and rich-fat forage.
Transcription factor Nrf2 is activated in response to electrophiles and reactive oxygen species. During oxidative stress, Nrf2 is released from sequestration in the cytoplasm and translocates to the nucleus[35]. It has been demonstrated that Nrf2 is a key transcription factor for both the inducible and constitutive expression of phase II enzymes. Previous research has shown that Nrf2 knockout mice are more susceptible to acetaminophen-induced hepatocellular injury and that Nrf2 may also be important in protecting against liver fibrosis[36]. In liver fibrosis, HSCs serve as the major source of extracellular matrices in liver fibrosis. During the activation process, HSCs undergo phenotypic changes in cellular morphology to a myofibroblast-like cell type with expression of α-SMA[12]. In the current study, immunohistochemistry results showed that treatment with CAPE at 6 mg/kg and 12 mg/kg significantly activated Nrf2, and inhibited α-SMA. These data indicate that CAPE may be able to protect liver cells from oxidative stress induced damage and inhibit activation of HSCs through up-regulated Nrf2 expression.
It should be considered that the protective effect of CAPE against hepatotoxicity might not be solely dependent on the antioxidant effect of CAPE, which may exert its hepatoprotective effect by other means. For example, it was shown that CCl4 induces liver cell apoptosis by cytochrome C release and caspase 3 activation, and causes hepatoxicity by increasing mRNA expression of NF-κB dependent cyclooxygenase-2 and iNOS. Alternatively, CAPE shows an anti-inflammatory action as lipoxygenase and cyclooxygenase inhibitor[37,38]. Furthermore, CAPE is a potent mitochondrial protective agent that blocks Ca2+-induced cytochrome release and is a potent suppressor of pro-inflammatory cytokines by reducing caspase 1 activation and depressing NF-κB[39].
In conclusion, CAPE exhibits significant partial protection against hepatotoxicity as evidenced from the reversal of various biochemical indices and histopathology, which were altered due to CCl4, alcohol and high-fat forage induced toxicity. The protective effects against liver damage could be due to the antioxidant effect of CAPE. However, the protective effect of CAPE may not always be associated with its antioxidant effects. Owing to the hepatoprotective potential, CAPE has clinical importance and could be used to develop a safe hepatoprotective medicine.
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix proteins. Oxidative stress has been recognized as a fundamental factor in the pathological processes of liver fibrosis. Caffeic acid phenethyl ester (CAPE) has been widely accepted as a hepatoprotective folk medicine for many years. However, the hepatoprotective effects of CAPE on liver fibrosis and the underlying mechanism are unclear.
CAPE is one of the major components of honeybee propolis and has several biological and pharmacological properties, including cytoprotective, anti-oxidant, anti-proliferative, and anti-inflammatory effects. Moreover, it was reported that many CAPE activities were related to transcription factor inhibition of nuclear factor-kappa B.
Oxidative stress can cause excessive damage to hepatocytes through lipid peroxidation and protein alkylation. NF-E2-related factor 2 (Nrf2) is a key transcription factor that regulates the induction of genes encoding antioxidant proteins and phase 2 detoxifying enzymes, which is involved in drug metabolism, detoxification and antioxidant defenses. CAPE may inhibit liver fibrosis and oxidative stress by activating Nrf2 expression.
These findings show that the protective effects of CAPE against CCl4, alcohol and high-fat forage induced liver fibrosis may be due to its ability to suppress activation of HSCs by inhibiting oxidative stress.
CAPE is a flavonoid-like compound and is one of the major components of honeybee propolis.
This is an interesting study about CAPE in liver fibrosis. CAPE is a flavonoid-like compound and is one of the major components of honeybee propolis. In this study, the authors evaluated the hepatoprotective effects and antioxidant activity of CAPE in rats with liver fibrosis. The study design is good, and the results are interesting.
| 1. | Türkez H, Yousef MI, Geyikoglu F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem Toxicol. 2010;48:2741-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Simões LM, Gregório LE, Da Silva Filho AA, de Souza ML, Azzolini AE, Bastos JK, Lucisano-Valim YM. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J Ethnopharmacol. 2004;94:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Nakamura T, Ohta Y, Ohashi K, Ikeno K, Watanabe R, Tokunaga K, Harada N. Protective effect of Brazilian propolis against hepatic oxidative damage in rats with water-immersion restraint stress. Phytother Res. 2012;26:1482-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kus I, Colakoglu N, Pekmez H, Seckin D, Ogeturk M, Sarsilmaz M. Protective effects of caffeic acid phenethyl ester (CAPE) on carbon tetrachloride-induced hepatotoxicity in rats. Acta Histochem. 2004;106:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Pekmez H, Kus I, Colakoglu N, Ogeturk M, Ozyurt H, Turkoglu AO, Sarsilmaz M. The protective effects of caffeic acid phenethyl ester (CAPE) against liver damage induced by cigarette smoke inhalation in rats. Cell Biochem Funct. 2007;25:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Coban S, Yildiz F, Terzi A, Al B, Ozgor D, Ara C, Polat A, Esrefoglu M. The effect of caffeic acid phenethyl ester (CAPE) against cholestatic liver injury in rats. J Surg Res. 2010;159:674-679. [PubMed] |
| 7. | Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347-2352. [PubMed] |
| 8. | Wu WM, Lu L, Long Y, Wang T, Liu L, Chen Q, Wang R. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: A structure-activity insight. Food Chemistry. 2007;105:107-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Macías-Pérez JR, Beltrán-Ramírez O, Vásquez-Garzón VR, Salcido-Neyoy ME, Martínez-Soriano PA, Ruiz-Sánchez MB, Angeles E, Villa-Treviño S. The effect of caffeic acid phenethyl ester analogues in a modified resistant hepatocyte model. Anticancer Drugs. 2013;24:394-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro D, Savage J. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer. 2009;125:565-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hess A, Wijayanti N, Neuschäfer-Rube AP, Katz N, Kietzmann T, Immenschuh S. Phorbol ester-dependent activation of peroxiredoxin I gene expression via a protein kinase C, Ras, p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2003;278:45419-45434. [PubMed] |
| 12. | Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090-9095. [PubMed] |
| 13. | Ha J, Choi HS, Lee Y, Lee ZH, Kim HH. Caffeic acid phenethyl ester inhibits osteoclastogenesis by suppressing NF kappaB and downregulating NFATc1 and c-Fos. Int Immunopharmacol. 2009;9:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Bezerra RM, Veiga LF, Caetano AC, Rosalen PL, Amaral ME, Palanch AC, de Alencar SM. Caffeic acid phenethyl ester reduces the activation of the nuclear factor κB pathway by high-fat diet-induced obesity in mice. Metabolism. 2012;61:1606-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol. 2011;251:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1366] [Cited by in RCA: 1317] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 19. | Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 900] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 20. | Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol. 2007;35:459-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2217] [Cited by in RCA: 2133] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 22. | Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 383] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3306] [Cited by in RCA: 3536] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 24. | Lee KJ, Jeong HG. Protective effect of Platycodi radix on carbon tetrachloride-induced hepatotoxicity. Food Chem Toxicol. 2002;40:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Lee KJ, Panzera A, Rogawski D, Greene LE, Eisenberg E. Cellular prion protein (PrPC) protects neuronal cells from the effect of huntingtin aggregation. J Cell Sci. 2007;120:2663-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Wang H, Yu H. Thioacetamide-induced cirrhosis in selenium-adequate mice displays rapid and persistent abnormity of hepatic selenoenzymes which are mute to selenium supplementation. Toxicol Appl Pharmacol. 2007;224:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 465] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 28. | Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 432] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 29. | Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73 Suppl 1:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Bai H, Liu R, Chen HL, Zhang W, Wang X, Zhang XD, Li WL, Hai CX. Enhanced antioxidant effect of caffeic acid phenethyl ester and Trolox in combination against radiation induced-oxidative stress. Chem Biol Interact. 2014;207:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195-202. [PubMed] |
| 32. | Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Pascual C, Gonzalez R, Torricella RG. Scavenging action of propolis extract against oxygen radicals. J Ethnopharmacol. 1994;41:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Hünkar T, Aktan F, Ceylan A, Karasu C. Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin-diabetic rats. Cell Biochem Funct. 2002;20:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 715] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 36. | Xu W, Hellerbrand C, Köhler UA, Bugnon P, Kan YW, Werner S, Beyer TA. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88:1068-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, Ialenti A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73 Suppl 1:S53-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Celik S, Erdogan S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol Cell Biochem. 2008;312:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Orbell JH, Shaun C S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S