INTRODUCTION
Colorectal cancer is the third most common cancer worldwide, affecting 1.36 million people[1] and is the largest killer amongst non-smokers in the United Kingdom[2]. The greatest chance of cure is with disease confined to the bowel wall, hence, early diagnosis and prompt treatment are important[3]. It is generally accepted that cancers develop through accumulation of mutations in key genes[4,5]. Traditionally, a three step process comprising initiation, promotion and progression was proposed[6,7]. Later, it became apparent that the colonic epithelium undergoes an ordered sequence of genetic events with corresponding histological abnormalities on its journey to cancer formation[8]. However, several recent analyses have revealed that the mutations found in colorectal cancer occur long before the onset of a clinically visible lesion[9,10]. In many cancers, cells have been shown to acquire pro tumorigenic mutations that are not able to produce morphological change but predispose to subsequent malignant transformation[11-14]. These cells can expand creating patches of mucosa which have an increased risk of developing into cancer. This process has been described as “field cancerisation”[15,16]. It is a concept that has previously received little attention in the scientific literature. Most studies investigating colorectal carcinogenesis have focused solely on the cancer tissue or assumed that the mucosa adjacent to the neoplastic lesion is normal[17,18]. However, based upon the field cancerisation theory, characterization of the biological events that occur in the “mucosa at risk” could enable identification of the earliest steps in colorectal cancer formation. This could aid the scientist in discovery of how neoplasia develops and enable the clinician to develop more reliable tests to risk stratify patients. This article discusses the evidence for field cancerisation, its limitations and its potential clinical application to improve patient outcome.
FIELD CANCERISATION THEORY - DEFINITION AND MECHANISM
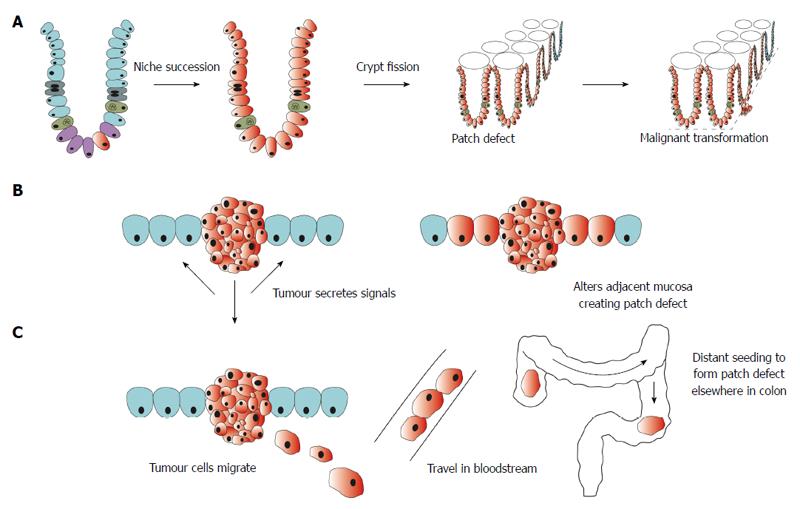
Field cancerisation was first described by Slaughter in 1953 for head and neck squamous cell carcinoma[15]. It was based upon the observation that a statistically significant proportion of oral cancers developed in multifocal areas and often had histologically abnormal cells surrounding the cancer. Since its inception, field cancerisation has been applied to several other cancers including cancer of the oesophagus[19], stomach[20], lung[21], bladder[22], pancreas[23] and skin[24]. In the colon, it has been described as “the process whereby colonic epithelial cells acquire pro-tumorigenic mutations that are insufficient to cause morphological change but which predispose to tumour”[25]. Multiple mechanisms have been proposed to explain how a patch of altered mucosa forms around a cancer (Figure 1). Genetic analysis has revealed that the field defect consists of a clonal proliferation of a mutated cell. Based on this observation, it was proposed that a mutation or epigenetic alteration in stem cells gives it reproductive advantage so that it generates clonal descendents that outcompete neighbouring stem cells[26]. These stem cells replace other stem cells through the process of niche succession[27] and eventually, the entire crypt is occupied by the mutant or epigenetically altered cells[28,29]. Crypt fission of this mutated or epigenetically altered crypt results in a patch defect (Figure 1A). Other mechanisms include alteration in the adjacent mucosa by the presence of the tumour itself or as a result of chemicals released by the tumour[30,31] (Figure 1B). Whilst others have proposed that, like oral cancer[32,33] and bladder cancer, colonic epithelial cells shed in the lumen at one place could migrate to another site, seed and give rise to synchronous cancer[16] (Figure 1C). Field changes could be more widespread which has led some authors to suggest that dietary exposure, for example, vitamin B and folate, could alter the methylation state of the entire colonic mucosa predisposing it to cancer[25]. Further investigation is required to elucidate the precise biological mechanism and causal events that underly field cancerisation. Despite this, however, a large body of evidence exists that supports the presence of a field defect in colorectal cancer.
Figure 1 Schematic representation of proposed mechanisms for formation of field defect.
A: A mutation or epigenetic alteration in a stem cell (depicted in red) is inherited by all cells within the crypt through niche succession. Crypt fission results in several crypts becoming biologically altered creating a patch defect. Further mutation within this field of altered mucosa leads to malignant transformation; B: Tumour secretes chemical signals that alter the adjacent mucosa resulting in a field defect; C: Malignant cells shed from a tumour travel in the bloodstream and seed in a distant site rendering the mucosa susceptible to malignant transformation.
EARLY EVIDENCE BASED ON THE TRANSITIONAL MUCOSA
The term “transitional mucosa” was used to describe the patch of mucosa around a cancer that was abnormal compared to the rest of the mucosa. Although field cancerisation had not been formally proposed at the time, these were some of the early studies supporting the concept.
In 1969, Filipe described abnormal histochemical properties in the transitional mucosa[34] with a decrease in sulfomucins, usually found in normal colorectal tissue and concurrent increase in sialomucin content. Sulfomucins protect against luminal insults by increasing mucus viscosity which increases the resistance against bacterial degradation and microbe adhesion[35]. Replacement by sialomucin has been previously observed in colorectal cancer tissue. Based on the finding of increased sialomucin content in the transitional mucosa, Filipe proposed that these changes could represent an early stage of carcinogenesis[36]. Further exploration using light microscopy revealed that there were alterations in crypt morphology and cell type within this mucosa. Saffos and Rhatigan[37] found an increase in the length of the crypts, increased distension and branching of the crypts and an increase in the number of goblet cells in the crypt. They were unable to demonstrate similar changes in tissue samples taken along the rest of the colon in these patients. They concluded that these changes were confined to the rim around the tumour.
In the late 1970’s, several investigators characterised the ultrastructural properties of the transitional mucosa[38,39]. They found that the crypts were larger in diameter and composed of larger cells with larger nuclei compared to those found in the normal colon. There was also a change in cell distribution with an increase in mature goblet cells in the lower half of the crypt and an increase in immature goblet cells and “intermediate” cells in the upper half of the crypt.
Subsequent studies have highlighted alterations in the nuclear morphology of cells in the transitional mucosa, often as far as 50 mm from the tumour[40,41]. Many nuclear features were found to differ in the transitional mucosa compared to that of healthy controls including total optical density, nuclear area, chromatin texture, chromatin coarseness, average optical density and increased tendency of peripherally placed chromatin[42]. However, because of the considerable inter-patient and inter gland variation in these parameters, the authors cautioned against the use of any single feature to identify those at risk. Instead, it was suggested that a combination of parameters be used to develop a tool for risk stratifying patients. More recent studies, using computer based karyometric analysis[43] or electron microscopy[44] have confirmed these earlier findings. Although there are differences in nuclear appearance of cells in the transitional mucosa, the variability seen between patients and between samples taken from the same patient preclude the use of nuclear analysis as a discriminant factor to risk stratify patients. Investigators have therefore sought to identify other biological changes that could be indicative of a field defect.
SUPPORTING EVIDENCE BASED ON CANCER BIOLOGY
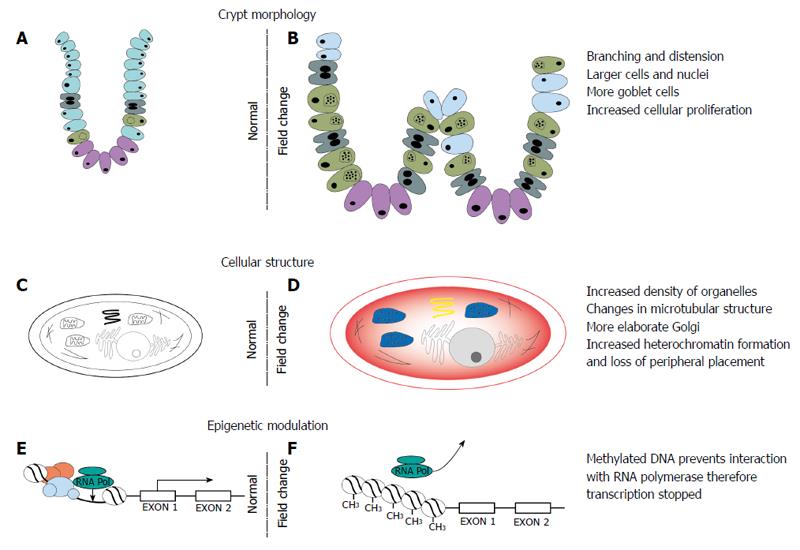
Colorectal cancer usually arises from multiple dysfunctional cellular processes which enable the cell to evade homeostatic signals and grow in an autonomous manner. Similarly, alterations at the genetic, epigenetic and protein level in a number of cellular processes and function have been described in the colonic field (Figure 2).
Figure 2 Changes in crypt morphology, cellular ultrastructure and epigenetic modulation in the field defect.
A, B: Changes in crypt morphology characterised by increased branching and distension of crypts, increased cell division and a change in proportion of cells with increase in goblet cells; C, D: Changes in the cell cytoskeleton, organelles and nuclear composition; E, F: Epigenetic modulation of DNA leading to transcriptional silencing of certain genes involved in regulation of cell division, apoptosis and DNA repair.
Cellular proliferation and apoptosis
Many studies have demonstrated that there is an increase in cellular proliferation and concurrent reduction in apoptosis in the macroscopically normal appearing mucosa around a malignant lesion[45-48]. Using 3H thymidine autoradiography, the rate of proliferation in normal mucosa was found to be significantly higher in patients harbouring a colorectal cancer or large adenoma compared to those with small adenomas or healthy individuals[45]. These changes were most prominent in the upper third of the mucosal crypt[47,48] and could potentially be utilised as a predictive marker to identify patients with a neoplastic lesion[49,50]. Not only have these changes in proliferation been linked to the presence of neoplastic lesions but they have also been shown to be predictive for the risk of polyp recurrence[49]. Epithelial cell proliferation in the macroscopically normal rectal mucosa of patients with and without polyps was assessed. The crypt was divided into five compartments along the longitudinal axis and the labelling index was calculated for the entire crypt and each of the five compartments. In the 22 patients whose polyps recurred, the upper compartments 3, 4 and 5 demonstrated a significantly higher labelling index compared to the 33 patients without recurrence. There was an upward shift in the proliferative zone of the crypt that was associated with polyp recurrence. Interestingly, there was no difference in the labelling index between the first and second biopsy suggesting that an underlying genetic defect or persisting environmental insult may have been responsible for the field defect detected in this study.
Genetic and epigenetic modulation
The genetic and epigenetic abnormalities found in colorectal cancer have also been shown to extend into the macroscopically normal mucosa supporting the field cancerisation theory. Early studies using flow cytometry confirmed that mucosa adjacent to diploid cancers is diploid in nature and in patients with aneuploid tumours, is often aneuploid[51,52]. Similarly, epigenetic modulation of genes has been found to differ in patients with classical adenomas compared to those with serrated polyps[53]. These lesions represent the two different pathways to colorectal cancer. Classical adenomas are linked to carcinogenesis that occurs along the CIN (chromosomal instability) pathway compared to serrated polyps which are the precursor for the CpG island methylator phenotype (CIMP) pathway. The authors demonstrated that age-related methylation was inversely associated with the presence of classical adenomas compared to methylation of cancer specific genes that was more likely in patients with serrated polyps. This suggests that the background mucosa of these patients had detectable epigenetic differences that conferred a predisposition to a specific pathway of cancer prior to the development of any discernible histological abnormality.
Differences in expression of genes across a wide number of cellular processes have been implicated in the field defect and may potentially play a role in early tumourigenesis. Based on a 15-gene signature encompassing genes that play a role in the APC/Beta catenin, NFκB, cell cycle and inflammation pathways, significant alterations in gene expression were found in the normal mucosa of human cancer resection specimens, often extending into the margins[54]. In a further study, based on analysis of a macroscopically normal appearing rectosigmoid biopsy, this 15-gene signature could discriminate between individuals with and without polyps[55].
Epigenetic silencing of genes has been implicated in both mismatch repair deficient and CIMP cancer. Several investigators have reported epigenetic changes in the normal colonic mucosa in patients with cancer. Reduced protein expression of the DNA repair proteins, mismatch repair endonuclease (Pms2), DNA excision repair protein (ERCC1) and DNA excision repair protein XPF (ERCC4) was found up to 10 cm longitudinally from the tumour edge supporting the field theory[56]. In a study by Shen et al[57], O-6-methylguanine-DNA methyltransferase (MGMT) methylation was found in the normal adjacent mucosa of 50% patients whose tumours also had methylated MGMT compared to only 6% when MGMT was not methylated in the tumour. In 10 out of 13 patients, methylation changes were seen as far as 10 cm away from the tumour and hypermethylation was more pronounced at 1 cm compared to 10 cm. These findings raise the possibility that MGMT methylation may play a role in the field defect representing an early step in the carcinogenesis pathway of tumours with hypermethylated MGMT. Similarly, others have also reported that MGMT hypermethylation is more likely to be found in the surrounding mucosa of microsatellite unstable tumours compared to microsatellite stable cancers[58,59]. Other studies have investigated the methylation profile of combinations of multiple genes confirming that the apparently normal mucosal field has undergone significant epigenetic change that could represent the earliest stages of colorectal cancer development[60,61].
Epigenetic modulation through methylation of micro-RNAs (miR) may also contribute to a field defect. Grady et al[62], found expression of hsa-miR-342, a microRNA encoded in an intron of the gene EVL, is commonly suppressed in human colorectal cancer. They found methylation at the EVL/has-miR-342 locus in 56% of histologically normal mucosa from patients with colorectal cancer compared to only 12% of patients without colorectal cancer. Similarly, methylation of miR-124a and miR-34b/c in the histologically normal mucosa, was observed in 59% and 26% of patients with cancer but was not found in patients without cancer or Ulcerative Colitis[63]. In another study, the level of methylation of miR-137 was found to be higher in the macroscopically normal mucosa in cancer patients compared to healthy controls[64] (10.3% vs 7.7%, P = 0.035). These findings suggest that changes at the micro-RNA level could also play an important role in field defect around a tumour.
Although, there are considerable genetic and epigenetic alterations in the “normal” mucosa surrounding a cancer, it is not yet clear which of these changes are most important. Epigenetic changes are particularly interesting as they can be modified by changes in diet or pharmacological agents unlike the germline mutations often linked with cancer. Elucidation of the specific epigenetic marker that underlies the field defect could enable specific chemopreventative agents to be designed to target these early changes prior to the development of any precancerous lesions such as adenomas.
FIELD CANCERISATION - POTENTIAL PITFALLS
Although there is sufficient evidence to support the field cancerisation theory in colorectal cancer, a number of pertinent questions remain.
Pre malignant change or a secondary phenomenon?
Similar changes in crypt and cellular morphology to those observed in the transitional mucosa have also been described in mucosa adjacent to squamous cell carcinoma of the anus[65,66], sarcoma of the colon[66,67] and in non-neoplastic lesions such as endometriosis[67]. This has led to the conclusion that these alterations do not represent premalignant change but rather, a reactive phenomenon in response to tumour or non-neoplastic injury such as that induced by inflammation or necrosis. Early studies showed that the width of the transitional mucosa was related to the size of the tumour[36] where it became larger with increasing stage of the tumour. However, if these changes precede cancer formation, it would be expected that as a tumour grows, it replaces the transitional mucosa from which it arose resulting in a smaller area containing the initial cellular changes. Therefore, one would expect that the area of the transitional mucosa would be inversely related to the size of the tumour and the changes seen in the transitional mucosa would be demonstrated throughout the entire colonic mucosa. Investigators were unable to provide evidence to support this hypothesis which led to the proposal that the transitional mucosa represents the neoplastic phenotype, however, is likely to be a secondary phenomenon as a result of factors released by the tumour. This may also explain how it was found adjacent to non-neoplastic lesions which would be expected to release similar growth factors, in response to the inflammatory process, to those secreted by the tumour. However, subsequent studies have successfully correlated genetic mutations found in the tumour with those demonstrated in the surrounding mucosa confirming that these tumour cells share a common clonal origin. Also, the reports of these changes persisting despite removal of the offending lesion[49,68] suggest that this is a primary phenomenon rather than reactive change in response to the presence of a neoplastic lesion. Hence, these field changes are most likely to be pre-malignant events that represent some of the very early steps along the path to colorectal cancer.
How far along the colon does a field defect extend?
If the macroscopically normal mucosa is biologically altered in response to the tumour, it would be limited to the area immediately in the vicinity of the tumour. In an early study investigating the field defect based on histochemical analysis, transitional mucosa was found in 90/100 cases, extending as far as 17 cm from the tumour[69]. The change in sialomucin content that was identified in the transitional mucosa was found at the resection margins and in a subset of patients, it was a direct extension of the zone of altered mucosa surrounding the tumour. Several other studies have described biological changes in mucosa as far as 10 cm from the tumour[43,70,71] whilst others have reported that the field defect extends as far as the rectum in these patients[49,72]. Some authors have shown that the hypermethylation changes observed in the field defect are more pronounced 1 cm away from the tumour compared to 10 cm[57] whereas others were unable to corroborate their findings with distance from the lesion[54] Some investigators have proposed that the field of altered mucosa does not occur in a contiguous manner but occurs in discrete patches. Bernstein et al[46] measured the bile salt induced apoptosis rate in 68 patients (17 colorectal cancer, 37 adenoma and 14 with neoplasia). Biopsies were taken 20 cm from the anal verge, caecum and descending colon. Site to site variability, both between regions of the colon and adjacent biopsies was greater than the inter-patient variability for individuals with a history of colorectal cancer suggesting that there was ‘‘patchiness’’ of the susceptibility of regions of the colon to bile acid induced apoptosis. In other words, the field defect was not continuous along the entire colon; there were areas which showed greater changes in rate of apoptosis, however, these areas did not correspond to site of previous neoplasia. If these changes occur as a consequence of the interplay between an underlying genetic predisposition and environmental insult, patchy mucosal alterations could be explained by differences in luminal factors along the colon. Hence, there would be areas that are more susceptible to carcinogens found in the lumen or areas where cells are defective at protecting against the harmful effects of carcinogens. Further study is required to characterise the nature of the field defect and examine the causative agents responsible.
Are these alterations passengers or drivers in carcinogenesis?
Colon cancers have been found to contain a median of 76 non-silent sequence mutations of which, only 15 represent driver mutations[73]. These are mutations in key oncogenes or tumour suppressor genes that confer a selective advantage to the cell enabling it to divide uncontrollably and survive in unfavourable conditions. In comparison, passenger mutations occur during normal cell division that takes place to replenish the colonic epithelium and have no role in driving carcinogenesis. They can be over-represented in cancer tissue due to aberrant DNA repair mechanisms and defective anti-apoptotic machinery. Similarly, it is difficult to discriminate which of the molecular changes found in the field defect are integral in driving cancer formation from those that are innocent bystanders. Roy et al[74], used 4-dimensional elastic light scattering fingerprinting (4D-ELF) to probe the nanoarchitecture of colonocytes in the Azoxymethane treated rat model vs the saline treated rat. They measured 4D-ELF at different time points and correlated the changes observed with the emergence of the aberrant crypt focus. Their finding that changes in 4D-ELF were apparent 2 wk prior to development of aberrant crypt foci (ACF) and that they correlated both spatially and temporally with subsequent development of ACF suggests that these changes were integral in early colorectal cancer formation.
Mathematical modelling suggests that it is not the rate of mutations which is important but rather the selection of clones of cells with specific advantages in autonomous growth that drives malignant transformation[75]. It has also become apparent that this selective advantage is not conferred by mutations in one or few genes but is the accumulated benefit of several genes that have low individual selective advantage[76]. Therefore, it is crucial that mechanistic studies are conducted based upon the gene targets found in the mucosal field to discern the driver mutations from those that are innocent bystanders.
CLINICAL APPLICATION
Despite some of these shortcomings, field cancerisation in colorectal cancer is a promising prospect upon which to develop potentially diagnostic and therapeutic modalities. Elucidation of the underlying molecular mechanism could enable more accurate screening tests to be designed that are able to identify individuals with a malignant lesion. Current research is focused on developing tools that are capable of identifying patients with colorectal cancer based on analysis of a “normal” biopsy from a distant site. Using light scattering technology, three manifestations of tissue alteration in the colonic field have been shown[77]: changes in microcirculation [early increase in blood supply (EIBS)], changes in the extracellular matrix from abnormal cross linking and alignment of collagen fibres [as assessed by low coherence backscattering (LEBS)] and differences in the internal structure of colonocytes [as assessed using partial wave spectroscopy (PWS)]. EIBS can be detected within 30 cm of a polyp using a spectroscopic probe on 222 patients undergoing colonoscopy. The magnitude of EIBS correlated with the size and proximity of the adenoma. Based on a rectal biopsy, EIBS was found to be increased in 50% patients with an adenoma. A logistic regression model using EIBS, mucosal oxyhaemoglobin and patient age gave a sensitivity of 83% and specificity of 82% with an AUC of 0.88 for the detection of advanced adenomas[72]. A progressive change from control patients to those with advanced adenomas was demonstrated using LEBS parameters[78]. LEBS was able to discriminate between patients with and without advanced adenomas with 100% sensitivity, 80% specificity and an AUC of 0.90. An in vivo study was subsequently performed where a fibre optic probe was used to measure LEBS parameters in the rectum of 574 subjects[79] and was shown to reliably identify patients with an advanced adenoma. Similarly, PWS has been shown to correlate with risk of developing colorectal cancer[80]. The differences in EIBS, LEBS and PWS parameters detected in these studies were not confounded by demographics, presence of non-neoplastic lesions or site of adenoma suggesting true potential for development into a screening tool.
The presence of a field defect may indicate a higher risk of metachronous neoplastic lesions and could help to identify which patients require more radical surgery. Field cancerisation could also be utilized to ascertain risk of disease progression, hence, could enable risk stratification of patients with inflammatory bowel disease or a family history of colorectal cancer. However, the most exciting use of field cancerisation theory is its potential application in chemoprevention. Individuals at risk of malignancy could be identified based on field defects in their mucosa. Pharmacological therapy could be developed, targeted at the underlying signaling pathway, to modify the field change and reduce the risk of subsequent malignant transformation.
FUTURE DIRECTIONS
There is considerable evidence in the literature to support the field cancerisation theory in colorectal cancer. However, important questions about the underlying mechanism and extent of the field defect require further investigation before it can be applied in a clinical setting.
In other conditions that results in an increased risk of colorectal cancer such as Ulcerative Colitis, mutations in KRAS, CDNK2A (p16) and TP53 have been detected in non-tumour, non-dysplastic and dysplastic epithelium. In two patients, these changes were detected 4 years before the development of tumour suggesting that they represent some of the very early genetic events that led to colorectal carcinogenesis[81].
Furthermore, a recent study using a mouse colitis model showed persisting epigenetic alteration in the mucosa despite removal of the toxic insult that initiated it[68]. Lessons learnt from these studies could shed light upon the interactions that take place between the environment and the mucosa in the journey along the cancer pathway.
Cancer research has traditionally focused on characterization of the genetic/epigenetic events that occur in a malignant cell to understand the processes that contribute to malignancy. This approach seems somewhat backwards, especially in a disease where early intervention is important. Future research needs to identify early events that occur along the cancer pathway. Hence, a paradigm shift in scientific enquiry is required which focusses on the temporal sequence of mutational events to elucidate early molecular targets in colorectal cancer. The field cancerisation theory offers such an approach whereby, based on the changes occurring in the surrounding mucosa, the initial events leading to colorectal carcinogenesis can be discerned.
P- Reviewer: Nishiyama M, Ritchie S, Stanojevic GZ S- Editor: Qi Y L- Editor: A E- Editor: Wang CH