Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3657
Peer-review started: September 19, 2014
First decision: October 29, 2014
Revised: November 10, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: March 28, 2015
Processing time: 194 Days and 23.4 Hours
AIM: To evaluate urine β2-microglobulin (β2-M), retinol-binding protein (RBP) excretion, and renal impairment with adefovir dipivoxil (ADV) for chronic hepatitis B.
METHODS: We enrolled 165 patients with chronic hepatitis B infection who were treated with ADV monotherapy (n = 90) or ADV plus lamivudine combination therapy (n = 75). An additional 165 chronic hepatitis B patients treated with entecavir were recruited as controls. We detected serum creatinine, urine β2-M, and RBP levels, and estimated the glomerular filtration rate (eGFR) at the initiation of antiviral therapy and every 6 mo for a period of five years.
RESULTS: Urine β2-M abnormalities were observed in patients during the first (n = 3), second (n = 7), third (n = 11), fourth (n = 16), and fifth (n = 21) year of ADV treatment. Urinary RBP abnormalities were observed in patients during the first (n = 2), second (n = 8), third (n = 12), fourth (n = 15), and fifth (n = 22) year of ADV treatment. eGFR decreased 20%-30% from baseline in 20 patients, 30%-50% in 12 patients, and > 50% in 3 patients during the five years of treatment. Further analysis indicated that decreases in eGFR of ≥ 30% relative to the baseline level correlated significantly with urine RBP and β2-M abnormalities. In contrast, both serum creatinine and eGFR remained stable in patients treated with entecavir, and only one of these patients developed a urine β2-M abnormality, and two developed urine RBP abnormalities during the five years of treatment.
CONCLUSION: Urine RBP and β2-M are biomarkers of renal injury during long-term ADV treatment for chronic hepatitis B, and indicate when treatment should be switched to entecavir.
Core tip: Identifying a reliable and sensitive biomarker of early renal dysfunction would be helpful for facilitating early intervention and evaluating the effectiveness of treatments for chronic hepatitis B. Urinary β2-microglobulin and retinol-binding protein are early markers of nephrotoxicity induced by nephrotoxic substances, cardiac surgery, diabetes mellitus, or hypertension. This study shows that long-term adefovir dipivoxil therapy in patients with chronic hepatitis B results in renal impairment that correlates with abnormalities in these markers.
- Citation: Jia HY, Ding F, Chen JY, Lian JS, Zhang YM, Zeng LY, Xiang DR, Yu L, Hu JH, Yu GD, Cai H, Lu YF, Zheng L, Li LJ, Yang YD. Early kidney injury during long-term adefovir dipivoxil therapy for chronic hepatitis B. World J Gastroenterol 2015; 21(12): 3657-3662
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3657.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3657
More than 350 million people worldwide are infected with the hepatitis B virus (HBV)[1]. Hepatitis B is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma, for which high levels of HBV DNA are an independent factor[2]. Therefore, the main goal of treatment is complete suppression of HBV replication to limit progressive liver damage and improve the natural history of chronic hepatitis B (CHB). Currently, oral nucleoside analogs have demonstrated success in suppressing virus replication, with few side effects. Evidence-based medicine has demonstrated that a slow virologic response after initiation of nucleoside analog treatment is associated with high rates of long-term drug resistance[3,4].
Among the available nucleoside analogs, adefovir dipivoxil (ADV) is a phosphonate acyclic nucleotide analog of AMP. It is a potent inhibitor of HBV reverse transcriptase and is effective for patients with hepatitis B e antigen (HBeAg)-positive and HBeAg-negative CHB[5,6]. ADV also shows no cross-resistance with other nucleoside analogs such as lamivudine (LAM), telbivudine, and entecavir (ETV), and thus is widely used as a rescue therapy for these drugs[7,8]. However, renal dysfunction associated with prolonged use of ADV has been reported recently[9-11]. For example, in a study of 10 mg ADV combined with 100 mg LAM, serum creatinine increased in 38% of patients following median treatment duration of 38 mo[12]. However, most routine renal function tests used in these studies were based on serum creatinine, blood urea nitrogen, and estimated glomerular filtration rate (eGFR), which failed to identify early stages of renal dysfunction and structural injury. Therefore, identification of a reliable and sensitive biomarker of early renal dysfunction would be helpful for facilitating early intervention and evaluating its effectiveness in CHB patients.
β2-Microglobulin (β2-M) is an 11.8-kDa protein, and is a light chain of major histocompatibility class I expressed on the surface of every nucleated cell[13]. Retinol-binding protein (RBP) is a protein of 21 kDa that is synthesized by the liver. Both β2-M and RBP are subject to glomerular filtration, the bulk of which undergo proximal tubular reabsorption and catabolism, which might be disrupted in renal dysfunction[14]. β2-M and RBP excretion in urine has been reported as an early marker of nephrotoxicity induced by nephrotoxic substances, cardiac surgery, diabetes mellitus, or hypertension[15]. At present, it remains unclear whether urine β2-M and RBP can be used as early markers to diagnose renal impairment in CHB patients with long-term ADV treatment. In this study, we aimed to evaluate the relationship between urine β2-M and RBP excretion and early renal impairment during long-term ADV treatment in CHB patients.
From January 2007 to March 2010, 165 patients diagnosed with CHB at the First Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) were treated with ADV monotherapy (n = 90) or ADV and LAM combination therapy (n = 75). An additional 165 CHB patients treated with ETV were also recruited as controls. All the patients had normal renal function at the outset of ADV and ETV treatment (serum creatinine < 59 μmol/L and eGFR of ≥ 50 mL/min per 1.73 m2). Urinary excretion of β2-M and RBP was not detected in any of the patients at the beginning of the study. We excluded patients infected with hepatitis delta virus, hepatitis C virus, or those who had HIV co-infection. Patients with hypertension, diabetes mellitus, hepatocellular carcinoma, autoimmune hepatitis, alcoholic liver cirrhosis, or severe heart, renal and brain diseases were also excluded. All patients who participated in this study provided informed consent and were aware of the procedures to be conducted. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University.
Serum HBV markers, including hepatitis B surface antigen, anti-HBs, HBeAg, hepatitis B e antibody (anti-HBe), and hepatitis B core antibody, were detected by commercially available enzyme immunoassays (Abbott Laboratories, Chicago, IL, United States). Serum HBV DNA was measured by PCR with a linear range between 1 × 103 copies/mL and 5 × 108 copies/mL (Shanghai ZJ Bio-Tech Co. Ltd., China).
Patients visited our hospital every 3-6 mo after the start of ADV and ETV treatment. Follow-up clinical assessments included physical examination, HBeAg and anti-HBe, quantitative HBV DNA, serum biochemistry, α-fetoprotein, renal function, and ultrasonography or CT. The eGFR (measured as mL/min per 1.73 m2) was calculated by the Chinese equation [175 × Pcr1.234× age0.179 (female × 0.79)]. Renal impairment was indicated by a decrease in eGFR to < 50 mL/min per 1.73 m2. Urine β2-M and RBP were tested in the First Affiliated Hospital of Zhejiang University. The normal values of urine β2-M and RBP were 0.000-0.025 g/mol Cr, respectively.
SPSS version 16.0 software (SPSS Inc., Chicago, IL, United States) was used for data analysis. Measurements are presented as mean ± SD and comparisons were conducted using the Student’s t test. Proportions are presented as percentages, and rate comparisons were performed using the χ2 test. The cumulative incidence of renal impairment and urine β2-M and RBP changes were calculated using the Kaplan-Meier method, and group data were calculated using the log rank test. The Cox proportional hazard regression model was used to estimate univariate and multivariate risk factors for urine microprotein (β2-M and RBP) abnormalities. P < 0.05 was considered significant.
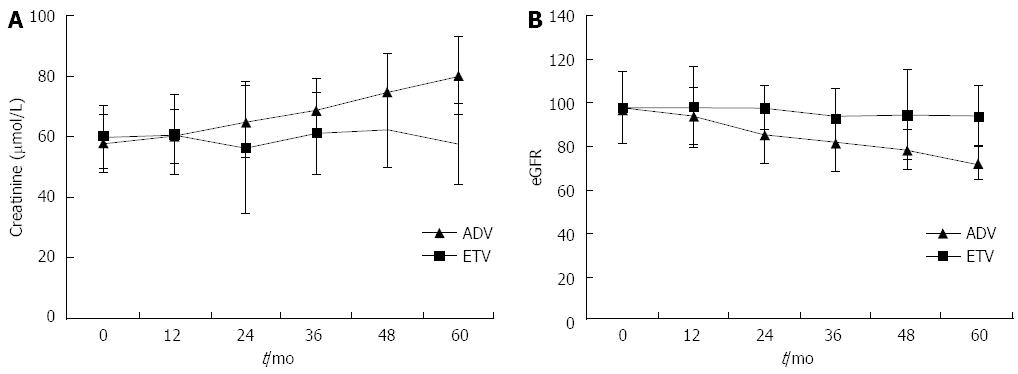
Baseline characteristics of the groups did not differ, and are presented in Table 1. Figure 1 shows the dynamic changes in the mean value of creatinine and eGFR from baseline during ADV (with or without LAM) and ETV treatment. The creatinine level increased gradually from the second year, and was increased 20%-30% from baseline in 32/165 (19.4%) patients, 30%-50% in 15/165 (9.1%), and > 50% in 4/165 (2.4%) patients during the five-year period. Serum creatinine was > 104 μmol/L in 5/165 (3.0%) patients treated with ADV. eGFR decreased 20%-30% from baseline in 20/165 (12.1%) patients, 30%-50% in 12/165 (7.2%), and > 50% in 3/165 (1.8%) in patients treated with ADV, and three patients displayed renal impairment (eGFR < 50 mL/min per 1.73 m2). In the ETV control treatment group, both creatinine and eGFR remained stable over the five-year period.
| Characteristic | ADV or ADV + LAM(n = 165) | ETV(n = 165) | P value |
| Age (yr) | 46.2 ± 9.2 | 48.6 ± 8.7 | 0.89 |
| Male | 115 (69.7) | 120 (72.7) | 0.68 |
| Treatment duration (mo) | 54.6 (12–95) | 53.8 (12–98) | 0.76 |
| HBeAg positive | 95 (57.6) | 93 (56.3) | 0.64 |
| HBV DNA (log10 copies/mL) | 6.65 ± 0.93 | 6.54 ± 0.89 | 0.88 |
| Total bilirubin (mmol/L) | 48.9 (23–71) | 45.7 (21–75) | 0.76 |
| ALT (U/L) | 146 (85–210) | 156 (89–216) | 0.54 |
| Albumin (g/L) | 45.6 ± 6.7 | 44.9 ± 7.1 | 0.32 |
| Creatinine (mmol/L) | 57.8 ± 4.6 | 59.7 ± 8.3 | 0.45 |
| eGFR (mL/min per 1.73 m2) | 97.8 ± 10.7 | 96.7 ± 13.5 | 0.23 |
| Inorganic phosphate (mmol/L) | 1.43 ± 0.9 | 1.37 ± 0.8 | 0.12 |
| b2-M (g/mol Cr) | 1.4 ± 0.006 | 1.3 ± 0.005 | 0.55 |
| RBP (g/mol Cr) | 1.6 ± 0.004 | 1.7 ± 0.008 | 0.51 |
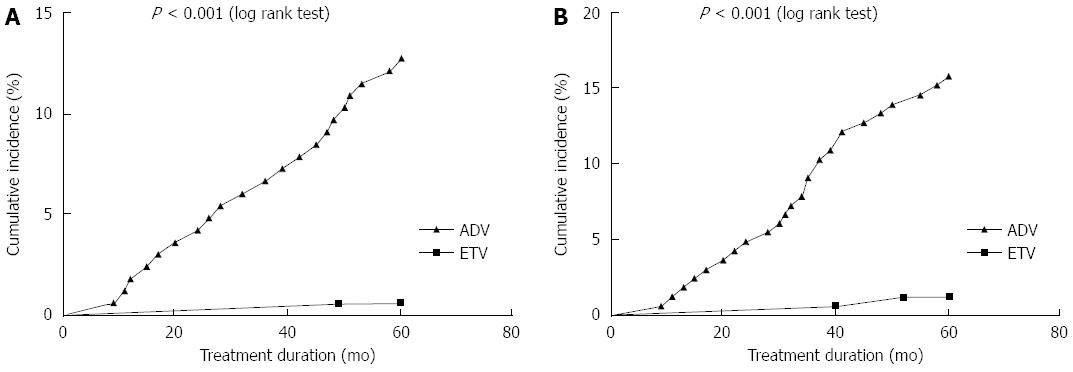
Cumulative incidences of urine microprotein abnormalities are shown in Figure 2. Urine β2-M abnormality developed in 3/165 (1.8%), 7/165 (4.2%), 11/165 (6.7%), 16/165 (9.7%), and 21/165 (12.7%) patients in the first, second, third, fourth, and fifth year of ADV or ADV plus LAM treatment, respectively. Urine RBP abnormality developed in 2/165 (1.2%), 8/165 (4.8%), 12/165 (1.8%), 15/165 (9.1%), and 22/165 (13.3%) patients in the first, second, third, fourth, and fifth year of treatment, respectively. Only 1/165 (0.1%) patient developed a urine β2-M abnormality, and 2/165 (1.2%) patients developed urine RBP abnormalities during the five years of ETV treatment. The incidence of urine β2-M abnormality in the ADV treatment group was higher than that in the ETV group (P < 0.001).
Further analysis indicated that a ≥ 30% decrease in eGFR relative to the baseline level after five years correlated significantly with urine RBP and β2-M abnormality (P = 0.006 and 0.005, respectively).
The results of univariate and multivariate analyses are indicated in Table 2. Univariate analysis showed that old age (≥ 50 years), ADV treatment, and baseline mild renal dysfunction (eGFR < 80 mL/min per 1.73 m2) were associated with the development of urine β2-M abnormality (all P < 0.001). Multivariate analysis indicated that old age (P = 0.003) and ADV treatment (P = 0.005) were significant predictors of urine β2-M abnormality.
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age ≥ 50 yr | 5.237 (2.687-10.742) | < 0.001 | 3.675 (1.612-7.865) | 0.003 |
| Male | 1.523 (0.876-3.487) | 0.782 | - | - |
| Body weight | 1.323 (0.712-3.237) | 0.574 | - | - |
| Baseline eGFR < 80 mL/min per 1.73 m2 | 3.879 (1.657-7.986) | 0.001 | - | - |
| ADV treatment | 5.178 (2.358-9.867) | < 0.001 | 3.078 (1.328-6.871) | 0.005 |
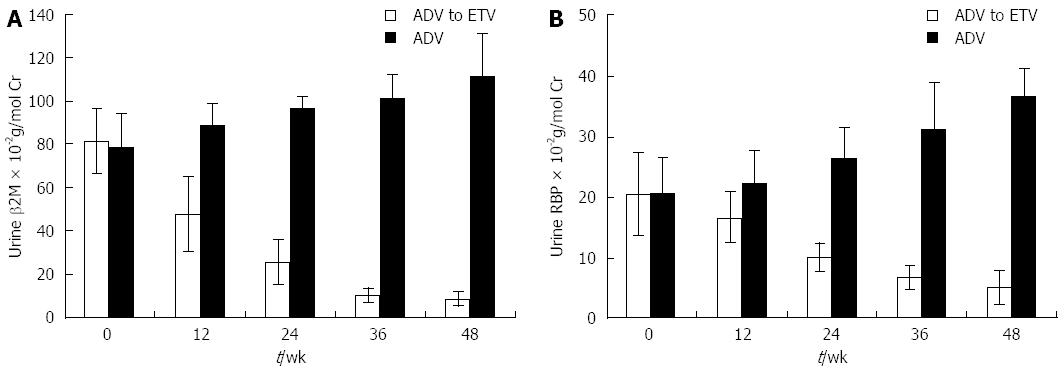
Among 22 patients with urine β2-M abnormalities, 11 switched to ETV treatment while the other 11 continued ADV treatment. As indicated in Figure 3, both urine RBP and β2-M decreased gradually after switching to ETV treatment for 48 wk, while both urine RBP and β2-M increased gradually in the ADV or ADV plus LAM treatment group. eGFR increased from 78.5 ± 11.8 mL/min per 1.73 m2 to 89.5 ± 13.7 mL/min per 1.73 m2 after switching to ETV treatment for 48 wk. eGFR deteriorated from 79.8 ± 14.3 mL/min per 1.73 m2 to 68.5 ± 14.3 mL/min per 1.73 m2 if ADV was continuously applied.
Renal dysfunction is one of the serious adverse effects of ADV. In a previous retrospective study of 687 patients, during a median treatment duration of 27 mo, 10.5% of patients developed renal impairment, which was defined as a decrease in eGFR > 20% relative to baseline[16]. In another study of 292 patients treated with ADV plus LAM combination therapy, 9.6% patients developed renal impairment during a median treatment duration of 64.3 mo[9]. Our results show that during the five years of ADV or ADV plus LAM treatment, creatinine increased by 20%-30% from baseline in 19.4%, 30%-50% in 9.1%, and > 50% in 2.4% of patients, while the eGFR decreased 20%-30% from baseline in 12.1%, 30%-50% in 7.2%, and > 50% in 1.8% of patients. Furthermore, five patients had serum creatinine > 104 μmol/L and three had eGFR < 50 mL/min per 1.73 m2 during the five years of ADV or ADV plus LAM treatment. These rates are less than those reported previously, because all the CHB patients with hypertension, diabetes mellitus, and liver cirrhosis were excluded from our study. In contrast to ADV treatment, both creatinine and eGFR remained stable in the ETV treatment group.
Routine renal function tests based on serum creatinine and blood urea nitrogen fail to identify early stages of renal dysfunction and structural injury. Creatinine change is not specific because it can also occur in non-renal disease, reflecting changes in muscle mass and nutrition intake[17]. eGFR mainly reflects the renal filtration capacity, and cannot detect the early stage of renal tubular disorders. Therefore, other biomarkers have been examined, such as β2-M, RBP, kidney injury molecule-1, neutrophil gelatinase associated lipocalin, interleukin-18, and sodium/hydrogen exchange form 3[18]. These biomarkers show increased levels in the urine at an early stage after renal dysfunction occurs. Our results indicate that 1.8%, 4.2%, 6.7%, 9.7% and 12.7% of patients developed urine β2-M abnormalities, and 1.2%, 1.8%, 4.8%, 9.1%, and 13.3% of patients developed urine RBP abnormalities in the first, second, third, fourth, and fifth year of treatment, respectively. Only 3% of patients displayed serum creatinine > 104 μmol/L and 1.8% eGFR < 50 mL/min per 1.73 m2 at the same time. This indicates that the urine β2-M and RBP abnormalities preceded creatinine abnormality renal impairment.
The mechanism of ADV-induced nephrotoxicity may be related to drug accumulation in renal proximal tubules after long-term administration, which may reduce the reabsorption capacity of microproteins (β2-M and RBP), amino acids, glucose, phosphorus, and calcium[19,20]. Detection of these biomarkers in serum or urine might be important, because at this stage, positive results should raise awareness of the risk of renal damage at the moment when it is still reversible with prophylactic or therapeutic intervention. In our study, among 11 patients with urine β2-M abnormality, urine RBP and β2-M decreased gradually, and eGFR increased after switching to ETV treatment for 48 wk, whereas urine RBP and β2-M continued to increase, and the eGFR decreased in those remaining on ADV treatment. Our results strongly suggest that ADV should be switched to ETV treatment immediately if urine RBP or β2-M is detected in CHB patients.
We also analyzed the risk factors of urine β2-M abnormality after long-term ADV treatment. Consistent with previous reports, our univariate analysis indicated that age was a significant and independent factor of urine β2-M abnormality, together with long-term ADV administration and baseline mild dysfunction (eGFR < 80 mL/min per 1.73 m2)[9,10]. Multivariate analysis suggested that age and ADV administration were independent factors of urine β2-M abnormality.
In conclusion, both urine RBP and β2-M are sensitive biomarkers for detecting early renal injury during long-term ADV treatment. Therefore, when RBP or β2-M are detected in urine, ADV should be switched to ETV immediately. Moreover, ADV should be avoided as a first-line treatment for CHB patients, especially for elderly people.
Ding F is a graduate student, now working in the Sixth People’s Hospital of Shaoxing, Zhejiang, China.
Renal dysfunction associated with prolonged use of adefovir dipivoxil (ADV) has been reported recently. However, most routine renal function tests used in these studies were based on serum creatinine, blood urea nitrogen, and estimated glomerular filtration rate (eGFR), which fail to identify early stages of renal dysfunction and structural injury. Therefore, identification of a reliable and sensitive biomarker of early renal dysfunction would be helpful in facilitating early intervention and evaluating its effectiveness for chronic hepatitis B (CHB) patients. The aim of this study was to evaluate the relationship between urine β2-microglobulin (β2-M) and retinol-binding protein (RBP) excretion and early renal impairment during long-term ADV in CHB patients.
β2-M is an 11.8-kDa protein, and is a light chain of major histocompatibility class I expressed on the surface of every nucleated cell. RBP is a protein of 21 kDa that is synthesized by the liver. Both β2-M and RBP are subject to glomerular filtration, and undergo proximal tubular reabsorption and catabolism, which might be disrupted in renal dysfunction. β2-M and RBP excretion in urine has been reported as an early marker of nephrotoxicity induced by nephrotoxic substances, cardiac surgery, diabetes mellitus, or hypertension. At present, it remains unclear whether urine β2-M and RBP can be used as early markers to diagnose renal impairment in CHB patients with long-term ADV treatment.
Routine renal function tests based on serum creatinine and blood urea nitrogen fail to identify early stages of renal dysfunction and structural injury. Recently, several biomarkers have been found, such as β2-M, RBP, kidney injury molecule-1, neutrophil gelatinase associated lipocalin, interleukin-18, and sodium/hydrogen exchange form 3, which show increased levels in the urine at an early stage after renal dysfunction occurs. These results indicated that a proportion of patients developed urine β2-M and RBP abnormalities during long-term ADV treatment, which occur before creatinine abnormality and impaired eGFR. Thus, detection of urine β2-M and RBP can help identify early renal dysfunction during long-term ADV treatment.
The study results suggest that urine β2-M and RBP can be used as early biomarkers to indicate renal dysfunction during long-term ADV treatment.
ADV is a phosphonate acyclic nucleotide analog of AMP. It is a potent inhibitor of hepatitis B virus reverse transcriptase and is effective for patients with chronic hepatitis B infections.
This is a good descriptive study in which the authors analyzed the β2-M and RBP excretion induced by long-term ADV treatment in the CHB patients. The results are interesting and suggest that urine RBP and β2-M are sensitive biomarkers of early renal injury during long-term ADV treatment. ADV should be switched to ETV as soon as urine RBP or β2-M is detected. ADV should be avoided as first-line treatment for CHB.
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1765] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 2. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2395] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 3. | Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, Han S, Poynard T, Myers M, Chao G. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Locarnini S. Primary resistance, multidrug resistance, and cross-resistance pathways in HBV as a consequence of treatment failure. Hepatol Int. 2008;2:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1020] [Article Influence: 44.3] [Reference Citation Analysis (1)] |
| 6. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 735] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 7. | Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Mukaide M, Tanaka Y, Shin-I T, Yuen MF, Kurbanov F, Yokosuka O, Sata M, Karino Y, Yamada G, Sakaguchi K. Mechanism of entecavir resistance of hepatitis B virus with viral breakthrough as determined by long-term clinical assessment and molecular docking simulation. Antimicrob Agents Chemother. 2010;54:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Tanaka M, Suzuki F, Seko Y, Hara T, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y. Renal dysfunction and hypophosphatemia during long-term lamivudine plus adefovir dipivoxil therapy in patients with chronic hepatitis B. J Gastroenterol. 2014;49:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Gara N, Zhao X, Collins MT, Chong WH, Kleiner DE, Jake Liang T, Ghany MG, Hoofnagle JH. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Eguchi H, Tsuruta M, Tani J, Kuwahara R, Hiromatsu Y. Hypophosphatemic osteomalacia due to drug-induced Fanconi’s syndrome associated with adefovir dipivoxil treatment for hepatitis B. Intern Med. 2014;53:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tamori A, Enomoto M, Kobayashi S, Iwai S, Morikawa H, Sakaguchi H, Habu D, Shiomi S, Imanishi Y, Kawada N. Add-on combination therapy with adefovir dipivoxil induces renal impairment in patients with lamivudine-refractory hepatitis B virus. J Viral Hepat. 2010;17:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Che M, Xie B, Xue S, Dai H, Qian J, Ni Z, Axelsson J, Yan Y. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron Clin Pract. 2010;115:c66-c72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Hoet P, Haufroid V, Deumer G, Dumont X, Lison D, Hantson P. Acute kidney injury following acute liver failure: potential role of systemic cadmium mobilization? Intensive Care Med. 2012;38:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Molitoris BA, Melnikov VY, Okusa MD, Himmelfarb J. Technology Insight: biomarker development in acute kidney injury--what can we anticipate? Nat Clin Pract Nephrol. 2008;4:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Kim YJ, Cho HC, Sinn DH, Gwak GY, Choi MS, Koh KC, Paik SW, Yoo BC, Lee JH. Frequency and risk factors of renal impairment during long-term adefovir dipivoxil treatment in chronic hepatitis B patients. J Gastroenterol Hepatol. 2012;27:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Devarajan P. Neutrophil gelatinase-associated lipocalin--an emerging troponin for kidney injury. Nephrol Dial Transplant. 2008;23:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, Fidalgo P, Frade F, Devarajan P. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi’s syndrome. Am J Kidney Dis. 2003;41:292-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Imazeki F S- Editor: Yu J L- Editor: AmEditor E- Editor: Wang CH