Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.2918
Peer-review started: August 4, 2014
First decision: August 15, 2014
Revised: September 10, 2014
Accepted: November 30, 2014
Article in press: December 1, 2014
Published online: March 14, 2015
Processing time: 224 Days and 16.5 Hours
AIM: To improve an asialoglycoprotein receptor (ASGPR)-based enrichment method for detection of circulating tumor cells (CTCs) of hepatocellular carcinoma (HCC).
METHODS: Peripheral blood samples were collected from healthy subjects, patients with HCC or various other cancers, and patients with hepatic lesions or hepatitis. CTCs were enriched from whole blood by extracting CD45-expressing leukocytes with monoclonal antibody coated-beads following density gradient centrifugation. The remaining cells were cytocentrifuged on polylysine-coated slides. Isolated cells were treated by triple immunofluorescence staining with CD45 antibody and a combination of antibodies against ASGPR and carbamoyl phosphate synthetase 1 (CPS1), used as liver-specific markers, and costained with DAPI. The cell slide was imaged and stained tumor cells that met preset criteria were counted. Recovery, sensitivity and specificity of the detection methods were determined and compared by spiking experiments with various types of cultured human tumor cell lines. Expression of ASGPR and CPS1 in cultured tumor cells and tumor tissue specimens was analyzed by flow cytometry and triple immunofluorescence staining, respectively.
RESULTS: CD45 depletion of leukocytes resulted in a significantly greater recovery of multiple amounts of spiked HCC cells than the ASGPR+ selection (Ps < 0.05). The expression rates of either ASGPR or CPS1 were different in various liver cancer cell lines, ranging between 18% and 99% for ASGPR and between 9% and 98% for CPS1. In both human HCC tissues and liver cancer cell lines, there were a few HCC cells that did not stain positive for ASGPR or CPS1. The mixture of monoclonal antibodies against ASGPR and CPS1 identified more HCC cells than either antibody alone. However, these antibodies did not detect any tumor cells in blood samples spiked with the human breast cancer cell line MCF-7 and the human renal cancer cell line A498. ASGPR+ or/and CPS1+ CTCs were detected in 29/32 (91%) patients with HCC, but not in patients with any other kind of cancer or any of the other test subjects. Furthermore, the improved method detected a higher CTC count in all patients examined than did the previous method (P = 0.001), and consistently achieved 12%-21% higher sensitivity of CTC detection in all seven HCC patients with more than 40 CTCs.
CONCLUSION: Negative depletion enrichment combined with identification using a mixture of antibodies against ASGPR and CPS1 improves sensitivity and specificity for detecting circulating HCC cells.
Core tip: We previously described asialoglycoprotein receptor (ASGPR)-based positive selection for the enrichment and further detection of circulating tumor cells (CTCs) of hepatocellular carcinoma (HCC). However, expression of ASGPR is heterogeneous in human HCC, and ASGPR- cells are missed by this method. In this study, we describe an improved method using depletion enrichment with identification using antibodies against ASGPR and carbamoyl phosphate synthetase 1. The improved method significantly improved sensitivity for CTC enrichment, and also provided high specificity for CTC detection in patients with HCC, thereby minimizing false negative/positive results.
- Citation: Liu HY, Qian HH, Zhang XF, Li J, Yang X, Sun B, Ma JY, Chen L, Yin ZF. Improved method increases sensitivity for circulating hepatocellular carcinoma cells. World J Gastroenterol 2015; 21(10): 2918-2925
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/2918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.2918
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third leading cause of cancer-related deaths[1,2]. There are an estimated 100 million newly diagnosed patients with HCC per year, with 548600 deaths from liver cancer reported in the year 2000[3]. For patients with adequate hepatocellular function and anatomically resectable tumors, liver resection is generally the first treatment consideration. However, up to 40% of those patients develop recurrences within the first year and there is a 25%-40% expected five-year survival rate[4,5]. The spread of disseminated circulating tumor cells (CTCs) in the blood plays a major role in tumor recurrence and the initiation of metastases after surgery[6,7]. CTCs may represent an active source of HCC metastasis or recurrence, as transplanted allografts are the most common site of early tumor recurrence[8,9].
Currently, the detection and molecular characterization of CTCs are utilized in a variety of malignancies, and have become one of the most active areas of translational cancer research[10-12]. Various technologies for CTC detection and enrichment have been developed in the past decade, including strategies that mainly depend on epithelial cell-surface markers, such as epithelial cell adhesion molecules[13,14]. However, only a small proportion of HCC cells express this marker[15-18], which only identifies low numbers of CTCs in approximately 30%-40% of patients[19-21]. Moreover, Morris et al[21] used a marker-independent isolation approach to successfully identify CTCs in all patient samples, demonstrating the poor concordance between epithelial cell adhesion molecule-positive CTCs and HCC CTCs.
We recently developed and validated an asialoglycoprotein receptor (ASGPR)-based magnetic cell separation method for detection of HCC CTCs[22,23]. ASGPR is an abundant cell surface receptor specific to mammalian hepatocytes that recognizes and internalizes desialylated glycoproteins with exposed terminal N-acetylgalactosamine or galactose residues[24,25]. However, several studies reported that the expression of ASGPR is heterogeneous in human HCC[26-28]. Therefore, we evaluated a modified strategy to increase analytical sensitivity of the assay. The modified method involves an initial depletion of CD45+ leukocytes from the sample, followed by detection of CTCs with a combination of two antibodies against liver-specific markers, ASGPR and carbamoyl phosphate synthetase 1 (CPS1; a newly identified antigen for Hep Par 1)[29].
Participants in this study included patients with HCC (n = 32), 17 patients with other types of cancer, including breast (n = 3), lung (n = 2), esophageal (n = 3), gastric (n = 5) and colorectal (n = 4) cancer, patients with other liver diseases, including benign intrahepatic space-occupying lesions (n = 12), acute hepatitis A (n = 3), chronic hepatitis B (n = 6), chronic hepatitis C (n = 4) and cirrhosis (n = 15), as well as healthy volunteers (n = 20). Peripheral venous blood samples (5 mL) from each subject were collected into VACUETTE polyethylene tubes containing ethylene diaminetetraacetic acid (Greiner Bio-One GmbH; Frickenhausen, Germany). The study was approved by the Biomedical Ethics Committee of Eastern Hepatobiliary Surgery Hospital (Shanghai, China) and written informed consent was obtained from all participants.
Human liver cancer cell lines (HepG2, Hep3B, Huh7, MHCC-97H, MHCC-97L, PLC/PRF/5, and SMMC-7721), the human breast cancer cell line MCF-7, and the human renal cancer cell line A498 were obtained from American Type Culture Collection (Manassas, VA, United States) and cultured according to their instructions.
A total of 4 × 105 cells were incubated at 37 °C for 45 min with monoclonal mouse anti-ASGPR and/or monoclonal anti-CPS1 antibodies (Abcam; Cambridge, United Kingdom) followed by staining with fluorescein isothiocyanate-conjugated secondary antibody (Beyotime; Shanghai, China) at 4 °C for 30 min in the dark. Flow cytometric analysis was then performed using a FACSCalibur system (Becton, Dickinson and Co.; Franklin Lakes, NJ, United States). For spiking experiments, various numbers of tumor cells were added to the 5 mL blood sample aliquots.
HCC tissue sections were incubated with anti-ASGPR and rabbit anti-CPS1 (Abcam) antibodies at 4 °C overnight, and then stained with Cy3-conjugated goat anti-rabbit and fluorescein isothiocyanate-conjugated goat anti-mouse IgG secondary antibodies (Beyotime) with DAPI at room temperature for 30 min.
Cell slides were incubated with mouse anti-cytokeratin (CK) antibody (CK3-6H5; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) or a mouse monoclonal antibody cocktail against ASGPR and CPS1 and a rat anti-human CD45 monoclonal antibody (Santa Cruz Biotechnology Inc., Dallas, TX, United States). Slides were then stained with Cy3-conjugated goat anti-mouse and Alexa Fluor 488-conjugated rabbit anti-rat (Invitrogen of Thermo Fisher Scientific Inc., Waltham, MA, United States) IgG secondary antibodies.
After enriching mononuclear cells and tumor cells from the whole blood samples by density gradient with Ficoll-Paque PLUS (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom), CD45+ leukocytes were depleted from the enriched cells with 25 μL of beads coated with anti-CD45 monoclonal antibody (Miltenyi Biotec) according to the manufacturer’s instructions. The remaining CD45- cells were cytocentrifuged on polylysine-coated slides, which were dried and stored at 4 °C for subsequent immunofluorescence staining.
The cell slides were imaged and CTCs counted according to the method previously described[22].
SPSS statistical software (SPSS Inc., Chicago, IL, United States) was used to conduct Student’s t-tests and ANOVA. Data are presented as mean ± standard deviation, and a two-sided P < 0.05 was considered statistically significant.
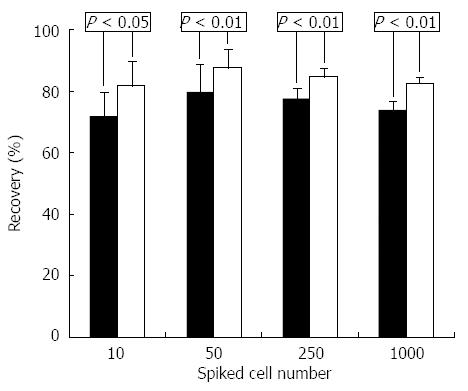
To compare two methods of HCC enrichment, one of the healthy volunteer’s blood samples was spiked with various amounts of HepG2 cells and recovery was measured by enumeration of spiked HepG2 cells after enrichment. The results show that a significantly greater proportion of HepG2 cells were recovered with CD45+ depletion than by ASGPR+ selection at each spiking level (Ps < 0.05) (Figure 1).
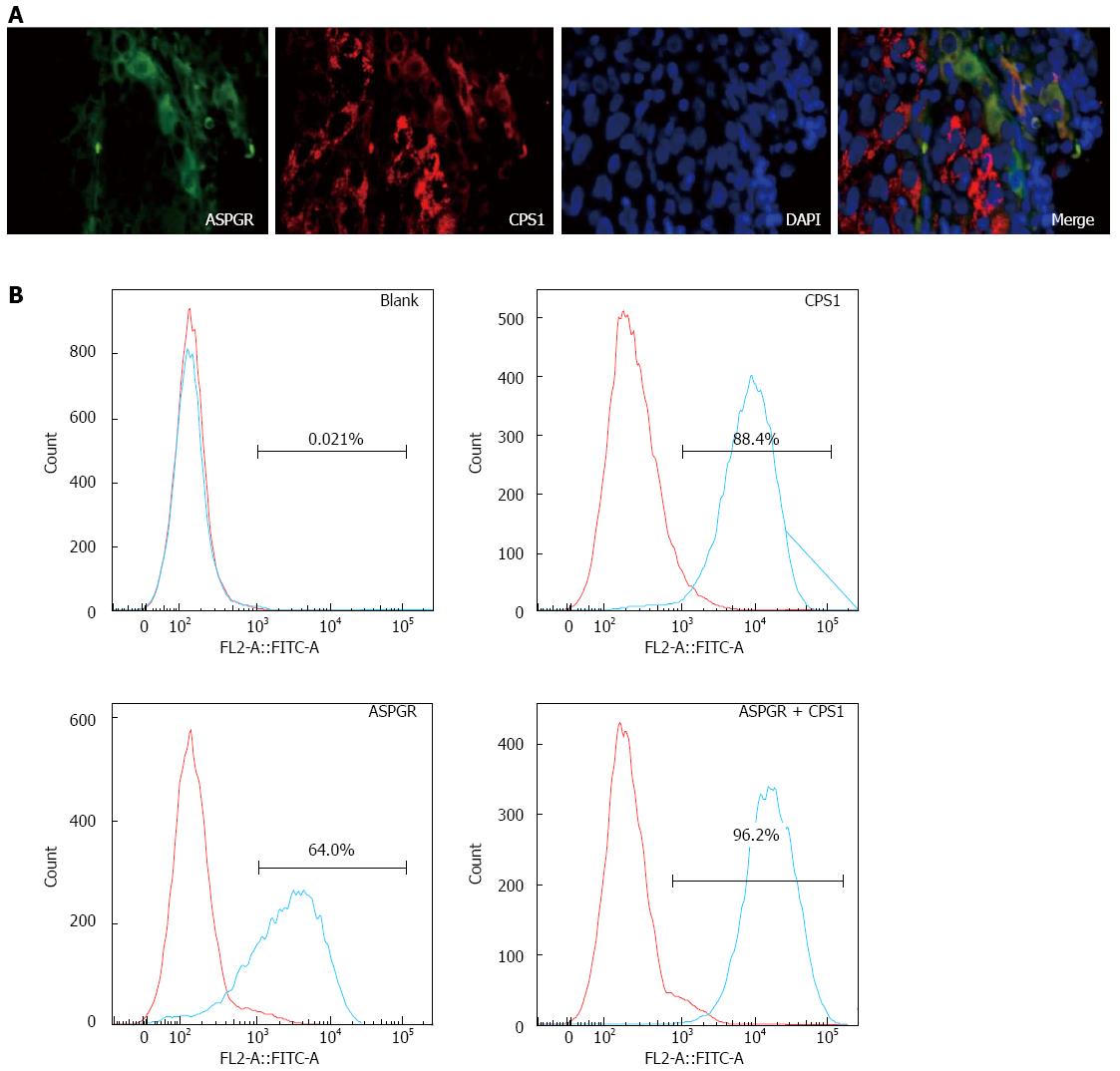
Immunofluorescence analysis of human HCC tissues showed that, based on DAPI staining, almost all of the HCC cells stained positive for both ASGPR and CPS1, whereas there were cells that did not stain with ASGPR or CPS1 separately (Figure 2A). Flow cytometry was performed on multiple cancer cell lines (HepG2, Hep3B, Huh7, MHCC-97H, MHCC-97L, PLC/PRF/5, and SMMC-7721), revealing that the expression rates of ASGPR varied between 18% and 99%, and between 9% and 98% for CPS1. A typical flow cytometry experiment from Hep3B cells is shown in Figure 2B. These results demonstrate heterogeneous expression of ASGPR and CPS1 in human HCC.
We then used the antibody cocktail containing both anti-ASGPR and anti-CPS1 to analyze a healthy volunteer’s blood sample spiked with HepG2 cells, and identified more cells compared with either antibody alone (Table 1). However, neither the ASGPR nor the CPS1 antibody detected tumor cells in blood samples spiked with cell lines MCF-7 and A498 (100 cells spiked), though CD45 depletion of leukocytes enriched them from the spiked blood samples (data not shown).
| Spiked cells, n | ASGPR | CPS1 | ASGPR + CPS1 | |||
| Detected cells, n | Detection rate | Detected cells, n | Detection rate | Detected cells, n | Detection rate | |
| 10 | 7 ± 1 | 74% ± 11% | 6 ± 1 | 64% ± 11% | 8 ± 1 | 84% ± 9% |
| 50 | 36 ± 3 | 72% ± 6% | 31 ± 3 | 62% ± 6% | 44 ± 3 | 89% ± 7% |
| 250 | 150 ± 16 | 60% ± 6% | 140 ± 16 | 56% ± 6% | 215 ± 11 | 86% ± 4% |
| 1000 | 573 ± 24 | 57% ± 3% | 531 ± 21 | 53% ± 2% | 824 ± 21 | 83% ± 2% |
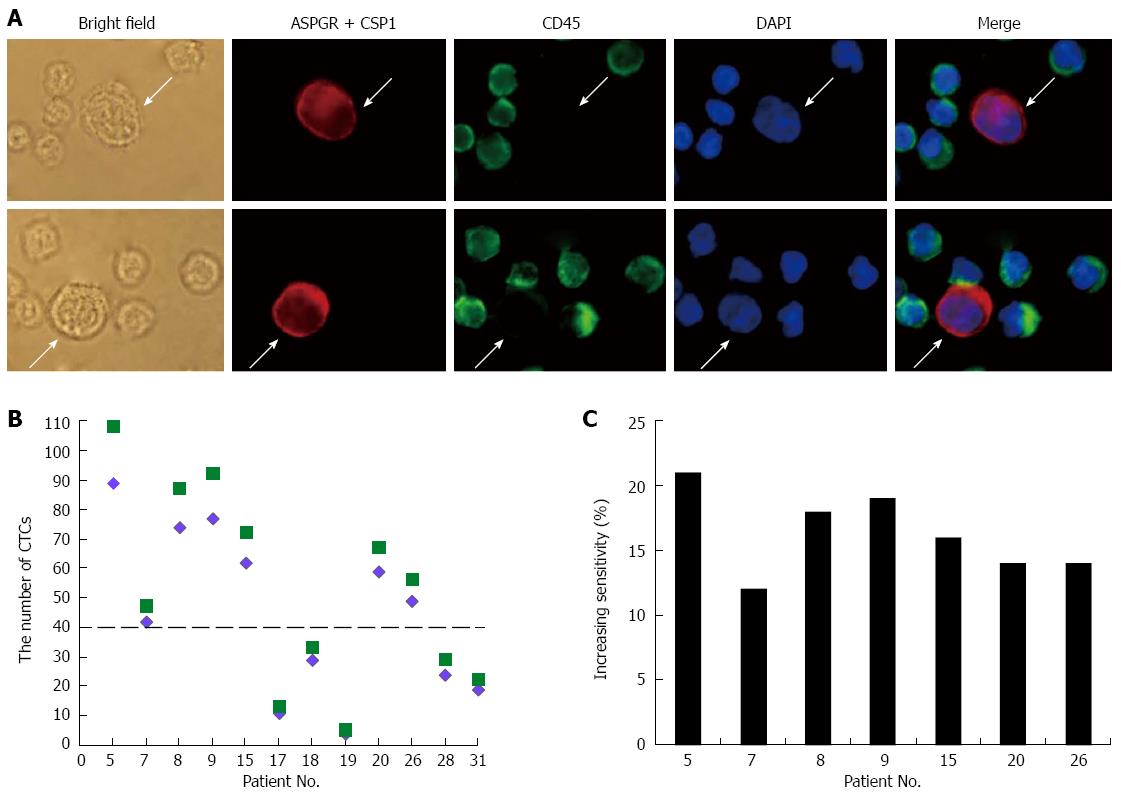
HCC CTCs were defined as large cells with a morphologically intact DAPI-stained nucleus that were CD45- and ASGPR+ and/or CPS1+ (Figure 3A). Using the antibody cocktail in CD45-depleted blood samples, CTCs were detected in 29/32 (91%) patients with HCC (Table 2). On the contrary, no CTCs were detected in samples from the healthy volunteers, or from any of the other cancer or hepatitis patient samples. This method was then compared with the previously reported method (ASGPR+ selection followed by identification with CK and CPS1 antibodies[23]). The current method detected a significantly higher CTC count than did the previous method (P = 0.001), with 12%-21% increased sensitivity of CTC detection in HCC patients with more than 40 CTCs (Figure 3B and C).
| Patient ID | Age/sex | Tumor size, cm | Portal vein tumor thrombus | TNM | CTCs, n |
| 1 | 70/M | > 5 | + | IV | 56 |
| 2 | 49/M | > 5 | + | III | 72 |
| 3 | 27/M | > 5 | - | III | 29 |
| 4 | 51/M | < 5 | - | I | 11 |
| 5 | 49/M | > 5 | + | IV | 108 |
| 6 | 48/F | < 5 | + | III | 43 |
| 7 | 53/M | > 5 | + | IV | 47 |
| 8 | 64/M | > 5 | + | III | 87 |
| 9 | 47/F | > 5 | + | IV | 92 |
| 10 | 62/M | > 5 | + | III | 51 |
| 11 | 35/F | < 5 | - | I | 0 |
| 12 | 42/F | < 5 | - | II | 23 |
| 13 | 57/M | < 5 | - | II | 26 |
| 14 | 41/F | < 5 | - | II | 23 |
| 15 | 71/M | > 5 | + | III | 72 |
| 16 | 37/M | < 5 | - | II | 15 |
| 17 | 47/F | < 5 | - | I | 13 |
| 18 | 65/M | > 5 | - | III | 33 |
| 19 | 65/M | < 5 | - | I | 5 |
| 20 | 63/M | > 5 | + | IV | 67 |
| 21 | 46/F | < 5 | - | II | 29 |
| 22 | 43/M | < 5 | - | II | 9 |
| 23 | 48/M | < 5 | - | I | 0 |
| 24 | 37/M | > 5 | + | III | 50 |
| 25 | 35/F | > 5 | + | III | 38 |
| 26 | 56/F | < 5 | - | II | 56 |
| 27 | 77/F | < 5 | - | II | 17 |
| 28 | 49/M | < 5 | - | II | 29 |
| 29 | 58/M | < 5 | - | I | 0 |
| 30 | 38/M | > 5 | + | III | 50 |
| 32 | 75/F | < 5 | - | II | 22 |
| 32 | 74/M | < 5 | - | II | 28 |
ASGPR is highly expressed in the human liver but not in other organs[24,25]. Given the generally accepted absence of normal hepatocytes in circulation, blood cells labeled with the ASGPR antibody are thus considered to be circulating HCC cells. However, not all HCC tissues and human liver cancer cell lines express ASGPR, which is consistent with previous reports[26,27]. Alternatively, detection can be enhanced by the removal of normal hematopoietic cells, such as CD45+ cells, thereby enriching the blood cell suspension for the rare tumor cells. Additional advantages to this type of negative depletion include potential time/cost-efficiency and improved sample yield and purity, allowing subsequent multiple biomarker analysis[30]. A number of authors have used either cell lysis or gradient separation to remove red blood cells, followed by CD45+ depletion[31-34]. Our strategy utilized a Ficoll gradient to remove red blood cells and targeted CD45+ cells with magnetic particles for further removal, which is expected to enrich all nucleated cells, including HCC CTCs that lack expression of ASGPR.
CPS1 is a mitochondrial urea cycle enzyme found in hepatocytes[29] that can be detected with the Hep Par 1 antibody, which is commonly used to determine the hepatocellular origin of neoplasms in diagnostic surgical pathology practice. However, several studies reported heterogeneous expression of CPS1 in human HCC[35-37]. For example, Timek et al[37] reported that 17/18 small tissue biopsy specimens of HCC were positive for Hep Par 1, but only 19/29 fine-needle aspiration biopsy specimens were positive. Because of this, we previously utilized a mixture of two antibodies (against CPS1 and CK) to stain ASGPR+ HCC CTCs. However, CK was not employed in the present method, as it can detect viable circulating epithelial cells in healthy people and patients with benign diseases. To decrease the occurrence of false-positive results, we used a modified mixture of two antibodies against liver-specific antigens (ASGPR and CPS1) that are downregulated in HCC cells.
The combination of ASGPR and CPS1 antibodies allows for increased detection of cells that may express only one of the two markers, as was observed in our immunohistochemical analyses. Furthermore, when combined with CD45+ depletion, this method obtained greater than 82% recovery of HepG2 cells from blood samples. This method was specific for HCC CTCs, as breast cancer tumor cells were not detected. Consistent with these results, blood tests showed that the current system detected a higher CTC count in almost all patients examined than did the previous system[25], further indicating that the previous ASGPR-based capture process underestimated the CTC population.
Collectively, the results reported here demonstrate that negative depletion enrichment combined with identification using an antibody cocktail against ASGPR and CPS1 not only significantly improves sensitivity for CTC enrichment, but also provides high specificity for CTC detection in patients with HCC, thereby minimizing false negative/positive results.
The development of overt metastasis is preceded by the dissemination of tumor cells into blood circulation, bone marrow or the lymphatic system. The spread of circulating tumor cells (CTCs) in the blood plays a major role in the initiation of metastases and tumor recurrence after surgery.
CTCs provide a readily accessible real-time liquid biopsy of tumors, serving as biomarkers of the metastatic disease process. Currently, the detection and molecular characterization of CTCs are applied in a variety of malignancies, and have become one of the most active areas of translational cancer research.
The authors previously described asialoglycoprotein receptor (ASGPR)-based positive selection for enrichment and further detection of CTCs of hepatocellular carcinoma (HCC). In this study, they improve upon this method by combining depletion enrichment with identification using an antibody cocktail of liver-specific markers (ASGPR and carbamoyl phosphate synthetase 1). The current method significantly improves sensitivity for CTC enrichment, and provides high specificity for CTC detection in patients with HCC, thereby minimizing the possible false negative/positive results.
The improved method will allow for effective enrichment and accurate enumeration of circulating HCC cells, which has great potential for predicting HCC patient prognosis and for monitoring treatment response.
CTCs are cancer cells shed from either the primary tumor or its metastases that circulate in the peripheral blood.
The paper is well written, clear and concise. The topic is interesting. The methods are sound. Conclusions are consistent with the results.
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2564] [Article Influence: 183.1] [Reference Citation Analysis (3)] |
| 2. | Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115-4127. [PubMed] |
| 3. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1338] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 4. | Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508-518; discussion 518-519. [PubMed] |
| 5. | Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 821] [Article Influence: 63.2] [Reference Citation Analysis (3)] |
| 7. | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 2017] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Li J, Cao L, Xu W, Yin Z. Circulating tumor cells in hepatocellular carcinoma: detection techniques, clinical implications, and future perspectives. Semin Oncol. 2012;39:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Shi ZL, Yang X, Yin ZF. Targeting of circulating hepatocellular carcinoma cells to prevent postoperative recurrence and metastasis. World J Gastroenterol. 2014;20:142-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother. 2013;62:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Lianidou ES, Markou A, Strati A. Molecular characterization of circulating tumor cells in breast cancer: challenges and promises for individualized cancer treatment. Cancer Metastasis Rev. 2012;31:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol. 2015;141:189-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Jacob K, Sollier C, Jabado N. Circulating tumor cells: detection, molecular profiling and future prospects. Expert Rev Proteomics. 2007;4:741-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Attard G, de Bono JS. Utilizing circulating tumor cells: challenges and pitfalls. Curr Opin Genet Dev. 2011;21:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Proca DM, Niemann TH, Porcell AI, DeYoung BR. MOC31 immunoreactivity in primary and metastatic carcinoma of the liver. Report of findings and review of other utilized markers. Appl Immunohistochem Mol Morphol. 2000;8:120-125. [PubMed] |
| 17. | Porcell AI, De Young BR, Proca DM, Frankel WL. Immunohistochemical analysis of hepatocellular and adenocarcinoma in the liver: MOC31 compares favorably with other putative markers. Mod Pathol. 2000;13:773-778. [PubMed] |
| 18. | Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 639] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 19. | Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 20. | Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Morris KL, Tugwood JD, Khoja L, Lancashire M, Sloane R, Burt D, Shenjere P, Zhou C, Hodgson C, Ohtomo T. Circulating biomarkers in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 22. | Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH, Kang XY, Zhang Y, Liao J, Shi LH. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17:3783-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 23. | Li J, Chen L, Zhang X, Zhang Y, Liu H, Sun B, Zhao L, Ge N, Qian H, Yang Y. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLoS One. 2014;9:e96185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Stockert RJ, Morell AG. Hepatic binding protein: the galactose-specific receptor of mammalian hepatocytes. Hepatology. 1983;3:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 25. | Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Hyodo I, Mizuno M, Yamada G, Tsuji T. Distribution of asialoglycoprotein receptor in human hepatocellular carcinoma. Liver. 1993;13:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Trerè D, Fiume L, De Giorgi LB, Di Stefano G, Migaldi M, Derenzini M. The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells. Br J Cancer. 1999;81:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Shi B, Abrams M, Sepp-Lorenzino L. Expression of asialoglycoprotein receptor 1 in human hepatocellular carcinoma. J Histochem Cytochem. 2013;61:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Butler SL, Dong H, Cardona D, Jia M, Zheng R, Zhu H, Crawford JM, Liu C. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest. 2008;88:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Lustberg M, Jatana KR, Zborowski M, Chalmers JJ. Emerging technologies for CTC detection based on depletion of normal cells. Recent Results Cancer Res. 2012;195:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Balasubramanian P, Lang JC, Jatana KR, Miller B, Ozer E, Old M, Schuller DE, Agrawal A, Teknos TN, Summers TA. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS One. 2012;7:e42048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Guo J, Yao F, Lou Y, Xu C, Xiao B, Zhou W, Chen J, Hu Y, Liu Z. Detecting carcinoma cells in peripheral blood of patients with hepatocellular carcinoma by immunomagnetic beads and rt-PCR. J Clin Gastroenterol. 2007;41:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Wu C, Hao H, Li L, Zhou X, Guo Z, Zhang L, Zhang X, Zhong W, Guo H, Bremner RM. Preliminary investigation of the clinical significance of detecting circulating tumor cells enriched from lung cancer patients. J Thorac Oncol. 2009;4:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Liu H, Dong H, Robertson K, Liu C. DNA methylation suppresses expression of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular carcinoma. Am J Pathol. 2011;178:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Siddiqui MT, Saboorian MH, Gokaslan ST, Ashfaq R. Diagnostic utility of the HepPar1 antibody to differentiate hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration samples. Cancer. 2002;96:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Timek DT, Shi J, Liu H, Lin F. Arginase-1, HepPar-1, and Glypican-3 are the most effective panel of markers in distinguishing hepatocellular carcinoma from metastatic tumor on fine-needle aspiration specimens. Am J Clin Pathol. 2012;138:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lichtor T, Mocellin S, Usta J, Yan SL S- Editor: Gou SX L- Editor: Logan S E- Editor: Zhang DN